Original Article
, Volume: 17( 3)Adsorption Kinetics and Isotherms Studies of Acid Azo Dye based on Anthranilic Acid and ÃÂ-Naphthol on Jute Fabric
- *Correspondence:
- Pallavi Sarkhel
Department of Chemistry, Birla Institute of Technology-Mesra, Ranchi, Jharkhand, India
Tel: +919863023938
E-mail: sarkhelprashanta@gmail.com
Received: January 29, 2023, Manuscript No. TSPC-23-88162; Editor assigned: January 31, 2023, PreQC No. TSPC-23-88162 (PQ); Reviewed: February 14, 2023, QC No. TSPC-23-88162; Revised: April 18, 2023, Manuscript No. TSPC-23-88162 (R); Published: April 25, 2023, DOI: 10.37532/0974-7524.2023.18(1).166
Citation: Sarkhel P, Srivastava PK, Sarkhel P. Adsorption Kinetics and Isotherms Studies of Acid Azo Dye based on Anthranilic Acid and Β-Naphthol on Jute Fabric. Phys Chem Ind J. 2023;18(1):166.
Abstract
Jute is a long, soft, silky shiny natural fiber that can be spun into coarse and strong threads and is also called “the golden fiber” for its golden colour and high cash value. Dyeing experiments were carried out on bleached and unbleached jute fabrics with an Azo dye 2-hydroxynaphthaazo benzoic acid at various concentrations i.e. 2%, 4%, 6%, 8% and 10% (owf) at 80°C. It was seen that a concentration of 8% (owf), pH of 9.5-10.5 and temperature of 80°C is the ones that produced the most effective result in bleached and unbleached jute dyeing. The kinetic data showed that the mechanism of dyeing bleached and unbleached jute fabric follows the first order kinetics which is related to the chemisorption process as the rate-controlling step. The equilibrium data for bleached and unbleached jute were adjusted to the Langmuir-Freundlich model indicating a considerable contribution to the chemisorption process in a monolayer fashion.
Keywords
Jute; 2-hydroxynaphthazobenzoic; Efficiency; Decolourisation; Adsorption; Kinetic studies; Langmuir-Freundlich adsorption isotherm
Introduction
The emergence of the largest class of synthetic Azo dyes occurred due to constant efforts to find a specific dye or a particular class of dye for application on diverse materials of industrial importance for dyeing viz. jute, cotton, silk, wool, paper printing, biomedical and cosmetic industries [1-4]. Out of different classes of dyes, Azo dyes cover a major and important area due to their brilliant shades of colour, excellent light, washing and sublimation fastness and simplicity of synthesis which are the causes for their applicability to different materials as high level dyeing agents in the dyestuff industries viz. textiles, leather, metals etc.[5].
Jute fiber is biodegradable, recyclable and shows antibacterial properties thus it is environmentally friendly [6-8]. Jute also known as golden fiber find it’s applications on various non-traditional diversified products viz. upholstery, curtains, soft luggage, design handbags, furnishing textiles and apparel textiles sectors due to its high tensile strength, excellent thermal conductivity, low extensibility, coolness and ventilation function which ensures better breathability of fabrics [9-12]. Jute fabric requires bleaching, dyeing and finishing for bright shades of attractive look and better feel in the production of colourful, decorative value added items [13]. The use of Azo dyes for dyeing jute fabric heralds a new era in the dyeing industry while considering it’s fastness and brilliancy of shades [14,15].
Literature survey reveals that the kinetics and adsorption studies at equilibrium of different adsorbents like Azo dyes on fabrics viz. silk, wool, jute etc., were being investigated and also have shown great interest among the researchers [16-19].
In this work dyeing experiments were being carried out on bleached and unbleached jute fabrics with an Azo dye 2-hydroxynaphthazobenzoic acid at various concentrations i.e. from 2%, 4%, 6%, 8% and 10% (owf) and at a constant temperature i.e. 80°C. The amount of dye adsorbed along with its efficiency was studied at various concentrations. The dyeing process and the kinetic data at equilibrium follow the first order kinetics which was in conformity with the investigation and the results obtained in the experiment. The equilibrium data for bleached and unbleached jute were adjusted to the Langmuir-Freundlich model and a study of the considerable contribution of the chemisorption process in a monolayer fashion was investigated [20].
Materials and Methods
Chemicals and instruments
Analytical grade chemicals (~99% purity) from S.D. fine chem Ltd. were used for the preparation of dye, bleaching and dyeing of the jute fabric: viz. anthranilic acid, naphthol, sodium nitrite, sodium hydroxide, hydrogen peroxide(30 vol), trisodium phosphate, sodium hydroxide, sodium carbonate, sodium silicate, sodium sulphate, acetic acid and concentrated hydrochloric acid (12 N). Non-ionic detergent was used for soaping dyed fabric. Distilled water was used in all the experiments performed.
The UV visible spectra were recorded in doubly distilled water on a Perkin Elmer, lambda-250, a pH meter Mettler Delta 320 was used to measure the pH values of the Azo dye solutions. Carbon, hydrogen and nitrogen analysis were measured in M/s Elementar, Vario EL-III model.
Fabric
The following specifications of a plain weave grey jute fabric were used as presented in Table 1.
| Conc. (%, owf) | Bleached jute fabric | Unbleached jute fabric | ||
|---|---|---|---|---|
| Wrap (ends/dm) | Weft (ends/dm) | Wrap (ends/dm) | Weft (ends/dm) | |
| 2 | 28 (count, 188 tex) | 26 (count, 174 tex) | 30 (count, 193 tex) | 27 (count, 174 tex) |
| 4 | 25 (count, 203 tex) | 25 (count, 203 tex) | 25 (count, 167 tex) | 23 (count, 153 tex) |
| 6 | 27 (count, 199 tex) | 24 (count, 177 tex) | 27 (count, 210 tex) | 25 (count, 195 tex) |
| 8 | 30 (count, 173 tex) | 29 (count, 167 tex) | 27 (count, 198 tex) | 26 (count, 191 tex) |
| 10 | 27 (count, 151 tex) | 26 (count, 145 tex) | 30 (count, 181 tex) | 29 (count, 175 tex) |
TABLE 1. Specifications of bleached and unbleached jute fabric.
