Original Article
, Volume: 8( 2)The Present Anomalous Testing of Water Purity: Setting a New Standard for the Future in Producing Nanometer-Sized Metals or Better Still, What Can Water Tell Us About Itself?
- *Correspondence:
- Laroo H, As Honorary Research Adviser to University of Queensland and Griffith University, Brisbane, Queensland, Australia, Tel: 61732023767; E-mail: hlaroo@bigpond.com
Received: October 19, 2017; Accepted: October 31, 2017; Published: November 08, 2017
Citation: Hans Laroo. The Present Anomalous Testing Of Water Purity: Setting A New Standard For The Future in Producing Nanometer Sized Metals or Better Still, What Can Water Tell Us About Itself? Res Rev Electrochem. 2017;8(2):110
Abstract
For a number of years my activities have included: determining exactly how to produce nanometer sized silver clusters in an aqueous medium using physics and photonic light at 420 nm. This wavelength is known to be absorbed by silver and has proven successful, resulting in producing atomic silver clusters in a narrow band distribution just 6.5 nm wide (3.6 to 10.1 nm). However soon after, it came to realization that an even bigger challenge was waiting to be explained: How to properly test the properties of water?
So-called colloidal silver as a potential biocide can only exist in water and in particular uncontaminated water. For that reason water, in addition to the silver used, requires to be very pure indeed. Water itself is a dielectric and thus an insulator. It does not conduct electric current. Any conduction of current or charge carrying that does occur is done exclusively by the ionic matter contained in the water. For that reason it is a prudent decision to use the purest possible water. Deionized water was chosen. In comparison, distilled water, containing much foreign matter, does allow too much current to flow and is not suitable. For example, when testing the properties of distilled water, the lowest conduction ever measured is only 100, 000 Ohm. More often than not the conduction is even higher at 56, 000 Ohm. This is well short of the expected quality of distilled water at 1 million Ohm. According to the Science of Conductance, 1 micro Siemens/cm should provide an equivalent reciprocal or inverse proportionality of 1 million Ohm resistivity/cm for any type of water and that should include distilled water. It is clear that in the case of distilled water, this is not true. In comparison, good quality deionized water of a purity of between 0.01 and 0.022 Micro Siemens/cm will have a closer resemblance to its Ohmic reciprocals although not exactly. However, the values are close enough in Ohmic resistance to allow current to flow between electrodes at a set voltage of either 300 or 600 Volts DC for a current of 500 and 1, 500 micro ampere/h respectively. It has been noted after numerous batch productions, a steadily increasing carbonation due to carbon dioxide (CO2) entering the water seems to have no serious side effects. However nothing is ever that simple! In this manuscript we will present our findings that conventional water purity testing using the concept of Siemens or its reciprocal in resistivity are flawed, inadequate and confusing by not embracing proper science and Ohm’s law and especially ignoring the voltage potential, as if irrelevant.
Keywords
Equilibrium voltage of water; Conductance; Resistivity; High input resistance; 10 K Million Ohm
Introduction
Ever since my research started on a new method of producing a high quality colloidal silver in early 2008, the most questionable aspect of this material proved to be the water. Water forms the bulk of colloidal silver, that incidentally is not a colloid at all and better identified as nanometre sized atomic silver clusters in an electrical suspension. More and more used in clinical trials, the effective concentration, in line with the MIC (minimum inhibitory concentration), is generally no more than 10 ppm or 10 mg/litre. For that concentration to be true, the ratio of water will be 999, 990 to 10. Using silver at a purity of 99.998% in order to avoid too high a percentage of hazardous substances such as arsenic and lead, the water is expected to be equally pure. Very soon this presented a problem. How is water purity tested? Other than analysing the operation of pH and conductance meters displaying either MHO or micro Siemens, no specific information could be found anywhere and it became suspicious about such absent information. For years a vacuum tube voltmeter (VTVM) had been part of my arsenal of test equipment and particularly valuable for its maximum resistance reading at full scale deflection (FSD) at 1, 000 million Ohm. None of the dozen or so other multimeters, including the latest digital multi-meters could not even come close, realising that officially the maximum resistance of water had been established years ago as being 18.24 million Ohm, with a reciprocal of 0.0549 micro Siemens at a temperature of 25 degrees Centigrade across two 1 cm2 metal foils at 1cm distance [1-3]. My resistance measurements however were much higher with readings in the hundreds of millions of OHMs and confirming these readings with 1% precision resistors at an equal value. After repeated and consistent testing, it was realised that something was amiss and initiated an enduring search for the truth. This presented itself recently with a report from NIST (National Institute of Standards Testing). The values quoted and relied on by the Industry at large, were theoretically calculated and found not to be related to actual physical conditions, science and the real world [4].
