Research
, Volume: 21( 1)Viscosity, Density and Ultrasonic Interferometric Investigations of 3-(Chloroaryl)-5-Aryl-1-Substituted Pyrazolines in Dioxane Medium
- *Correspondence:
- AD Deshmukh
Department of Chemical Engineering,
PES College of Engineering,
Aurangabad,
Maharashtra,
Tel: 9763936966;
E-mail: deshmukhashwini16@gmail.com
Received date: April 15, 2023, Manuscript No. TSIJCS-23-96146; Editor assigned date: April 17, 2023, PreQC No. TSIJCS-23-96146 (PQ); Reviewed date: May 01, 2023, QC No. TSIJCS-23-96146; Revised date: July 05, 2023, Manuscript No. TSIJCS-23-96146 (R); Published date: July 12, 2023, DOI: 10.37532/0972-768X.2023.21(1).434
Citation:Deshmukh AD. Viscosity, Density and Ultrasonic Interferometric Investigations of 3-(Chloroaryl)-5-Aryl-1-Substituted Pyrazolines in Dioxane Medium. Int J Chem Sci. 2023;21(1):434
Abstract
Viscosity is one of the important physical property of liquid and gases and it implies resistance to flow as fluids (liquid and gases) exhibit a characteristic property of flowing under applied force of their own weight. The study of viscosity is widely used to obtain the molecular interaction in case of gases and liquids. In liquids and binary mixtures, the additional forces between molecules are important substituted pyrazolines. Rymantas Kazys, et al., measurement of viscosity of highly viscous non-Newtonian fluids by means of ultrasonic guided waves in order to perform monitoring of the polymerisation process, it is necessary to measure viscosity. However, in the case of non-Newtonian highly viscous fluids, viscosity starts to be dependent on the vibration or rotation frequency of the sensing element. Also, the sensing element must possess a sufficient mechanical strength.
Keywords
Acoustic parameters; Interferometry; Solute solvent interaction; Resistance; Viscosity
Introduction
The viscosity of a fluid is a measure of its resistance to gradual deformation by shear stress or tensile stress. For liquids, it corresponds to the informal notion of “thickness” for example; honey has higher viscosity than water. Viscosity is governed by the strength of intermolecular forces and especially by the shapes of the molecules of a liquid [1]. Liquids whose molecules are polar or can form hydrogen bonds are usually more viscous than similar non-polar substances. Concentrated sulphuric acid is a good example of a liquid which owes its viscosity of hydrogen bonding. The coefficient of viscosity is concerned with the viscous forces generated by compression or dilatation. In the absence of knowledge of its magnitude in liquids it has been customary in hydrodynamics to assume that the coefficient of dilatational viscosity, n′, could be approximated by the ideal gas value n= -2n/3, where n is the coefficient of shear viscosity [2]. A method has been developed for obtaining values for the dilatational viscosity which is based on Eckhart’s theory of acoustical streaming; the non-periodic motion of the fluid in the vicinity of a sound source is dependent on the two coefficients of viscosity. A method to measure non-Newtonian fluids viscosity using inertial viscometer with a computer vision system. A method to measure non-Newtonian fluids viscosity using inertial viscometer with a computer vision system [3].
Elena P Kornaeva, the theory of rheology of non-Newtonian fluids is based on the generalized Newtonian hypothesis of viscosity. The viscometers for non-Newtonian fluids should implement fluid flows with the known stress and strain state parameters distributions. Ideally, the distributions should be homogeneous in the flow domain [4]. The idea of the proposed method is based on a combination of a capillary and rotational viscometers implemented in the torus shaped capillary viscometer. Analysis of the mathematical model of the inertial non-Newtonian fluid flow in the torus allowed to determine the conditions of homogeneity of the mechanical and thermal parameters in the flow domain and to develop method of viscosity measurement [5].
Present work
The present study deals with the study of intermolecular interactions in terms of viscosity β-coefficient of substituted pyrazolines in different percentage and temperature of dioxane water mixture which will be prepared by change in the volume of solvent and keeping the volume of ligand fixed. The data obtained have been used to compute relative viscosity and viscosity β coefficient, 1, 4-dioxane water mixture at different temperature (293 K, 297 K and 300 K). The Ostwald’s viscometer was used to carry out viscosity measurements [6].