Fabric mass of bleached jute fabric 363 (2%), 407 (4%), 376 (6%), 341 (8%) and 296 (10%) g/sqm and for unbleached jute fabric 368 (2%), 334 (4%), 406 (6%), 389 (8%) and 356 (10%) g/sqm were taken for dyeing.
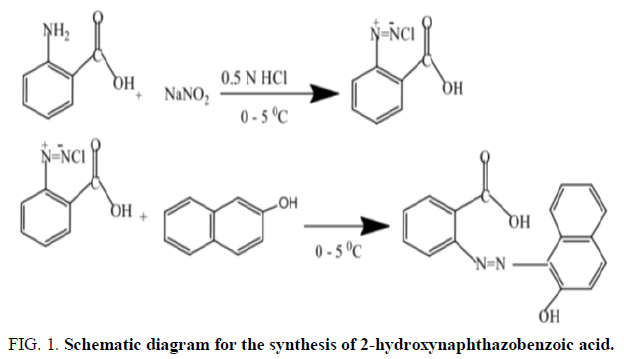
Preparation of 2-hydroxynaphthazobenzoic acid
The Azo dye was prepared by the interaction of diazonium salt from anthranilic acid with β-naphthol in an acidic medium as per the standard procedure [21].
Yield: 0.675 g (52.8%): M.P=245 ± 2°C; Elemental results (%): C: 69.96 (69.86); H: 4.65 (4.11); N: 9.21 (9.59); UV-Vis (λmax, nm): 488, 402 (sh), 310.
Bleaching of jute fabric
In a scouring vessel bleaching of appropriately weighed grey jute fabric and mother liquor was performed with a material to liquor ratio 1: 100 containing hydrogen peroxide (2 vol), trisodium phosphate (5 g/L), sodium hydroxide (1 g/L), sodium silicate (10 g/L) and non-ionic detergent (2 ml/L) for 2 hr at 80°C-90°C. The pH of the mother liquor of the dye bath was maintained at 9.5-10.5 throughout the experiment. After bleaching, the fiber samples were washed thoroughly with cold water by using non-ionic detergent and finally dried.
Dyeing of bleached jute fabric: In the dyeing process, bleached or unbleached jute fabric samples were dyed with an Azo dye viz. 2-hydroxynaphthazobenzoic acid. The sodium salt of the Azo dye was used for solubilization and then the solubilized Azo dye was applied to the fabric by direct dyeing process. The following steps were followed during dyeing of the jute fabric.
Solubilization of Azo dye: Azo dye of different concentrations viz. 2%, 4%, 6%, 8% and 10% (owf) were prepared by pasting with a non-ionic detergent (2 ml) and little warm water. About half the weight of the Azo dye appropriately weighed sodium carbonate was added to the paste, hot water was added to make the volume up to 250 ml. The pH of the dye solution was maintained between 9.5-10.5. The Azo dye solution was further heated for 10 min at 80°C.
Dyeing: Dye bath of different concentrations viz. 2%, 4%, 6%, 8% and 10%, (owf) were made with the dissolved Azo dye and diluted to a volume of 200 ml with doubly distilled water. The bleached jute fabric sample taken in the dye bath with the material to mother liquor ratio 1:100 was treated in the dye bath at 80°C for six hours for dyeing. The sample was then washed thoroughly with the cold water and non-ionic detergent (2 g/l) in a water bath at 50°C for 15 min followed by washing thoroughly with cold water aging and drying it in air.
Results and Discussion
Azo dye 2-hydroxynaphthazobenzoic acid was prepared by the direct diazotization reaction of anthranilic acid with an alkaline solution of β-naphthol. 2-hydroxynaphthazobenzoic acid Azo dye is an orange coloured, air stable and soluble in organic solvents and highly soluble in an alkaline aqueous medium. The 2-hydroxynaphthazobenzoic acid Azo dye has one Azo (-N=N-) group as chromophore, a carboxy (-COOH) group and one hydroxyl (-OH) group as auxochrome and the presence of these chromophoric and auxochromic group in the Azo compound which shows that the Azo dye has good affinity for textile applications towards the jute fabric at a higher concentration. The analytical results were in conformity with the molecular formula of 2-hydroxynaphthazobenzoic acid. The melting point of 2-hydroxynaphthazbenzoic acid was found to be 245 ± 2°C which is indicative of its covalent nature (Figure 1).
The UV visible spectrum shows one strong absorption maxima (λmax) at ~488 nm with a shoulder at ~402 nm and one weak absorption peak at ~310 nm in the visible region for 2-hydroxynaphthazobenzoic acid Azo dye. These absorption maxima may be due to the charge transfer transitions which are indicative of the presence of chromophore and auxochrome groups.
Jute fabric was bleached according to the standard procedure. In this experiment, the effect of 2-hydroxynaphthazobenzoic acid Azo dye on the bleached and unbleached jute fabric was investigated and compared.
Azo dye decolorization study
In the process of dyeing the sample i.e. jute fabric at different concentrations viz. 2%, 4%, 6%, 8% and 10%, (owf) were dipped in a dye bath for 6 hours using a fabric to liquor ratio of 1:100 and the pH of the solution was adjusted with hydrochloric acid. After every 30 minutes interval, 5 ml of aliquot samples was removed from the dye bath to assess the dye decolorization rate of the Azo dye at a particular concentration. 1 ml samples were taken out from the 5 ml sample and further diluted to 25 ml with doubly distilled water. At an absorption maximum (λmax=488 nm) of 2-hydroxynaphthazobenzoic acid, the concentrations (g/L or moles/L) of the diluted samples were determined spectrophotometrically to study the dye decolorization.
