Review
, Volume: 19( 9)Total synthesis of (-)-cryptocaryalactone, (R)-(+)-goniothalamin, 7-epi-goniodiol and (+)-kavalactones
- *Correspondence:
- R Koorella Department of Synthetic Organic Chemistry, Indian Institute of Chemical Technology, Hyderabad, India, E-mail: koorella.rajasekhar@gmail.com
Received: September 23, 2021; Accepted: September 30, 2021; Published: October 13, 2021
Abstract
A flexible stereoselective synthesis of styryl lactones (-) cryptocaryalactone, 7-epi-goniodiol, (R)-(+)-goniothalamin, (R)-(+)-kavain and (S)-(+)-7, 8-dihydrokavain is synthesized from inexpensive and readily available cinnamaldehyde by applying enantioselective allylation and dihydroxylation reactions. The attractive features of the synthesis is using commercially available inexpensive single starting material for the all the styryl lactones.
Keywords
Synthesis; Cryptocaryalactone; 7-epi-goniodiol; (R)-(+)-goniothalamin; (R)-(+)-kavain and (S)-(+)-7; 8-dihydrokavain; Cinnamaldehyde
Introduction
Styryl lactones represent a class of natural and synthetic compounds with significant cytotoxicity including antitumour, antifungal and antibiotic properties. In continuation of their importance, many styryl lactones have been isolated from plants and fungi. (-) Cryptocaryalactone 1 and R-(+)-goniothalamin 3 are the styryl lactones isolated from the dried bark of Cryptocarya species. (R)-(+)-Goniothalamin 3 displays cytotoxic effects against colon cancer, breast cancer, lung carcinoma and normal cell lines. Similarly, 7-epi-goniodiol 2 isolated from the ethanolic extract of stem barks of Goniothalamus leiocarpus (Annonaceae), a tropical plant found in south Yunnan province in China showed strong inhibition against HL-60 in very low concentration of 1μg/mL. α-Pyrone derivatives known as the kavalactones comprise approximately 15% of the Kava plant (Piper methysticum) rootstock. The more widespread of these include kavain 4 and dihydrokavain 5. The kavalactones displays anaesthetic, sedative, analgesic, anticonvulsive, antispasmodic, antimycotic, antifungal, antithrombotic and central muscular relaxing properties. Although many successful approaches to the synthesis of cryptocaryalactone, (R)-(+)-goniothalamin, 7-epi-goniodiol and kavalactones have been reported their biological importance and activity, prompted us to design a concise and flexible stereo selective route toward the total synthesis of these lactones. The proposed route describes the synthesis of all the five styryl lactones from inexpensive, commercially available starting material cinnamaldehyde and makes it useful and inexpensive route for the synthesis of styryl lactones.
Literature Review
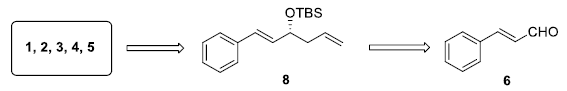
The retro synthetic analysis shows that all these lactones can be synthesized from tert-butyldimethylsilyl (TBS) ether 8 and this could be synthesized from the inexpensive and commercially available cinnamaldehyde 6 (Scheme 1).