Experimental
Research over a number of years relating to the purity of silver and the hazardous substances it may contain, has also placed a spotlight on the questionable purity of water and the equally unorthodox but established manner of water testing. Since the days of Michael Faraday in the early 1800s the conventional measurements of electrolysis was through the use of Direct Current. It was then that Faraday formulated his first and second laws of electrolysis, determined the atomic weights of hydrogen, silver and copper and that the equilibrium of voltage was 1.25 volts DC. This has now been more accurately established at 1.23 volts DC. The application of this voltage level will cause the molecules that make up water to separate into their constituent components of hydrogen and oxygen gasses. This will also cause unwanted ionic polarization of the water and disrupts any accurate testing. Some explanations of the problems associated with water testing are listed as follows:
The parallel resistance factor
Any instrument and associated measuring probes need to have an input resistance FAR in excess of the resistance of the water its content and other factors involved. In the electronic instrumentation of the 1900s and the use of high value resistors in use, i.e. 1 million Ohm, it was considered prudent to have an instrument and probes at a resistance factor at least a 100 times higher than the subject measured. The VTVM provided this at 1.000 million Ohm input resistance so that a close precision of 1% accuracy could be obtained. The vacuum tube Voltmeter was designed offering a full scale deflection of maximum 1, 000 million Ohm. With my claim that the actual resistance of water is dependent on purity, quantity and even the container it is in, measured 300 million Ohm using a purpose built acrylic probe 10 × 10 mm square and fitted with two foils of silver measuring 10 mm × 10 mm and facing each other at a distance of 10 mm. Using silver electrodes distanced at 1, 000 mm, a 1, 000 million ohm resistance reading was obtained. There was 40 litres of deionised water in a 1200 mm long tank. Each step of the way, the measured resistance of the water was compared with a 1% resistor of that same value, all the way from 1, 000 million ohms to 300 million Ohm respectively.
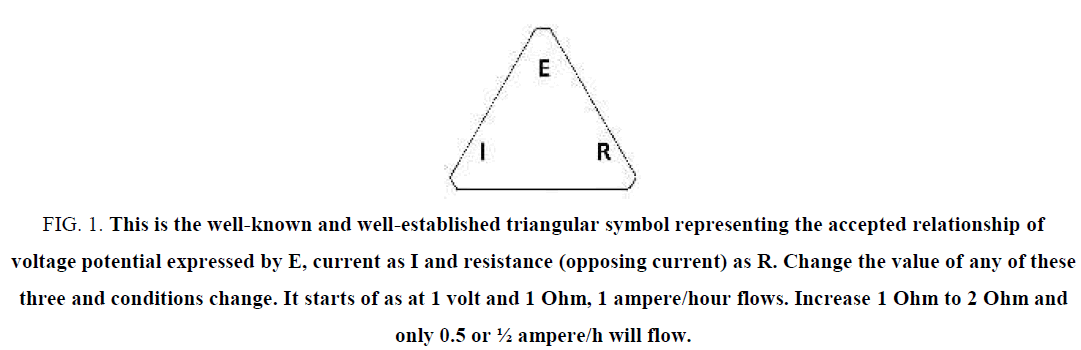
Author’s note: Over the years there has been plenty of argument about using this type of measurement practice, i.e. Ohm’s law. Comments like “You are not supposed to measure water that way. You are supposed to use a water conductivity meter calibrated in micro Siemens and its inversely proportional resistivity”. Guess what? Why argue with Ohm’s law that has a well-established relationship with Resistance in R, current in ampere/h as I and a voltage potential as E. Change the value of one and you change the values of the other two, i.e. 1 volt allows 1 ampere to flow through a resistor of 1 Ohm. Increase the resistor to 2 Ohm and the current is halved to only 500 mA/h. Guess what some more? The term Siemens originated from Werner von Siemens when establishing a standard form of resistance of 0.95 ohm for a solid metal length of conducting telegraph cable in the 1880s. The original word Resistivity was also designed with a solid metal conductor in mind. To use these terms for the non-conducting properties of water seems totally inappropriate! With the use of micro Siemens/cm the level of voltage potential is not even considered (Figures 1 to 4).