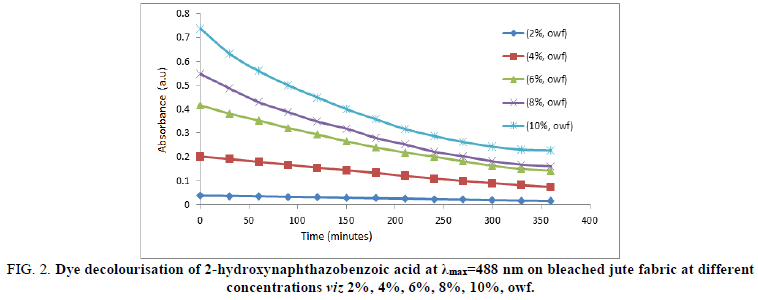
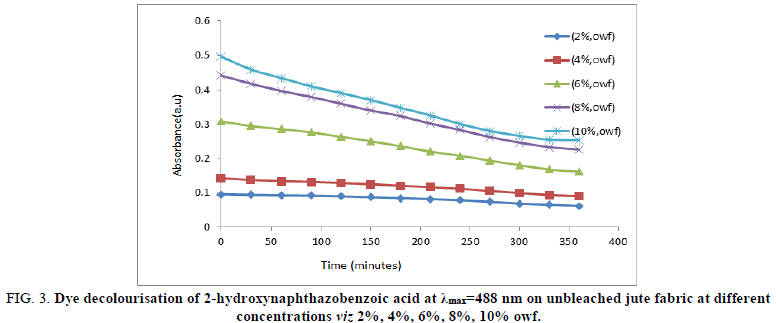
The dye decolorization performance of 2-hydroxynaphthazobenzoic acid in an aqueous medium was observed at different time intervals of i.e. 0, 30, 60, 90, 120, 150, 180, 210, 240, 270, 300, 330 and 360 minutes at an absorption maximum(λmax=488 nm) during dyeing on the bleached and unbleached jute fabric is shown in Figures 2 and 3 respectively.
The decolorization of 2-hydroxynaphthazobenzoic acid at a particular concentration for both bleached and unbleached jute fabric decreases with time at all the concentrations. Absorption maxima values reaches to a minimum ~300 minutes for all the concentrations of the 2-hydroxynaphthazobenzoic acid on both the bleached and unbleached jute fabric which indicates that the process of dying completes within 300 minutes approximately and after that the absorption maxima remains almost constant even though time was increased. The absorption maximum at λmax=488 nm for 2-hydroxynaphthazobenzoic acid on the bleached and unbleached jute fabric was found to be concentration dependent i.e. it increases with the increase in concentrations from 2% (owf) to 10% (owf) even considering at any particular time. This confirms the decolorization of the Azo dye. The dyeing process at higher concentrations viz. 8% and 10% of the Azo dye solution shows a sharp decline of slope which again indicates that at an initial stage the process of decolourisation was quite fast but at the later stage of the experiment the decolourisation of the Azo dye slows down with time before the completion of dyeing for both the bleached and unbleached jute fabric. At lower concentrations of the Azo dye i.e. from 2% (owf) and 4% (owf) in which the slope of the curve was found to be small which is indicative of a slow rate of decolorization of the dye solution.
FIG 2: Dye decolourisation of 2-hydroxynaphthazobenzoic acid at λmax=488 nm on bleached jute fabric at different concentrations viz 2%, 4%, 6%, 8%, 10%, owf.
FIG 3: Dye decolourisation of 2-hydroxynaphthazobenzoic acid at λmax=488 nm on unbleached jute fabric at different concentrations viz 2%, 4%, 6%, 8%, 10% owf.
Dye efficiency
The concentration of the dye solution was determined in g/L and moles/L at all concentrations for bleached and unbleached jute fabric. The absorbance maximum of 2-hydroxynaphthazo benzoic acid dye was measured at a wavelength (λmax) of 488 nm. The jute fabric without dye was used as the control to assess the dye effects on the jute fabric.
The efficiency of dye decolorization was determined at all concentrations and was expressed in percentage absorption of the dye concentration at any time with respect to initial dye concentration and was calculated as follows:
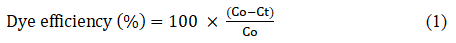
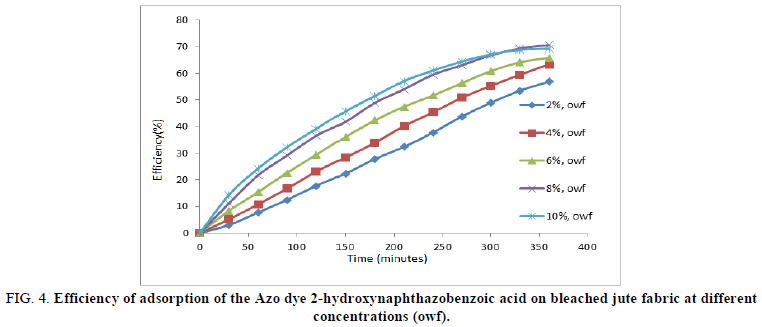
Where C0 and Ct were the initial absorbance value and the absorbance value at any time t respectively. The efficiency of the dye 2-hydroxynaphthazobenzoic acid on the bleached and unbleached adsorbents (jute) is shown in Figures 4 and 5.