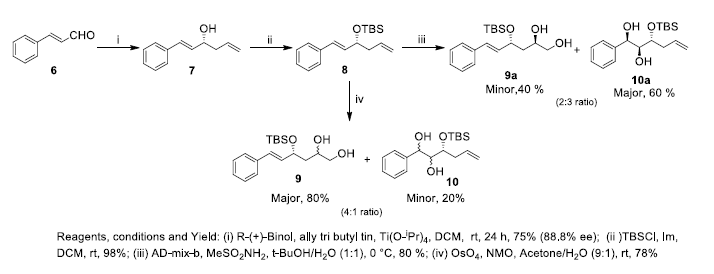
Based on this disconnection, we began the synthesis by enantioselective allylation of cinnamaldehyde 6 using allyl tri butyltin to afford alcohol 7 in 88.8% ee as determined by chiral HPLC. The alcohol 7 was protected as tert-butyldimethylsilyl (TBS) ether and further subjected to Sharpless asymmetric dihydroxylation to yield the two diols 9a and 10a in 4:6 ratio respectively in 80% yield. Compounds 9a and 10a were used as key starting material for the synthesis of (-)-Cryptocaryalactone 1 and 7- epi-goniodiol 2 respectively. The intermediate 8 was further used for conversion of diols 9 and 10. Thus, dihydroxylation of TBS protected alcohol 8 using OsO4/NMO gave the two diols 9 and 10 in 8:2 ratio and 78% yield. The compound 9 thus obtained was used as key intermediate for the synthesis of (R)-(+)-goniothalamin 3, (R)-(+)-kavain 4 and (S)-(+)-7, 8- dihydrokavain 5 (Scheme-2).
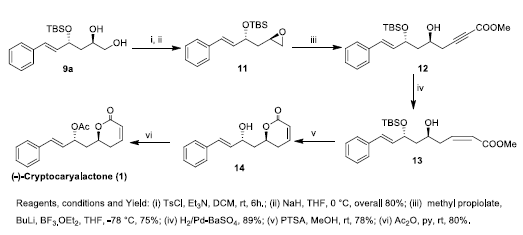
The synthesis of (-)-Cryptocaryalactone 1 was initiated with diol 9a, which was synthesized via Scheme 2. The diol 9a was mono tosylated using TsCl and further converted to epoxide 11 using NaH in 95% overall yield. The epoxide 11 was subjected to nucleophilic ring opening with methyl propiolate using BuLi and BF3.OEt2 to afford the δ-hydroxy ester 12 in 75% yield. The partial reduction of triple bond of the ester 12 using Lindlar’s catalyst yielded cis α,β-unsaturated ester 13 in 95% yield. The cis α,β-unsaturated ester 13 was cyclized with concomitant loss of silyl group to yield lactone 14 using catalytic amount of p-toluene sulphonic acid in 80% yield. Acylation of the hydroxy group using acetic anhydride in pyridine yielded cryptocaryalactone 1 in 98 % yield (Scheme 3).
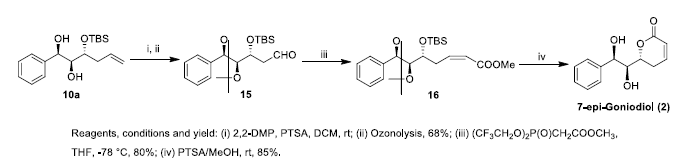
Similarly, 7-epi-goniodiol 2 was synthesized from diol 10a. The compound 10a was protected with 2,2-dimethoxy propane (DMP) and then subjected to ozonolysis to afford the aldehyde 15 in 68% overall yield. The aldehyde was subjected to Horner-Emmons reaction and obtained the cis ester 16 in 80% yield. The ester 16 was cyclized to 7-epi-goniodiol 2 using catalytic amount of PTSA in methanol to afford 7-epi-goniodiol 2 in 85 % yield (Scheme 4).