Figure 1: This is the well-known and well-established triangular symbol representing the accepted relationship of voltage potential expressed by E, current as I and resistance (opposing current) as R. Change the value of any of these three and conditions change. It starts of as at 1 volt and 1 Ohm, 1 ampere/hour flows. Increase 1 Ohm to 2 Ohm and only 0.5 or ½ ampere/h will flow.
Figure 2: This is a simple but high input resistance analogue conductance meter that actually measures current in micro ampere/h and seen measuring the conductanace of nano metre silver in water in a 100 ml flask. The actual measurement reads about 12 micro ampere/h for the sample of colloidal silver. The input resistance is 10, 000 million Ohm and operates at a potential of just 1 volt direct current, to ensure it will remain well under the equilibrium voltage of water at 1.23 volt DC. The other 100 ml flask is filled with pure water and reading just 2 micro ampere.
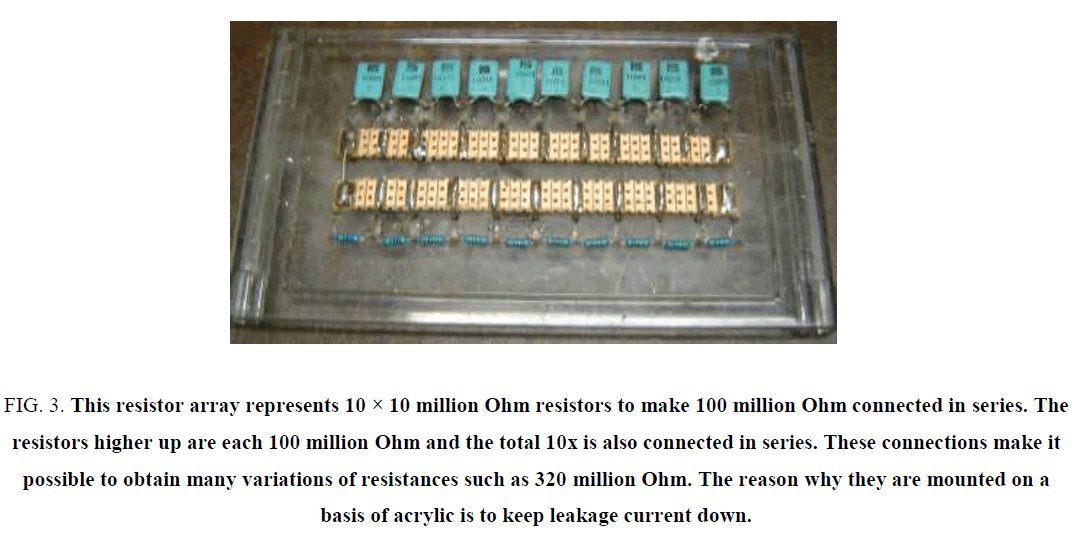
Figure 3: This resistor array represents 10 × 10 million Ohm resistors to make 100 million Ohm connected in series. The resistors higher up are each 100 million Ohm and the total 10x is also connected in series. These connections make it possible to obtain many variations of resistances such as 320 million Ohm. The reason why they are mounted on a basis of acrylic is to keep leakage current down.
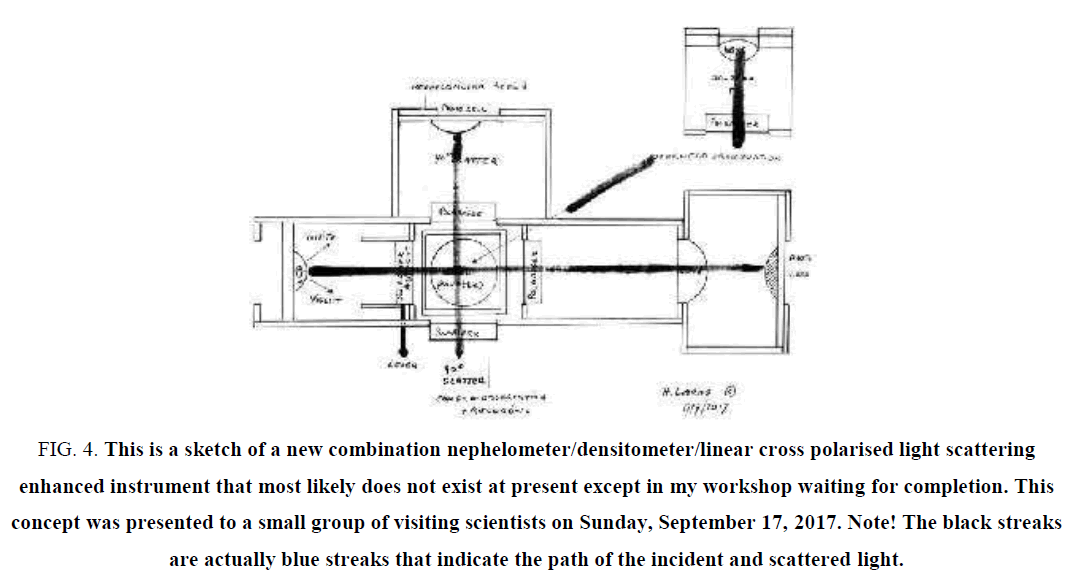
Figure 4: This is a sketch of a new combination nephelometer/densitometer/linear cross polarised light scattering enhanced instrument that most likely does not exist at present except in my workshop waiting for completion. This concept was presented to a small group of visiting scientists on Sunday, September 17, 2017. Note! The black streaks are actually blue streaks that indicate the path of the incident and scattered light.