Materials and Methods
Experimental and instrumentation
Ligands: The 0.01 M solution of each ligand was prepared in different percentage (70%, 75%, 80%, 85% and 90%) of 1,4-dioxane water mixture at different temperature (293 K, 297 K and 300 K). All the solutions of ligands were always used a fresh in the present investigation. The ligands were synthesized as given in part-I and are used for viscosity measurements.
• 3-(2-Hydroxy-5-chlorophenyl)-5-phenyl-1-thiocarboxamido pyrazoline (L1).
• 3-(2-Hydroxy-5-chlorophenyl)-5-(3-chlorophenyl)-1-thiocarboxamido pyrazoline (L2).
• 3-(2-Hydroxy-3-bromo-5-chlorophenyl)-5-phenyl-1-thiocarboxamido pyrazoline (L3).
M El Malkia, et al., measured the viscosity of different fluids, including edible oils and soaps, at a constant temperature, using two experimental methods. Namely, the falling ball and the oscillation of the mass spring system inside a fluid. Three different masses were used to evaluate the viscosity obtained by the falling ball method. The results were very similar. More precision of the viscosity value is done using a calibration curve. The results are compared with those measured by the mass spring system and they show good agreement. This simple way of viscosity measurement using mechanical physics concepts can be used for educational purposes such as the practical work of a bachelor.
Alcohols are commonly used as additives in biodiesel to improve its viscous and dense properties for better applicability in the industrial field. In this work, experimental investigations are conducted on the dynamic viscosities for the binary mixture of methyl octanoate (one component of biodiesel) with one 1-alcohol containing 1-propanol, 1-butanol, 1-pentanol, 1-hexanol and 1-heptanol from 293.15 K to 333.15 K at atmospheric pressure.
Results and Discussion
Study of viscosity shows the relation and also the effect of temperature on solution. These parameters are used in different types of industries such as oil, organic, inorganic, daily life, food processing plants, hopper level switches, tank level indicators etc.
The viscosity of each solution is determined by the following empirical formula:
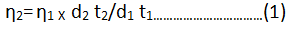
Where;
η2=Viscosity of ligand solution
η1=Viscosity of solvent.
d1 and d2=Densities of solvent and ligand respectively.
t1 and t2=Time of flow for solvent and ligand respectively.
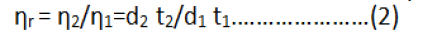
Where;
ηr=Relative viscosity.
Loria and their co-workers have studied prediction of density and viscosity of bitumen using the Pong-Robinson equation of state.
Mahapatra, et al., have shown that the hyperbranched polyamine containing trichloro-s-trizine exhibited low viscosity (η=0.15 dL/g) and was soluble only in highly polar solvents.
In the present study, viscosities of ligands L1, L2 and L3 are calculated by equations (1) and (2). The 0.01 M solutions of ligands were prepared in different percentage of dioxane water mixture. From the values all parameters like, relative viscosity and specific viscosities of solutions are calculated and listed in Tables 1-9 and Figures 1-9.
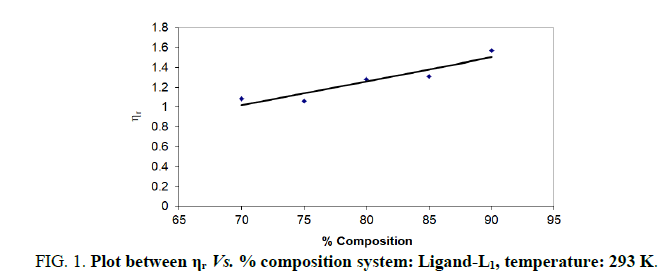
| % dioxane water | √C mole½/lit½ | Density d × 103 kgm-3 | Time flow ‘t’ sec | Relative viscosity (ηr) | 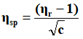 |
|---|---|---|---|---|---|
| 70 | 0.1 | 0.887 | 173.33 | 1.0860 | 0.860 |
| 75 | 0.1 | 0.889 | 167.66 | 1.0600 | 0.600 |
| 80 | 0.1 | 0.893 | 203.33 | 1.2800 | 2.800 |
| 85 | 0.1 | 0.893 | 191.33 | 1.3100 | 3.100 |
| 90 | 0.1 | 0.890 | 196.33 | 1.5687 | 5.687 |
Note: Temp.=293 K, Conc.=0.01 M.