Experimental
(R,E)-1-phenylhexa-1,5-dien-3-ol
A mixture of (R)-(+)-1,1’-bi-2-naphthol (0.429 g, 1.5 mmol), 1M Ti(O-i-Pr)4 in CH2Cl2 (0.447 mL, 1.5 mmol), and ovendried powdered 4 A0 molecular sieves (2 g) in CH2Cl2 (60 mL) was heated at reflux for 1h. The red-brown mixture was cooled to room temperature and cinnamaldehyde 6 (2 g, 15 mmol) was added. After being stirred for 10 min, the contents were cooled to –78°C, and allyl tri-n-butylstannane (5.16 mL, 16.6 mmol) was added. The reaction was stirred at -20°C for 5 h., warmed to 0°C and stirring was continued for 12 h. The reaction mixture was quenched with saturated NaHCO3 (5 mL). The contents were stirred for 1 h and then poured over Na2SO4 and filtered through a plug of celite. After evaporation of solvent, the crude material was purified by column chromatography to give the alcohol 7 as colorless oil. The compound has 88.8% ee as determined by chiral HPLC (Chiralcel OB hexane/2- propanol 95:5, 0,6 mL/min, 254 nm, tR= 15.14 min for minor enantiomer, tS= 16.51 min. for major enantiomer). Yield: 75%; [α]D25 (-) 10.6° (Et2O, c 1.0), lit. [α]D25 (-) 12.3° (Et2O, c 1.0); 1H NMR (200 MHz, CDCl3): δ 7.15-7.36 (m, 5H), 6.56 (d, J = 17.37 Hz, 1H), 6.19 (dd, J = 6.79, Hz, 1H), 5.75-5.90 (m, 1H), 5.10-5.20 (m, 2H), 4.26-4.34 (m, 1H), 2.32-2.42 (m, 2H); 13C NMR (300 mHz, CDCl3): δ 136.4, 134.3, 131.5, 130.2, 128.8, 127.4, 126.6, 118.6, 71.5, 42.3; [M+Na]+ found = 197.20, C12H14O requires 197.10.
(R,E)-tert-butyldimethyl((1-phenylhexa-1,5-dien-3-yl)oxy)silane
To a solution of alcohol 7 (2.0 g, 11.4 mmol) in dry CH2Cl2 (20 ml) and imidazole (1.16 g, 17.25 mmol) at room temperature under nitrogen atmosphere was added TBDMSCl (2 g, 13.8 mmol) and stirred for 4 h. The reaction mixture was diluted with water (3 ml) and extracted with CH2Cl2 (2 x 5 ml). The combined organic layers were washed with brine (1 x 5 ml), dried (Na2SO4), filtered and concentrated under vacuum to afford the crude product. Column chromatography of the crude product afforded compound 8 as a colorless liquid. Yield: 92%; 1H NMR (200 MHz, CDCl3): δ 7.17-7.37 (m, 5H), 6.47 (d, J = 16.24 Hz, 1H), 6.14 (dd, J = 5.90, 6.64 Hz, 1H), 5.74-5.90 (m, 1H), 5.01-5.13 (m, 2H), 4.25-4.36 (m, 1H), 2.27- 2.41 (m, 2H), 0.92 (s, 9H), 0.10 (s, 3H), 0.06 (s, 3H); 13C NMR (300 MHz, CDCl3): δ 137.4, 135.2, 132.6, 129.4, 128.1, 126.6, 117.3, 73.6, 43.5, 26.1, 18.4, -4.0, -4.4; [M+Na]+ found = 311.0, C18H28OSi requires 311.19.
(2R,4R,E)-4-((tert-butyldimethylsilyl)oxy)-6-phenylhex-5-ene-1,2-diol and (1R,2S,3R)-3-((tert-butyldimethylsilyl)oxy)- 1-phenylhex-5-ene-1,2-diol
To a well stirred solution of AD-mix β (5.2 g) and CH3SO2NH2 ( 3.50 g, 3.65 mmol) in (1:1) t-butanol:water (37 ml) at 0°C the compound 8 (1.1 g, 3.68 mmol) was added. After the reaction was completed (TLC), 3.5 g of Na2S2O5 was added and the stirring was continued for 30 min. The reaction mixture was extracted with ethyl acetate (4x50 ml). The combined organic layers were dried over anhydrous Na2SO4 and concentrated under reduced pressure. Purification by silicagel column chromatography afforded 9a (0.376 g) and 10a (0.56 g) as viscous liquids. Yield: 80% (overall); 9a: 1H NMR (200 MHz, CDCl3): δ 7.15-7.37 (m, 5H), 6.48 (d, J = 15.10 Hz, 1H), 6.11 (d, J = 6.79 Hz, 1H), 4.57-4.48 (m, 1H), 3.81-3.91 (m, 1H), 3.52-3.62 (m, 1H), 3.35-3.47 (m, 1H), 1.73-1.87 (m, 1H), 1.54-1.69 (m, 1H), 0.93 (s, 9H), 0.08 (s, 3H), 0.07 (s, 3H); 13C NMR (300 MHz, CDCl3): δ 134.62, 133.18, 126.25, 125.32, 73.84, 70.46, 63.98, 36.45, 26.77, 16.15 ; [M+Na]+ found = 345.1, C18H30O3Si requires 345.18; 10a: 1H NMR (200 MHz, CDCl3): δ 7.22-7.32 (m, 5H), 5.71-5.86 (m, 1H), 4.89-5.12 (m, 3H), 3.82-3.89 (m, 1H), 3.50-3.58 (m, 1H), 2.25-2.48 (m, 2H), 0.92 (s, 9H), 0.10 (s, 3H), 0.08 (s, 3H); 13C NMR (300 MHz, CDCl3): δ 143.24,126.55, 125.13, 124.41, 125.64, 117.33, 107.86, 72.34, 70.42, 68.75, 34.97, 27.26, 20.17; [M+Na]+ found = 345.1, C18H30O3Si requires 345.18.