Some more information about water
A molecule of water consists of just three atoms: one oxygen atom at 15.999 u and two hydrogen atoms totalling 2.015 u approximately, a ratio of 16 to 2. Fairly simple, but in reality quite complex, considering all of the unusual properties of water of which some are listed here: (a) Able to present either a hydrophilic (water loving) or hydrophobic (water hating) state, such as when water is confronted with silver. In fact the hydrophobicity water has with silver and many other metals as well, create a static electrical charge called the Zeta Potential (under the banner of Interfacial charges and science). This can reach a maximum of minus 100 mV DC (that is -1/10th of a volt) [5,6]. (b) Water forms cluster of molecules that constantly change by whatever it meets to allow the material to co-exist in the water. Water IS the great dissolver and a great solvent for almost anything. It does so by constantly breaking and remaking of the hydrogen bonds at a claimed time frame of 10-18 seconds [3,7-9]. (c) This complexity also makes it virtually impossible for current or charge carrier flow (cations and anions) to flow in a straight line between two electrodes placed in the water. Another reason for this current unable to follow the straightest route is that all current behaves in a similar way to the well-known magnetic lines of force, i.e. curved three dimensional trajectories, making a mockery of the so-called measurement per cm, for micro Siemens measurements. An even more bizarre factor about using Siemens or fractions thereof is the complete absence of a measurement per hour, such as with amperage. (d) Water and the ionic contents therein can also be measured on the actual current flow between two electrodes. Pure silver electrodes are to be preferred for silver’s high electrical conduction and secondly for not easily oxidised if the voltage across the probes is kept at 1 volt DC or less. An instrument is presently constructed, which is able to indicate flow of anions to the anode and cations to the cathode, completely independently of each other. That way more is learned about what is actually in the water by way of positive and negative ions instead of just measuring the difference. For some time both National Semiconductors and Radio Corporation of America (RCA) have designed easy to obtain affordable operational amplifiers with input resistance levels at 1 Tera Ohm (1012 Ohm) and higher as well as means to measure current down to nano and pico ampere (10-9 and 10-12) respectively. Unfortunately the feedback resistor for these operational amplifiers would need to be 10,000 Million Ohm. A difficult but not impossible task to obtain. For a number of years now, a custom designed simple inexpensive analogue water current meter with a input resistance of 10,000 million Ohm and operating at 1 volt, is able to tell me instantly (any time of the day) how much current is flowing through my water sample, i.e. deionised water at between 2 micro ampere/h and 3 micro ampere/h and between 70 micro ampere/h and 90 micro ampere/h for ordinary tap water fresh out of the tap.