TABLE 1. Ligand: 3-(2-hydroxy-5-chlorophenyl)-5-phenyl-1-thiocarboxzmido pyrazoline (L1).
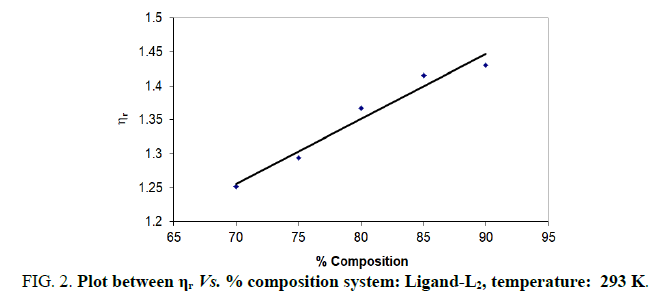
| % dioxane water | √C mole½/lit½ | Density d × 103 kgm-3 | Time flow ‘t’ sec | Relative viscosity (ηr) | 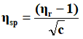 |
|---|---|---|---|---|---|
| 70 | 0.1 | 0.889 | 199.33 | 1.2516 | 2.516 |
| 75 | 0.1 | 0.893 | 208.00 | 1.2936 | 2.936 |
| 80 | 0.1 | 0.892 | 220.00 | 1.3670 | 3.670 |
| 85 | 0.1 | 0.892 | 192.66 | 1.4150 | 4.150 |
| 90 | 0.1 | 0.891 | 175.33 | 1.4300 | 4.300 |
Note: Temp.=293 K, Conc.=0.01 M.
TABLE 2. Ligand: 3-(2-hydroxy-5-chlorophenyl)-5-(3-chlorophenyl)-1-thiocarboxzmido pyrazoline (L2).
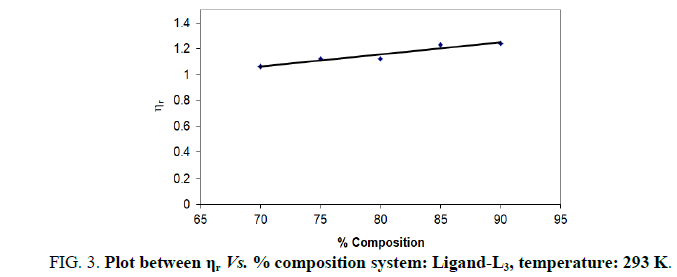
| % dioxane water | √C mole½/lit½ | Density d × 103 kgm-3 | Time flow ‘t’ sec | Relative viscosity (ηr) | 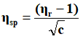 |
|---|---|---|---|---|---|
| 70 | 0.1 | 0.890 | 199.33 | 1.0630 | 0.630 |
| 75 | 0.1 | 0.893 | 189.33 | 1.1230 | 1.230 |
| 80 | 0.1 | 0.892 | 195.66 | 1.1230 | 1.230 |
| 85 | 0.1 | 0.892 | 188.00 | 1.2320 | 2.320 |
| 90 | 0.1 | 0.893 | 148.00 | 1.2420 | 2.420 |
Note: Temp.=293 K, Conc.=0.01 M.
TABLE 3. Ligand: 3-(2-hydroxy-3-bromo-5-chlorophenyl)-5-phenyl-1-thiocarboxzmido pyrazoline (L3).