(4R,E)-4-((tert-butyldimethylsilyl)oxy)-6-phenylhex-5-ene-1,2-diol and (3R)-3-((tert-butyldimethylsilyl)oxy)-1-phenylhex-5-ene-1,2-diol
To as stirred solution of N-Methylmorpholine N-oxide (2.98 g, 0.025 mol) in Acetone:Water (9:1, 30 mL), was added Osmium tetroxide (0.1 g, 0.39 mmol) followed by Compound 8 (5 g, 0.017 mol). The reaction mixture was stirred for 16 h at RT. After completion by TLC, the reaction mixture was quenched with water and extracted eith ethylacetate (4x50 ml). The combined organic layers were dried over anhydrous Na2SO4 and concentrated under reduced pressure. Purification by silicagel column chromatography afforded 9 (3.4 g) and 10 (0.85 g), (overall 4.25 g, 78% yield) as viscous liquids ; 9: 1H NMR (200 MHz, CDCl3): δ 7.17-7.33 (m, 5H), 6.51 (d, J = 15.10 Hz, 1H), 6.12 (d, J = 6.79 Hz, 1H), 4.55-4.47 (m, 1H), 3.83-3.94 (m, 1H), 3.51-3.61 (m, 1H), 3.33-3.42 (m, 1H), 1.72-1.86 (m, 1H), 1.55-1.72 (m, 1H), 0.95 (s, 9H), 0.09 (s, 3H), 0.08 (s, 3H); 13C NMR (300 MHz, CDCl3): δ 134.65, 133.23, 126.21, 125.29, 73.82, 70.44, 63.96, 36.47, 26.75, 16.18 ; [M+Na]+ found = 345.1, C18H30O3Si requires 345.18. 10: 1H NMR (200 MHz, CDCl3): δ 7.22-7.32 (m, 5H), 5.71-5.86 (m, 1H), 4.89-5.12 (m, 3H), 3.82-3.89 (m, 1H), 3.50-3.58 (m, 1H), 2.25-2.48 (m, 2H), 0.92 (s, 9H), 0.10 (s, 3H), 0.08 (s, 3H); 13C NMR (300 MHz, CDCl3): δ 143.22,126.53, 125.11, 124.42, 125.65, 117.36, 107.88, 72.38, 70.45, 68.76, 34.95, 27.25, 20.15; [M+Na]+ found = 345.1, C18H30O3Si requires 345.18 tert-butyldimethyl.