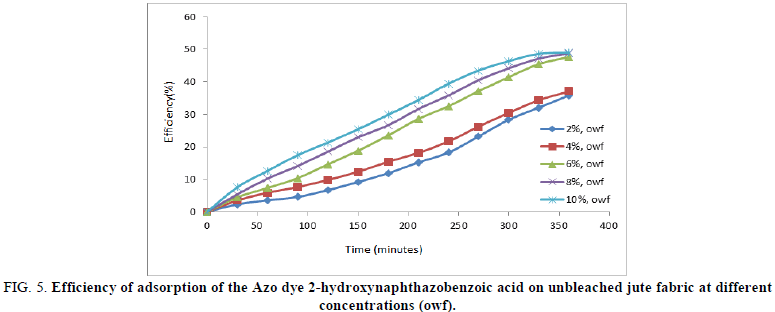
The dye efficiency of 2-hydroxynaphthazobenzoic acid on bleached and unbleached jute fabric was found to be increasing with the increase in concentrations i.e. from 2% owf to 10% owf which reaches a maximum for all concentrations ~330 minutes. The performance of the Azo dye observed can also be attributed to the fact that the efficiency of the dye on the bleached jute was much more in comparison to the unbleached jute fabric at any given concentration. This is indicative of dye uptake performance of 2-hydroxynaphthazobenzoic acid of bleached jute fabric is better than unbleached jute fabric at all concentrations and was in conformity with the maximum extent of dye efficiency at 8% owf.
FIG 4: Efficiency of adsorption of the Azo dye 2-hydroxynaphthazobenzoic acid on bleached jute fabric at different concentrations (owf).
FIG 5: Efficiency of adsorption of the Azo dye 2-hydroxynaphthazobenzoic acid on unbleached jute fabric at different concentrations (owf).
The maximum efficiency of 2-hydroxynaphthazobenzoic acid on bleached and unbleached jute fabric was presented in Table 2. The maximum extent of dye efficiency increases with the increase in concentration but the saturation reaches ~70% for bleached and ~48% for unbleached jute fabric at 8% owf which is consistent with the dye decolourisation study. It was also observed that the extent of efficiency remains almost the same even if the concentration was increased further up to 10% owf.
| Concentrations (%, owf) | Maximum efficiency (%) bleached jute fabric | Maximum efficiency (%) unbleached jute fabric |
|---|---|---|
| 2 | 56.83 | 35.83 |
| 4 | 63.36 | 37.04 |
| 6 | 65.80 | 47.62 |
| 8 | 70.61 | 48.71 |
| 10 | 69.22 | 49.01 |
TABLE 2. Maximum efficiency of 2-hydroxynaphthazobenzoic acid on bleached and unbleached bleached jute fabric at different concentrations viz. 2%, 4%, 6%, 8% and 10% owf.
Adsorption of Azo dye
The adsorption experiments were carried out by agitating 0.5 g-0.8 g of jute fabric with the Azo dye solution for the desired concentration for the time required to reach the equilibrium state and the pH of the solution was maintained in the range of 9-11. Adsorption studies were conducted by using a temperature controlled water bath at a temperature 80°C ± 2. The concentration of the Azo dye was measured spectrophotometrically at the wavelength corresponding to maximum absorbance of λmax=488 nm for 2-hydroxynaphthazobenzoic acid on bleached and unbleached jute fabric (Supplementary Tables 1-8). The samples were taken out from the flask at a predetermined time interval after every 30 minutes and then analyzed. The amount of dye adsorbed into the fabric Qe (mg/g), was calculated as follows:
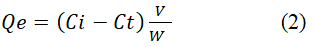
Where v is the volume (L) of the solution and W is the weight (g) of the dry jute fabric, Ci (mg/L) and Ct (mg/L) were the initial and final concentration of the Azo dye solution at any time respectively.
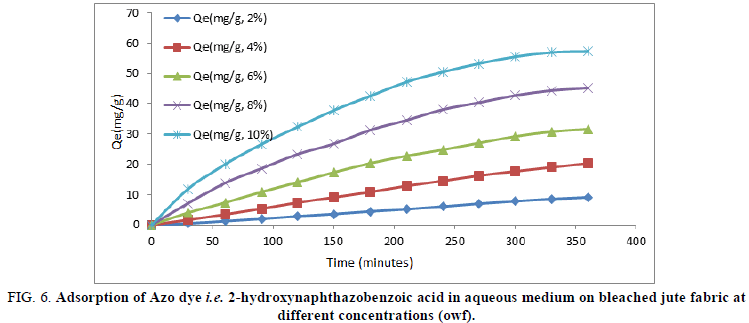
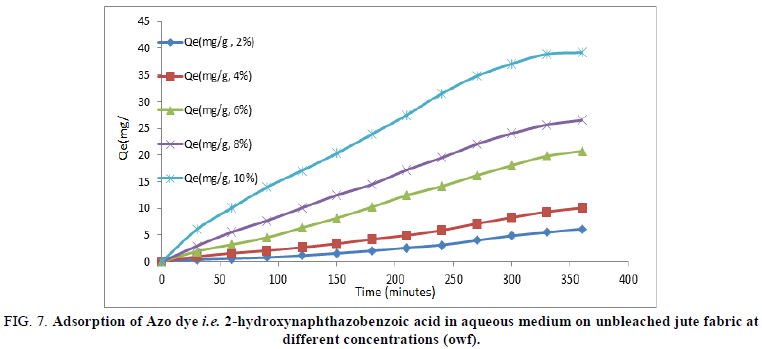
Fiber reactive Azo dyes were water soluble coloured organic compounds that were capable of forming a covalent bond between reactive groups of the adsorbate and the nucleophilic groups on the polymer chain within the adsorbent. Consequently, after adsorption of Azo dye becomes chemically part of the fiber by producing dye polymer linkage. The variation in adsorptions at different concentrations of 2-hydroxynaphthazobenzoic acid on a bleached and unbleached jute fiber with time at a constant temperature (80°C) was shown in Figures 6 and 7 respectively.