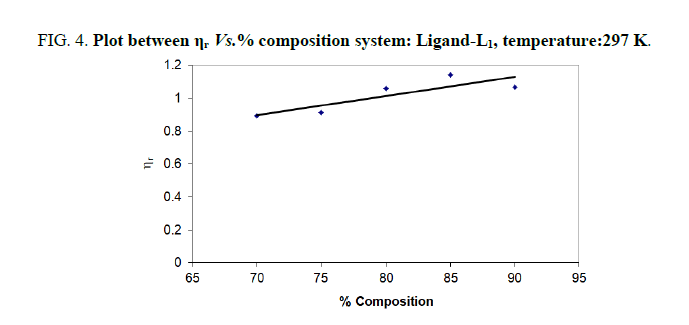
| % dioxane water | √C mole½/lit½ | Density d × 103 kgm-3 | Time flow ‘t’ sec | Relative viscosity (ηr) | 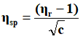 |
|---|---|---|---|---|---|
| 70 | 0.1 | 0.887 | 147.00 | 0.8921 | -1.079 |
| 75 | 0.1 | 0.886 | 150.00 | 0.9117 | -0.883 |
| 80 | 0.1 | 0.891 | 153.33 | 1.0603 | 0.603 |
| 85 | 0.1 | 0.890 | 150.33 | 1.1412 | 1.412 |
| 90 | 0.1 | 0.889 | 132.33 | 1.0656 | 0.656 |
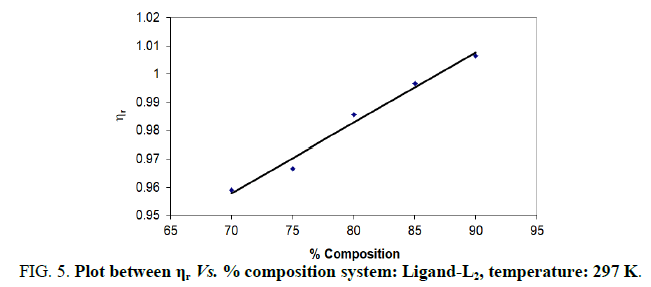
Note: Temp.=297 K, Conc.=0.01 M.
TABLE 4. Ligand: 3-(2-hydroxy-5-chlorophenyl)-5-phenyl-1-thiocarboxzmido pyrazoline (L1).
| % dioxane water | √C mole½/lit½ | Density d × 103 kgm-3 | Time flow ‘t’ sec | Relative viscosity (ηr) | 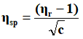 |
|---|---|---|---|---|---|
| 70 | 0.1 | 0.887 | 158.00 | 0.9590 | -0.410 |
| 75 | 0.1 | 0.890 | 157.66 | 0.9667 | -0.333 |
| 80 | 0.1 | 0.888 | 143.00 | 0.9855 | -0.145 |
| 85 | 0.1 | 0.886 | 140.66 | 0.9965 | -0.035 |
| 90 | 0.1 | 0.885 | 125.00 | 1.0064 | 0.064 |
Note: Temp.=297 K, Conc.=0.01 M.
TABLE 5. Ligand: 3-(2-hydroxy-5-chlorophenyl)-5-(3-chlorophenyl)-1-thiocarboxzmido pyrazoline (L2).
| % dioxane water | √C mole½/lit½ | Density d × 103 kgm-3 | Time flow ‘t’ sec | Relative viscosity (ηr) | 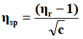 |
|---|---|---|---|---|---|
| 70 | 0.1 | 0.891 | 161.00 | 0.9560 | -0.440 |
| 75 | 0.1 | 0.889 | 151.33 | 0.9583 | -0.417 |
| 80 | 0.1 | 0.888 | 139.00 | 0.9670 | -0.330 |
| 85 | 0.1 | 0.887 | 133.66 | 0.9786 | -0.214 |
| 90 | 0.1 | 0.887 | 123.00 | 0.9882 | -0.118 |
Note: Temp.=297 K, Conc.=0.01 M.
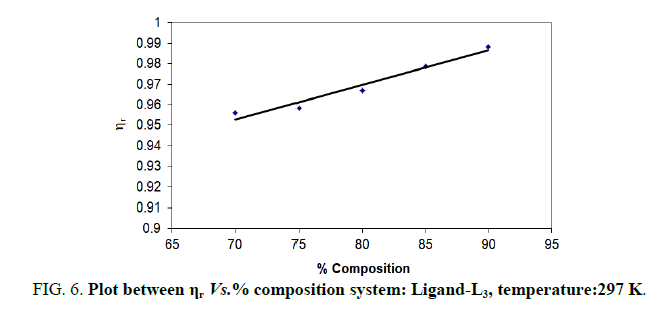
TABLE 6. Ligand: 3-(2-hydroxy-3-bromo-5-chlorophenyl)-5-phenyl-1-thiocarboxzmido pyrazoline (L3).