Results and Discussion
Current conductance measurement practices
Water is far from stable and is subject to freezing, heating, boiling and turning into steam. Present conductance meters, some of which are operating at a variety of frequencies, each one with their own impedance (AC resistance), cannot possibly compare their measurements with similar instruments operating at a different frequency. These then possess a different impedance and loading. When conductivity and resistivity were introduced for measuring ionic content, the term MHO was used for indicating the reciprocal value of Ohm. A reciprocal value however is based on an inversion, i.e. inverse proportion and to do the Ohm justice it should have been written upside down. Instead of doing that, it was spelt backward, which is not really the same thing. Measuring conductance of ionic matter in water is not an easy task as there are positive and negative flows in opposite direction and it is doubtful if this aspect has ever been fully investigated. Also it would seem that the equilibrium voltage of water has been ignored as well. Voltages well above this value have been used for the measurement of water testing, most likely with adverse consequences, i.e. breaking up of the he water molecules. This may have been the reason for adopting alternating current and impedance to try and circumvent this problem. It cannot do so, as alternating current consisting of pure sine waves (just like our mains power supplies) and are subject to the concept of ‘Root Mean Square’. This means that the voltage starts of at zero, rises to a maximum, drops to zero again and continues the other half of the cycle (Hertz) in a negative value and then from zero rises again to a positive mode. At no time is there a consistent voltage value as during each cycle the voltage rises and drops. Use of Direct current is more precise as it simply does not vary over time. There are other conditions as well. These and the Author’s comments are listed below: (a) The argument that water conductance should be measured at a pH of 7 and at a temperature of 25 degrees Centigrade. It is further claimed that at exactly those conditions, the resistance should be 18.24 Million Ohm and no higher when the conductivity is exactly 0.0549 micro Siemens.
Author’s comments: After searching for years for an explanation as to why just these figures, especially since a major supplier of pure and ultra-pure water and instrumentation for testing these waters allows for micro Siemens as low as 0.01 micro Siemens/cm and a reciprocal resistance of 100 million Ohm. Nowhere however it could be found a technical answer until recently, when it was presented with a report from the National Institute of Standards Testing (NIST) in the USA. This report can be found bearing the following identification: NIST Special Publication 260-142, 2004 Ed. Standard Reference Materials: Primary Standards and Standard Reference Materials for electrolytic Conductivity by R. H. Shreiner and K.W. Pratt. Under section 1.3, electrolytic conductivity of water, pages 2 and 3, claims that in order to calculate the theoretical electrolytic conductivity of water standards (these are derived from the H+ and OH- ions from dissociated water molecules as H+ at 349.81 Siemens/cm2 and 198.3 Siemens/cm2 for OH-). These two figures are then added together in order to obtain 549.11 Siemens/cm2. This was then converted by the calculation 549 × 10-7 divided by 1000 Siemens/cm as 5.5 10-8 Siemens/cm into 0.055 micro Siemens/cm the final figure that is actually used. They also add the note: This theoretical value is difficult to obtain experimentally because atmospheric CO2 is readily absorbed into water. As a result of the CO2 reaching equilibrium level with the water, the conductivity had increased to 1.05 micro Siemens/cm. This is an increase in conductivity by a factor of more than 19 times. How the actual H+ and OH- figures were calculated was not shown and is still a mystery. Note! From a standpoint of pico and nano metre sized atomic silver clusters in an electrical suspension, it would not be prudent to heat up this product to 25 degrees Centigrade, just to test the water. Repeated tests have shown that this silver product from which all ions have been reduced to neutral silver, i.e. Ag+ to Ag0, no silver ions were present [10].
Other peculiarities of water
As pointed out, water is a dielectric and an insulator and thus opposing any deliberate and casual current flow. Only a DC voltage level exceeding 1.23 volt is able to start some current flowing. In our research we deliberately control the current electronically to a set figure of 500 micro ampere per hour AND a deliberate DC voltage potential known to be able to allow this particular current to flow from start to finish. Others however use whatever voltage is available, such as 9 volts to 24 volts DC and expect a 100 milli ampere per hour (0.1 ampere) to flow. This just does not happen. It starts as a trickle of a few micro ampere and over many hours slowly creeps up to and ever increasing current until the maximum allowable current is reached. What is interesting is the fact that using a voltage doubling circuit where both the voltage and double the voltage is available, i.e. 30 and 60 volts DC, the current flowing is double that at the higher voltage level. Multiplying this voltage by a factor often, shows a logarithmic rise in current from 300 volts DC at 500 micro ampere to 1500 micro ampere at 600 volts DC. This provides proof of opposition to high initial currents at low voltage and only seeing a rise in current over time that is not really controlled and somewhat unpredictable in its output of ionic matter. Water is also the great absorber of energy and storing that energy until such time as the environment cools sufficiently to again release this stored energy back to the environment. There is one notable exception and that is the region of energy still in the visible range of violet light at between 417 nm and 420 nm. In fact water subjected to these wavelengths becomes super transparent. Water also recognises what actually is contained within itself and taking the appropriate action as to how to deal with each substance. When there is a foreign intrusion or contamination, for lack of a better description, it may orientate each dipolar nature of negative oxygen and positive hydrogen to hold the contaminating matter captive. It does so also with the so-called hydrated or solvated electrons [11-14]. Only irradiation with photonic energy starting with violet light at 420 nm (2.95 eV) can release these hydrated electrons from the clutches of the water. Recently it was discovered that clusters of water molecules formed three specific shapes, the crate, a pyramid and something representing an open book. The first two are able to contain matter other than the water itself [10,15-17]. Another peculiarity of water is creepiness, i.e. getting into smaller confines than what the size of the combined oxygen atom and two hydrogen atoms would allow. It appears it can do so by spreading its atoms further apart from each other. Such quantum confined water was detected initially inside a crystal of beryllium. The paper did not say if this was an aquamarine or an emerald. Such creepiness of water may also more closely explain some aspects of capillary action, another confined space. [12,18].