(((R,E)-1-((R)-oxiran-2-yl)-4-phenylbut-3-en-2-yl)oxy)silane
To a stirred solution of diol 9a (2.0 g, 6.21 mmol), triethylamine (1.3 ml, 9.31 mmol) in dry DCM (30 ml) at 0°C was added tosyl chloride (1.18 g, 6.21 mmol) portionwise. After stirring for 6 h at room temperature, the reaction mixture was quenched with saturated aqueous NaHCO3 solution. The organic layer was separated and the aqueous layer was extracted with DCM (2 ne and dried over anhydrous Na2SO4. Removal of solvent under reduced pressure gave crude tosylated product (2.6 g), which was taken in 50 mL of anhydrous THF, cooled to 0°C and NaH (0.33 g [60% w/w in paraffin oil], 8.2 mmol) was added pinch wise. The reaction mixture was stirred at the same temperature for 2h. After completion by TLC, quenched with saturated NH4Cl and extracted with ethyl acetate (2x20 ml). The combined organic layers were dried (Na2SO4) and concentrated under reduced pressure. Purification by silicagel column chromatography afforded the epoxide 11 as colorless liquid. Yield: 80%; 1H NMR (200 MHz, CDCl3): δ 7.14-7.38 (m, 5H), 6.48 (d, J = 14.98 Hz, 1H), 6.14 (dd, J = 6.04 and 6.79 Hz, 1H), 4.44-4.57 (m, 1H), 2.93-3.04 (m, 1H), 2.69-2.74 (m, 1H), 2.43-2.48 (m, 1H), 1.74-1.90 (m, 2H), 0.92 (s, 9H), 0.07 (s, 3H), 0.06 (s, 3H); 13C NMR (300 MHz, CDCl3): δ 132.68, 130.26, 125.45, 124.35, 72.78, 48.64, 45.08, 36.88, 27.37, 18.74; [M+Na]+ found = 327.10, C18H28O2Si requires 327.19.
(5S,7R,E)-methyl 7-((tert-butyldimethylsilyl)oxy)-5-hydroxy-9-phenylnon-8-en-2-ynoate
A solution of n-butyl lithium in hexane (1.85 ml, 4.8 mmol, 2.6 M solution in hexane) was added to a solution of methyl propiolate (0.55 g, 6.57 mmol) in THF (20 ml) at –78 oC under nitrogen atmosphere, and the mixture was stirred for 15 min. Then, BF3.OEt2 (0.4 ml, 3.3 mmol) was added to the solution and the stirring was continued for 15 min. at –78 oC. Finally, a solution of epoxide 11 (1.0 g, 3.3 mmol) in dry THF (5 ml) was added, and after stirring the reaction mixture for 3 h at –78 oC, the reaction was quenched by adding saturated aqueous NH4Cl solution (40 ml). The reaction mixture was extracted with ethyl acetate and dried (Na2SO4). Evaporation of the solvent resulted in crude alcohol, which was purified by column chromatography to afford pure alcohol 12 (0.960 g, 75% yield) as a yellow color oil. Yield: 75%; 1H NMR (200 MHz, CDCl3): δ 7.18-7.44 (m, 5H), 6.45 (d, J = 14.98 Hz, 1H), 6.15 (dd, J = 6.04 and 6.79 Hz, 1H), 4.56-4.71 (m, 1H), 3.93-4.17 (m, 1H), 3.70 (s, 3H), 2.44-2.54 (m, 2H), 1.75-1.88 (m, 2H), 0.91 (s, 9H), 0.10 (s, 3H), 0.06 (s, 3H); 13C NMR (300 MHz, CDCl3): δ 150.27, 131.66, 130.22, 127.42, 125.66, 84.86, 76.54, 74.42, 66.75, 51.39, 38.54, 27.98, 24.82, 22.34.; [M+Na]+ found = 411.20, C22H32O4Si requires 411.20.