| % dioxane water | √C mole½/lit½ | Density d × 103 kgm-3 | Time flow ‘t’ sec | Relative viscosity (ηr) | 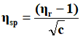 |
|---|---|---|---|---|---|
| 70 | 0.1 | 0.886 | 155.66 | 1.0415 | 0.415 |
| 75 | 0.1 | 0.885 | 153.33 | 1.0802 | 0.802 |
| 80 | 0.1 | 0.887 | 138.00 | 1.1003 | 1.003 |
| 85 | 0.1 | 0.885 | 126.00 | 1.1197 | 1.197 |
| 90 | 0.1 | 0.884 | 122.33 | 1.1652 | 1.652 |
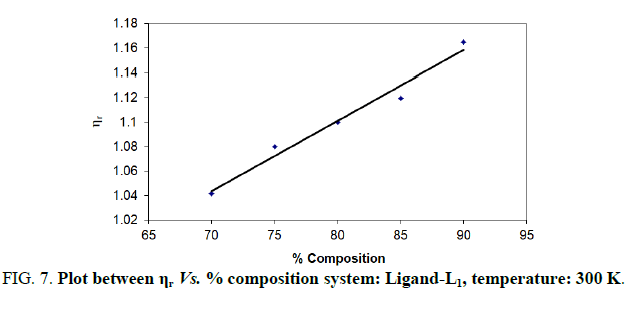
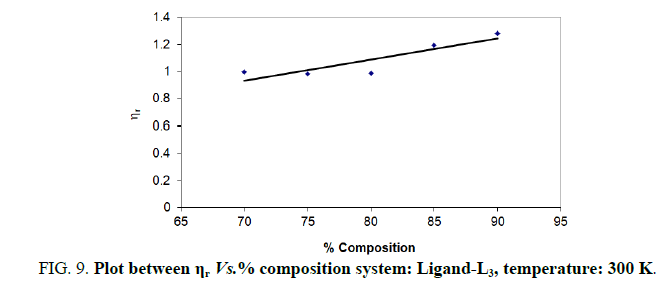
Note: Temp.=300 K, Conc.=0.01 M.
TABLE 7. Ligand: 3-(2-hydroxy-5-chlorophenyl)-5-phenyl-1-thiocarboxzmido pyrazoline (L1).
| % dioxane water | √C mole½/lit½ | Density d × 103 kgm-3 | Time flow ‘t’ sec | Relative viscosity (ηr) | 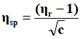 |
|---|---|---|---|---|---|
| 70 | 0.1 | 0.882 | 147.66 | 0.9835 | -0.165 |
| 75 | 0.1 | 0.883 | 141.33 | 0.9933 | -0.067 |
| 80 | 0.1 | 0.883 | 129.66 | 1.0291 | 0.291 |
| 85 | 0.1 | 0.880 | 126.00 | 1.0480 | 0.480 |
| 90 | 0.1 | 0.881 | 112.00 | 1.0632 | 0.632 |
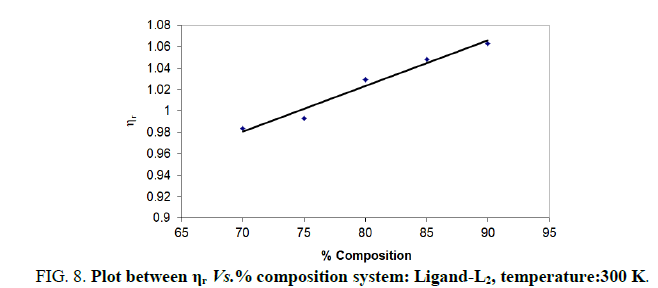
Note: Temp.=300 K, Conc.=0.01 M.