FIG 6: Adsorption of Azo dye i.e. 2-hydroxynaphthazobenzoic acid in aqueous medium on bleached jute fabric at different concentrations (owf).
FIG 7: Adsorption of Azo dye i.e. 2-hydroxynaphthazobenzoic acid in aqueous medium on unbleached jute fabric at different concentrations (owf).
Adsorption of 2-hydroxynaphthazobenzoic acid on bleached and unbleached jute fabric at different concentrations was found to be increasing with time, but after about ~300 minutes, the extent of adsorption (Qmax) almost becomes constant at equilibrium for the bleached and unbleached jute fabric and reaches to a saturation. The adsorption of 2-hydroxy-naphthazobenzoic acid was found to be increasing with the increase in concentration which indicates the dependency of concentration on dyeing of this Azo dye on jute. The data relating to the maximum extent of adsorption Qmax(mg/g) for both the bleached and unbleached jute were shown in Table 3. The maximum extent of adsorption (Qmax) for bleached and unbleached jute fabric at lower concentrations of 2-hydroxynaphthazobenzoic acid was found to be less in comparison to higher concentrations, which indicates the affinity of the dye is more towards the jute fabric at higher concentrations. The variation in the maximum extent of adsorption during the process of dying on bleached and unbleached jute fabric with 2-hydroxynaphthazobenzoic acid may be due to the presence of hydroxyl group in their chemical structure. Moreover, the dye adsorption was more on bleached jute fabric in comparison to the unbleached jute fabric at any given concentration. The maximum efficiency and extent of adsorption were found to be in consistent with each other for both the bleached and unbleached jute fabric at different concentrations.
| Concentration (%, owf) | Extent of adsorption (bleached jute fabric) (Qmax, mg/g) | Extent of adsorption (unbleached jute fabric) (Qmax, mg/g) |
|---|---|---|
| 2 | 9.09 | 6.14 |
| 4 | 20.27 | 10.08 |
| 6 | 31.58 | 20.72 |
| 8 | 45.19 | 26.53 |
| 10 | 57.22 | 39.20 |
TABLE 3. Extent of adsorption of 2-hydroxynaphthazobenzoic acid on bleached and unbleached jute fabric at different concentrations viz. 2%, 4%, 6%, 8% and 10% owf.
Kinetics of Azo dye adsorption
The study of sorption kinetics describes the rate of adsorbate (Azo dye) uptake on the adsorbent (fabric viz jute). For evaluating the adsorption of an Azo dye, Lagergren's first order kinetic model was used. The first order equation is represented as follows:

Where Co and Ct are the initial concentration of the solution and the concentration at any time t, k (min-1) is the rate constant of the first order kinetic equation model. The slopes and intercepts were determined form the plot of log (Ct) versus time (t).
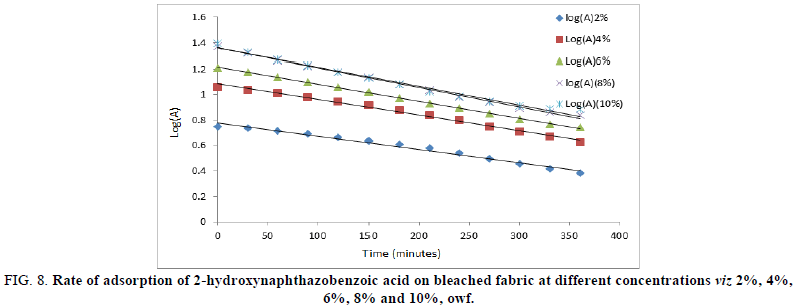
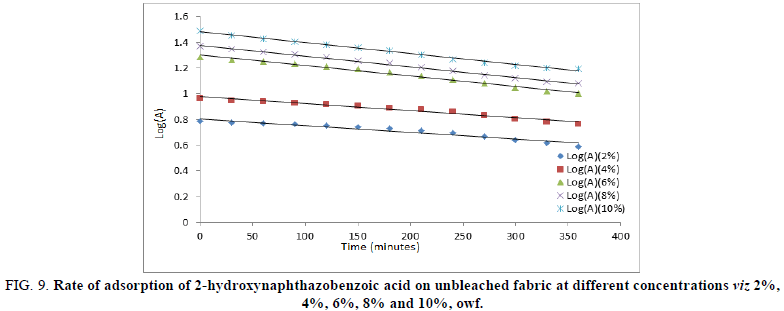
For evaluating the kinetics of adsorption for 2-hydroxynaphthazobenzoic acid, Lagergren’s first order models were used to fit in the experimental data. K1 (exp, min-1) and K2 (exp, min-1) are the rate constants for adsorption of 2-hydroxynaphthazobenzoic acid on the bleached and unbleached jute fabric respectively and K1 (graphical) and K2 (graphical) are the rate constants for adsorption of 2-hydroxynaphthazobenzoic acid on the bleached and unbleached jute fabric obtained from the slopes of the plot between Log (A) versus time (t). The coefficient of correlations for the first order kinetic model obtained at different concentrations was between 93%-99%. However, the theoretical values of the rate constants were found to conform with the graphical rate constant values which indicate the acceptability of first order reaction kinetics for the adsorption of Azo dye on jute fabric. The values of rate constants experimental and graphical with regression values were presented in Table 4. Plots of log A versus time of adsorption shows a linear relationship between them which were shown in Figures 8 and 9 for the bleached and unbleached jute fabric respectively. With the increase in concentration, the value of rate constants also increases for bleached and unbleached jute fabric indicating the dependence of the rate of the reaction on the concentration of Azo dyes.