(2Z,5S,7R,8E)-methyl 7-((tert-butyldimethylsilyl)oxy)-5-hydroxy-9-phenylnona-2,8-dienoate
To a stirred solution of 0.512 g of alkyne 12 (0.512 g, 1.31 mmol) in absolute MeOH (10 mL) was added Lindlar’s catalyst (0.318 g). The suspension was stirred at rt under 1 atm of H2 pressure. After completion of reaction by TLC (~ 0.5 h), the catalyst was removed by filtration and the solvent was removed from the reaction mixture by rotary evaporation. The LCMS of crude product indicated the presence of isomers in 95:5 ratio. After column chromatography over silica gel to afforded the major Z-Isomer 13 as colorless oil. Yield: 89%; 1H NMR (200 MHz, CDCl3): δ 7.18-7.35 (m, 5H), 6.35-6.45 (m, 2H), 6.10 (dd, J = 7.36 Hz, 1H), 5.86 (d, J = 11.52 Hz, 1H), 4.48-4.58 (m, 1H), 3.90-4.01 (m, 1H), 3.68 (s, 3H), 2.75-2.88 (m, 2H), 1.62-1.84 (m, 2H), 0.93 (s, 9H), 0.13 (s, 3H), 0.08 (s, 3H); 13C NMR (300 MHz, CDCl3): δ 162.39, 148.19, 137.62, 135.45, 126.82, 124.58, 121.96, 77.97, 71.85, 53.58, 40.18, 36.66, 27.48, 20.73; [M+Na]+ found = 413.30, C22H34O4Si requires 413.21.
(S)-6-((R,E)-2-hydroxy-4-phenylbut-3-en-1-yl)-5,6-dihydro-2H-pyran-2-one
To as stirred solution of ester 13 (0.2 g, 0.51 mmol) in 2 mL of methanol was added p-toluene sulphonic acid mono hydrate (0.02 g, 0.1 mmol). The reaction mixture was stirred for 1h. and solvent was removed under reduced pressure. The residue was dissolved in CH2Cl2, washed with NaHCO3 (4x5 mL) and extracted with CH2Cl2 giving water, brine wash, dried (Na2SO4) and concentrated to afford the deacetyl cryptocaryalactone 14 as pale yellow colour oil. The crude product 14 was directly used in the next step without further purification.
(-)-Cryptocaryalactone (1). To a stirred solution of lactone 14 (0.1g, 0.4 mmol) in pyridine (1 mL) was added acetic anhydride (0.1 mL, 1 mmol). The reaction mixtutre was stirred for 6h. and extracted with ethyl acetate giving sat. CuSO4, water, brine wash. The combined organic layers were dried (Na2SO4) and concentrated. The crude residue was subjected to column chroamatography to afford the pure (-)-cryptocaryalactone 1. The compound has 93.4% ee as determined by chiral HPLC (0.09 g, 80% yield). Solid (mp 109-110°C); [α]D25 (-) 19.5° (CHCl3, c 0.42), lit. [α]D25 (-) 20.0° (CHCl3, c 0.42); 1H NMR (CDCl3, 300 MHz): δ 7.25-7.41(m, 5H), 6.88 (m, 1H), 6.67 (dd, J =15.7, 0.3 Hz, 1H), 6.11 (dd, J =15.9, 7.3 Hz, 1H), 6.03 (dt, J = 9.9 Hz, 1H), 5.66 (m, 1H), 4.55 (m, 1H), 2.35-2.42 (m, 2H), 2.11-2.18 (m, 1H). 2.08 (s, 3H), 2.04-2.09 (m, 1H); 13C NMR (CDCl3, 50 MHz): 170.0, 163.8, 144.7, 133.2, 135.9, 128.6, 128.2, 126.6, 121.5, 74.2, 70.7, 39.9, 29.5, 21.3; [M+Na]+ found = 309.23, C17H18O4 requires 309.12; Anal calcd for C17H18O4: C, 71.31; H, 6.34; O, 22.35; Found: C, 71.36; H, 6.38; O, 22.33.