TABLE 8. Ligand: 3-(2-hydroxy-5-chlorophenyl)-5-(3-chlorophenyl)-1-thiocarboxzmido pyrazoline (L2).
| % dioxane water | √C mole½/lit½ | Density d × 103 kgm-3 | Time flow ‘t’ sec | Relative viscosity (ηr) | 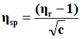 |
|---|---|---|---|---|---|
| 70 | 0.1 | 0.882 | 149.33 | 0.9946 | -0.054 |
| 75 | 0.1 | 0.884 | 139.66 | 0.9828 | -0.172 |
| 80 | 0.1 | 0.881 | 124.66 | 0.9872 | -0.128 |
| 85 | 0.1 | 0.882 | 134.66 | 1.1926 | 1.926 |
| 90 | 0.1 | 0.880 | 135.33 | 1.2832 | 2.832 |
Note: Temp.=300 K, Conc.=0.01 M.
TABLE 9. Ligand: 3-(2-hydroxy-3-bromo-5-chlorophenyl)-5-phenyl-1-thiocarboxzmido pyrazoline (L3).
The change in structure of solvent or solution resulted from hydrogen bonding formation or disruption leads to decrease or increase in the interactions. H-bond forming or disrupting properties can be correlated with changes in the density or viscosity; solute can occupy the interstitial space in solvent [7,8]. According to the temperature, at 297 K ligands L2 and L3 shows all negative values of specific viscosity it indicates weak solute solvent interaction and at 300 K ligands L2, L3 because in ligand L3 presence of -Br electron withdrawing group introducing to first benzene ring near -OH group. L3<L2<L1.
Conclusion
All results show decreasing the values of solute-solvent interactions with increasing solute-solute interaction. The solute-solute interaction of ligand L1 and L2 at different percentage is least due to unsaturation. This effect is due to the introduction of -Br group on first benzene ring near to -OH group in L3 ligand. Hence, due to steric hindrance solute-solute interaction increases and solute-solvent interaction decreases and vice versa.
Xiaojie Wang, et al., alcohols are commonly used as additives in biodiesel to improve its viscous and dense properties for better applicability in the industrial field. In this work, experimental investigations are conducted on the dynamic viscosities for the binary mixture of methyl octanoate (one component of biodiesel) with one 1-alcohol containing 1-propanol, 1-butanol, 1-pentanol, 1-hexanol and 1-heptanol from 293.15 K to 333.15 K at atmospheric pressure. The excessive viscosity shows negative deviation from the ideal solution for each binary system and is fitted with the Redlich-Kister equation. The McAllister three body models are used for the kinematic viscosity correlation of binary mixtures.
References
- Kazys R, Mazeika L, Sliteris R, et al. Measurement of viscosity of highly viscous non-Newtonian fluids by means of ultrasonic guided waves. Ultrasonics. 2014;54(4):1104-1112.
[Crossref] [Google Scholar] [PubMed]
- Kornaeva EP, Stebakov IN, Kornaev AV, et al. A method to measure non-Newtonian fluids viscosity using inertial viscometer with a computer vision system. Int J Mech Sci. 2023;242:107967.
- El Malki M, Bria A, Amraquib S, et al. A simple way to measure the dynamic viscosity of a fluid. New J Sci Vulgarization. 2021;1(2):38-48.
- Loria H, Pereira-Almao P, Satyro M. Prediction of density and viscosity of bitumen using the Peng-Robinson equation of state. Ind Eng Chem Res. 2009;48(22):10129-10135.
- Mahapatra SS, Karak N. Fluorescent hyper branched polyamine with s-triazine: Synthesis, characterization and properties evaluation. Polymer J. 2009;41(1):20-25.
- Wang X, Ma Y, Huo J, et al. Viscosity measurement and correlation research of one biodiesel component (methyl octanoate) with five 1-alcohols. Fuel. 2022;324:124441.
[Crossref]
- Ashima KC. Ultrasonic and viscometric investigation of ternary liquid mixtures of glycols in methanol solution of methyl parabene at different temperatures. Plant Arch. 2020;20(2):2792-800.
- Chimankar OP, Pawar NR. Ultrasonic investigation of bio-liquid mixtures of methanol with cinnamaldehyde by interferometric method operated in the frequency range 1 MHz-10 MHz. Int J Eng Res Technol. 2014;3(6):315-23. .