| Concentration (%, owf) | Bleached | Unbleached | ||||
|---|---|---|---|---|---|---|
| K1 (exp, min-1) | K1 (graphical) | r2 | K2 (exp, min-1) | K2 (graphical) | r2 | |
| 2 | 1.82 × 10-3 | 2.30 × 10-3 | 0.9829 | 0.82 × 10-3 | 1.15 × 10-3 | 0.9375 |
| 4 | 2.35 × 10-3 | 2.76 × 10-3 | 0.992 | 1.05 × 10-3 | 1.38 × 10-3 | 0.9675 |
| 6 | 2.98 × 10-3 | 2.99 × 10-3 | 0.9987 | 1.55 × 10-3 | 1.74 × 10-3 | 0.9875 |
| 8 | 3.73 × 10-3 | 3.45 × 10-3 | 0.9962 | 1.82 × 10-3 | 1.84 × 10-3 | 0.9961 |
| 10 | 4.05 × 10-3 | 3.47 × 10-3 | 0.9835 | 2.09 × 10-3 | 2.07 × 10-3 | 0.9832 |
TABLE 4. Rate constant (k) of adsorption of 2-hydroxynaphthazobenzoic acid on bleached and unbleached jute fabric at different concentrations.
FIG 8: Rate of adsorption of 2-hydroxynaphthazobenzoic acid on bleached fabric at different concentrations viz 2%, 4%, 6%, 8% and 10%, owf.
FIG 9: Rate of adsorption of 2-hydroxynaphthazobenzoic acid on unbleached fabric at different concentrations viz 2%, 4%, 6%, 8% and 10%, owf.
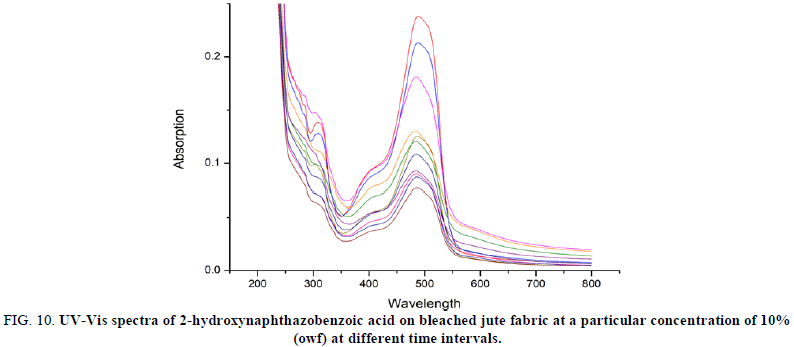
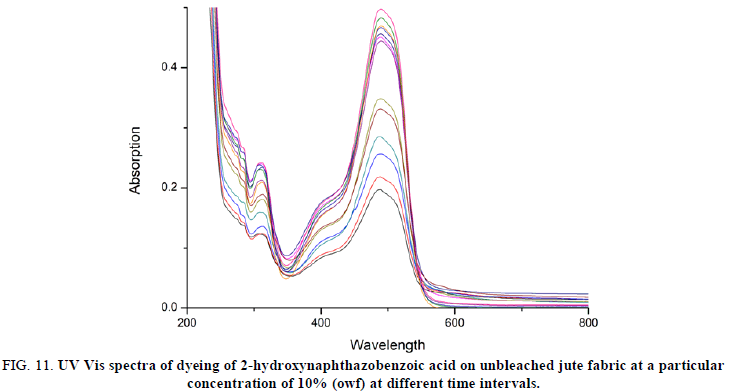
The UV-vis spectra of the dye solution of 2-hydroxynaphthazobenzoic acid on bleached and unbleached jute fabric at a particular concentration of 10% (owf) and at different time intervals of were shown in Figures 10 and 11. The intensity of the curve at a particular concentration (10% owf), the absorption maximum (λmax=488 nm) was found to be decreasing which confirms the rate of change of adsorption on the surface of the fabric increases for bleached and unbleached jute fabric. The same trend was also observed for all other concentrations on the jute fabric.
FIG 10: UV-Vis spectra of 2-hydroxynaphthazobenzoic acid on bleached jute fabric at a particular concentration of 10% (owf) at different time intervals.
FIG 11: UV Vis spectra of dyeing of 2-hydroxynaphthazobenzoic acid on unbleached jute fabric at a particular concentration of 10% (owf) at different time intervals.
Adsorption isotherms: Langmuir and Freundlich isotherm
The adsorption curves were applied to both Langmuir and Freundlich equations.
Langmuir adsorption isotherm: The linearized form of the Langmuir isotherm equation is represented as:
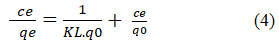
Where qe (mg/g) is the amount adsorbed per unit mass of adsorbent corresponding to complete coverage of adsorptive sites, Ce (mg/L) is the equilibrium concentration of the dye solution. The constants KL and q0 are known as Langmuir constants where qe (mg/g) is the monolayer adsorption capacity of the dye and KL (L/mg) is the adsorption energy.
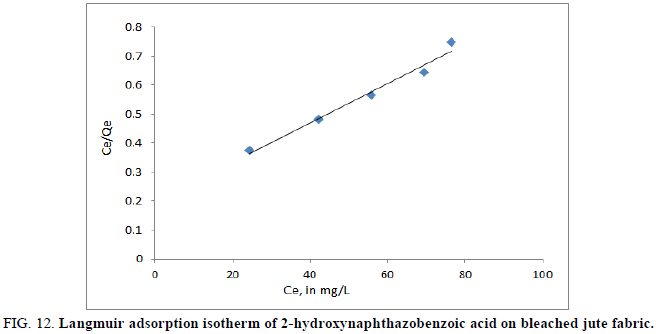
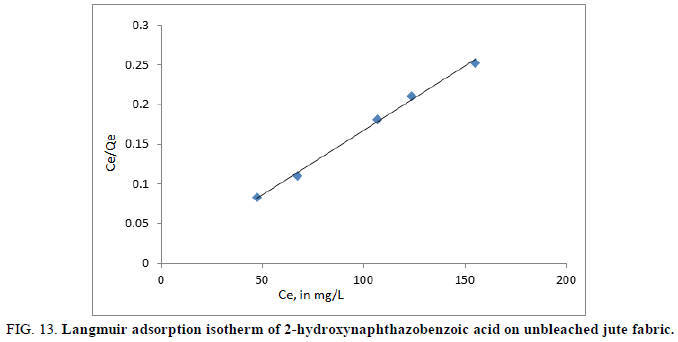
A plot of Ce/qe versus/Ce would give a straight line and the values of qe and KL were determined from the slope and intercept of the plots between Ce/Qe vs. Ce respectively (Figure 12 and 13).