(R)-3-((tert-butyldimethylsilyl)oxy)-3-((4S,5R)-2,2-dimethyl-5-phenyl-1,3-dioxolan-4-yl)propanal
To a solution of diol 10a (3 g, 9.3 mmol) in 30 mL of dry CH2Cl2, was added 2,2-dimethoxypropane (1.93 g, 18.6 mmol) and PTSA (100 mg, 0.58 mmol) and the reaction mixture was stirred at ambient temperature for 4 h. The organic layer was evaporated under reduced pressure to afford the crude acetonide. The crude acetonide was dissolved DCM and cooled to -78°C. Then Ozone gas was passed under stirring into the reaction mixture via pre-fitted glass tube. The appearance of a sky blue color in the solution indicates the completion of reaction. After completion, crushed Triphenyl Phosphene (4 g) was added, stirred for 30 min. and allowed to room temperature for 6h. Then the reaction mixture was concentrated under reduced pressure and the crude product 15 was directly used in the next step without further purification.
(R,Z)-methyl 5-((tert-butyldimethylsilyl)oxy)-5-((4S,5R)-2,2-dimethyl-5-phenyl-1,3-dioxolan-4-yl)pent-2-enoate
A solution of bis(2,2,2-trifluoroethyl) (methoxycarbonylmethyl) phosphonate (0.48 g, 1.50 mmol) in THF (10 ml) was treated with NaH (0.08 g [60% w/w in paraffin oil], 2 mmol) at –78°C for 15 min. Aldehyde 15 (0.5 g, 1.37 mmol), was then added and the resulting mixture was stirred at –78°C for 1h. The reaction mixture was quenched with saturated NH4Cl and extracted with ethylacetate (3x5 ml). The combined organic extracts were washed with water (2x15 ml) followed by brine, dried (Na2SO4) and concentrated under reduced pressure. The LCMS of crude product indicated the presence of isomers in 9:1 ratio. The resulting mixture was subjected to column chromatography to yield major product (Z)-α,β-unsaturated ester 16 as colourless oil. Yield: 82%; 1H NMR (200 MHz, CDCl3): δ 7.32-7.52 (m, 5H), 6.14-6.26 (m, 1H), 5.82 (d, J = 11.33 Hz, 1H), 5.00 (d, J = 8.30 Hz, 1H), 4.07-4.14 (m, 1H), 3.95 (dd, J = 3.02 Hz, 1H), 3.75 (s, 3H), 2.85-2.97 (m, 1H), 2.72-2.83 (m, 1H), 1.58 (s, 3H), 1.56 (s, 3H), 0.96 (s, 9H), 0.09 (s, 3H), 0.08 (s, 3H); 13C NMR (300 MHz, CDCl3): δ 162.19, 149.25, 138.62, 130.35, 127.58, 126.42, 123.28, 115.89, 82.86, 75.43, 71.67, 53.69, 37.76, 28.12, 26.49, 19.96; [M+Na]+ found = 443.20, C23H36O5Si requires 443.23.
Conclusion
In conclusion, we have developed a concise and flexible stereoselective route to synthesize the unsaturated lactones using inexpensive and readily available cinnamaldehyde.
Acknowledgement
The author gratefully acknowledges the support of Indian Institute of Chemical Technology (IICT), Hyderabad for carrying the research work.
General Remarks
The 1H and 13C NMR were recorded in CDCl3 at 300 MHz on a Bruker 300 MHz FT NMR spectrometer. The chemical shifts were reported in δ ppm relative to TMS. The mass spectrum (70 eV) was recorded on an Agilent-6310 LC-MS spectrometer. The solvents and reagents were used without any purification.
References
- Nicolaou KC, Yang Z, Liu JJ, et al. Total synthesis of taxol. Nature. 1994;367(6464):630-634.
- Nicolaou KC, Edmonds DJ, Bulger PG. Cascade reactions in total synthesis. 2006;45(43):7134-7186.
- Endo A, Yanagisawa A, Abe M, et al. Total synthesis of ecteinascidin 743. J Amer Chem Soc. 2002;124(23):6552-6554.
- Aicher TD, Buszek KR, Fang FG, et al. Total synthesis of halichondrin B and norhalichondrin B. J Amer Chem Soc. 1992;114(8):3162-3164.
- Danishefsky S. Siloxy dienes in total synthesis. Acc Chem Res. 1981;14(12):400-406.