The adsorption curves of the Azo dye on jute fabric were applied to the Langmuir equations. The linearized plots of Ce/qe versus Ce suggest the applicability of the Langmuir isotherms which were shown in Figures 14 and 15 for 2-hydroxynaphthazobenzoic acid on bleached and unbleached jute fabric respectively. The value of q0 (mg/g) and KL (L/mg) were determined and presented in Table 5, which were related to the adsorption efficiency and energy of adsorption respectively. The 2-hydroxynaphthazo-benzoic acid Azo dye uptake performance is better on bleached jute fiber as observed in dye efficiency studies.
The linearized form of the Freundlich isotherm equation is given by:
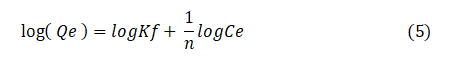
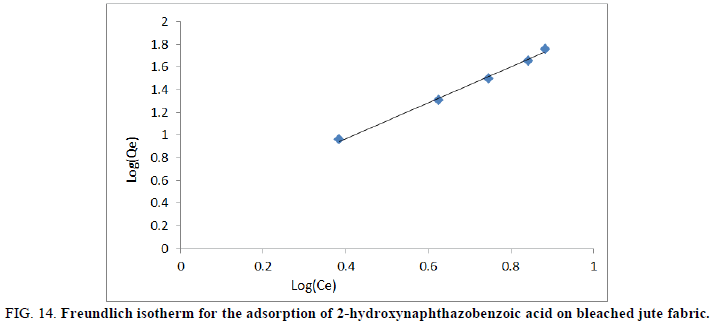
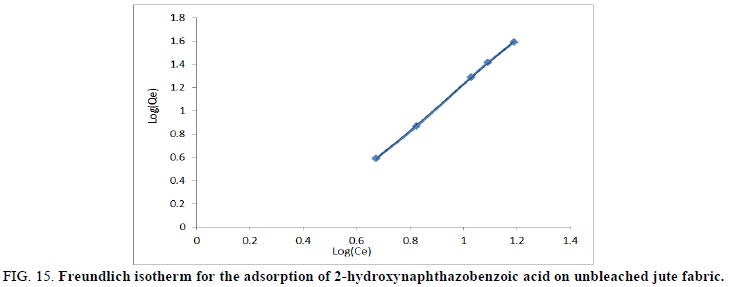
Where Kf (mg/g) is the Freundlich constant and n is the constant related to adsorption intensity. A plot of log (Qe) against log (Ce) would give the values of n and Kf, from the slope and the intercept, respectively.
FIG 14: Freundlich isotherm for the adsorption of 2-hydroxynaphthazobenzoic acid on bleached jute fabric.
FIG 15: Freundlich isotherm for the adsorption of 2-hydroxynaphthazobenzoic acid on unbleached jute fabric.
The Freundlich equation was also employed for the adsorption of 2-hydroxynaphthazo benzoic acid on bleached and unbleached jute fabric respectively. From equation 6 we have Kf and n values which were known as Freundlich constants and were presented in Table 5. Kf and n values indicates the adsorption capacity and intensity of adsorption respectively by incorporating all affecting factors. The linearity of the plots between Log (Qe) versus log (Ce) for the adsorption of the Azo dye on bleached and unbleached jute fabric shows the applicability of Freundlich isotherms as shown in Figures 14 and 15 respectively. According to Kf and n parameters, the Qe (mg/g)) increases with the increase of Ce (mg/L) but since n<1, therefore, Qe (mg/g) does not increase as rapidly as Ce (mg/L). Further, the values show the dominance of the adsorptive capacity of the dye.
| Jute fabrics | Langmuir constants | Freundlich constants | ||||
|---|---|---|---|---|---|---|
| qo | KL | r2 | Kf | n | r2 | |
| Bleached jute fabric | 147.06 | 29.34 | 0.9768 | 1.404 | 0.6350 | 0.9962 |
| Unbleached jute fabric | 625 | 3.25 | 0.9970 | 1.97 | 0.5087 | 0.9994 |
TABLE 5. Langmuir and Freundlich constants of Azo dye 1 and Azo dye 2 for adsorption by jute fabric.
The separation factor (RL) calculated for the adsorption process of the Azo dye viz 2-hydroxynaphthazobenzoic acid on jute fabric and were presented in Table 6. The separation factor values were found to be in the range between 0 to 1 which confirms that the ongoing process is favourable.
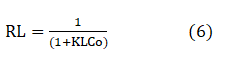
Where KL is the Langmuir constant and C0 is the initial concentration of the dye.
| Concentration (%, owf) | Bleached jute | Unbleached |
|---|---|---|
| 2 | 0.3777 | 0.8190 |
| 4 | 0.2286 | 0.7496 |
| 6 | 0.1732 | 0.5896 |
| 8 | 0.1261 | 0.5391 |
| 10 | 0.1208 | 0.4725 |
Conclusion
In this work, the adsorption process of the reactive acid Azo dye viz. 2-hydroxynaphthazobenzoic acid has been examined on bleached and unbleached jute fabric. It was shown that the adsorptive capacity and efficiency of the Azo dye increase with the increase in concentration from 2% (owf) to 8% (owf). The adsorptive efficiency and capacity remain almost constant even if the concentration was increased to 10% (owf). The best result of dyeing and efficiency of the Azo dye was achieved at a higher concentration i.e. from 8% (owf) to 10% (owf) in the pH range 9.5-10.5 at a temperature of 80°C. The Lagergren’s first order kinetics successfully represents the kinetic experimental data for bleached and unbleached jute fabric dyeing at all concentrations. The equilibrium data for both the bleached and unbleached jute fabric can be represented by the Langmuir-Freundlich model. According to the kinetic and equilibrium data, it was concluded that chemisorptions are quite important in the jute dyeing which satisfies the monolayer adsorption of the Azo dye on jute fabrics. The dimensionless separation factor (RL) reveals that the adsorption process of 2-hydroxynaphthazobenzoic on bleached and unbleached jute fabric is favourable.
Acknowledgement
The authors thank central instrumentation facility, BIT-Mesra, Ranchi for its technical assistance.
References
- Bhuiyan MA, Islam A, Islam S, et al. Improving dyeability and antibacterial activity of Lawsonia inermis L. on jute fabrics by chitosan pretreatment. Text Cloth Sustain. 2017;3:13.
- Arju SN, Ali MA. Chemical modification of jute fibers for improving its hydrophobicity and dyeability with reactive dyes. World J Res Rev. 2017;5(4):62-69.
- Ghosh P. Effect of selective chemical treatments of jute fiber on textile related properties and processibility. Indian J Fibre Text Res. 2004;229:85-89.
- Wang WM, Cai ZS, Yu JY. Study on the chemical modification process of jute fiber. J Eng Fibers Fabr. 2008;3(2):1-11.
- Cai Y, David SK, Pailthorpe MT. Dyeing of jute and jute/cotton blend fabrics with 2:1 pre-metallised dyes. Dyes Pigm. 2000;45(2):161-168.
- Dixit BC, Patel HM, Dixit RB, et al. Synthesis characterization and dyeing assessment of novel acid Azo dyes and mordent acid Azo dyes based on 2-hydroxy-4-methoxybenzophenone on wool and silk fabrics. J Serb Chem Soc. 2010;75(5):605-614.
- Bhuiyan MR, Shaid A, Bashar MM, et al. A novel approach of dyeing jute fiber with reactive dye after treating with chitosan. Open J Org Polym Mater. 2013;3(4):87-91.
- Augustine AA, Orike BD, Edidiong AD. Adsorption kinetics and modeling of Cu(II) ion sorption from aqueous solution by mercaptoacetic acid modified cassava (Manihot esculenta Cranz) wastes. Elec J Env Agricult Food Chem. 2007;6(4):2221-2234.
- Devi RP, Rathinamoorthy R, Moses JJ. Effect of jute proportion on the colour strength value of jute/cotton union fabric. Int J Eng Res. 2013;2(4):294-299.
- Basu G, Chattopadhyay SN. Ambient temperature bleaching of jute fiber its effect on yarn properties and dyeing behaviour. Indian J Fiber Text Res. 1996;21:217-222.
- Shah AB, Yang VW. Enzymatic polishing of jute/cotton blended fabrics. J Ferment Bioeng. 1996;81(1):18-20.
- Chattopadhyay SN, Pan NC, Day A. Reuse of reactive dyes for dyeing of jute fabric. Bioresour Technol. 2006;97(1):77-83.
[Crossref] [Google Scholar] [PubMed]
- Lv N, Wang X, Peng S, et al. Study of the kinetics and equilibrium of the adsorption of oils onto hydrophobic jute fibre modified via the sol-gel method. Int J Environ Res Public Health. 2018;15(5):969.
[Crossref] [Google Scholar] [PubMed]
- Alam J, Uddin MN. Kinetic and equilibrium studies of adsorption of Pb(II) on low cost agri waste adsorbent jute stick powder. Eur J Chem. 2019;10(4):295-304.
- Furniss BS, Hannaford AJ, Smith PWG. Vogel’s textbook of practical organic chemistry. 5th edition, Longman publication, London, England. 1989;957.
- Roy A, Adhikari B, Majumder SB. Equilibrium, kinetic and thermodynamic studies of Azo dye adsorption from aqueous solution by chemically modified lignocellulosic jute fiber. Ind Eng Chem Res. 2013;52(19):6502-6512.
- Roy A, Chakraborty S, Kundu SP, et al. Adsorption of anionic Azo dye from aqueous solution by lignocellulose biomass jute fiber: Equilibrium, kinetics and thermodynamics study. Ind Eng Chem Res. 2012;51(37):12095-12106.
- Sarkar PB, Chatterjee H, Mazumdar AK. Absorption of basic dyes by jute. Nature. 1946;157(3989):486.
- Varadarajan PR, Porkodi K, Subbhuraam CV. Adsorption of methylene blue onto jute fiber carbon: Kinetics and equilibrium studies. J Colloid Interface Sci. 2005;284(1):78-82.
[Crossref] [Google Scholar] [PubMed]
- Porkodi K, Kumar KV. Equilibrium, kinetics and mechanism modeling and simulation of basic and acid dyes sorption onto jute fiber carbon: Eosin yellow, malachite green and crystal violet single component systems. J Hazard Mater. 2007;143(1-2):311-327.
[Crossref] [Google Scholar] [PubMed]
- Furniss BS, Hannaford AJ, Smith PWG, et al. Vogel’s textbook of practical organic chemistry. 5th edition. Longman Scientific and Technical Publisher, (co published in the United States with John Wiley and Sons), Longman group UK Limited, Burnt hill, Harlow, England. 1989;957:1-1540.