Introducing new ways to test water in its totality
When testing water for its intended purpose, there are five recommended strategies:
a. Physical observation for visible impurities including micro sized life using the naked eye or a microscope.
b. Reading the content of the label if supplied.
c. The taste of the liquid if this is safe to do so.
d. Technical observation and/or quality analysing, by such techniques as light scattering and obscurity determination.
Note! Such an instrument or instruments can incorporate an incident light source. A laser diode pointer or a torch of fixed incident light shining through a cell filled with a sample of the water to be tested and measured with an appropriate photo sensitive diode quantifying either the total reflected light by scattering or the remaining light passing through being obscured. Metal parts can even be detected and distinguished from organic or inorganic matter through the use of linear cross polarising filters.
e. Determining the extent of water containing ionic matter of any type using a sensitive current measuring meter at micro ampere/h or alternatively the use of high input resistance meters of no less than 10,000 million Ohms (10 Giga Ohm or better).
Contamination of water
Water is capable of containing just about anything. As an illustration of such contamination, a 2009 study by the Environmental protection Agency in the USA, found at least 200 unregulated chemicals in the drinking water. In an analysis of 20 million tap water quality tests a total in excess of more than 300 contaminants from Industrial wastes were also found. Substantial evidence of the increased electrical conductivity of contaminated drinking water was found at home, when comparing ordinary tap water with a reasonably pure deionised water at a probable conductivity of 0.022 micro Siemens. We used a purpose built proto type current meter reading from 1 to 100 micro ampere (FSD). Using a specially constructed acrylic probe fitted with two silver foils measuring 10 × 10 mm and facing each other 10 mm apart, the deionised water measured between 2 and 3 micro amperes. In contrast to this low current value, the tap water gave a deflection indicating a current between 70 and 90 micro ampere. The readings were instantaneous and did not vary with changes in temperature. The contamination of distilled water is different again and although thought to be relatively pure may still contain pico and nano metre sized organic and inorganic contaminants that came from the original water but transferred to the new container with the super-heated steam. Distilled water may also contain copious amounts of CO2 and ammonia, both substances that are claimed difficult to detect using a standard conductivity meter [5].
Two Actual Physical Experiments
A. Experiment to determine if the quantity or volume of water reduces conductivity
Equipment used:
* A 10 mm thick walled acrylic tank measuring L=1200 mm × H=200 mm × D=200 mm.
* Two 99.998% pure silver electrodes L=150 mm Diameter 3.5 mm.
* 40 litres of deionised water of 0.022 micro Siemens/cm.
* A 1900s electronic Vacuum tube voltmeter (VTVM) with a maximum resistance range of 1, 000 million Ohm. 100 million ohm reading at about 75% deflection, thus a bit cramped at the top.
* The 10, 000 million Ohm to 100 micro Ohm FSD (full scale deflection) mentioned in this manuscript. See photo of the instrument in the text.
* A large number of high precision 1% and high value resistors ranging from 1 million ohm to 100 million Ohm (from RS components) and much higher such as 300, 1, 000, to as high as 10,000 million Ohm resistors at 4 KV. Ten of the 100 million Ohm resistors have been electrically joined end to end to give all the variation from 100 to 1, 000 million Ohm.
* A stainless steel rule of 1 metre metric divisions mounted along one side of the tank to indicate changing distances between the silver electrodes and only one of the electrodes actually shifting.
Details of the experiment
Initially, the electrodes were separated at 1 metre distance and the resistance measured on the VTVM was approximately 1, 000 million Ohm. As the separation between the electrodes was being diminished, the current measurements were also increasing. At each stage when a reading was made one or more of the 1% resistors was compared with the reading of the water, until a point was reached at about 400 mm separation, where the resistance would not drop any further. It was then shifted to my acrylic probe and plugged the leads of this probe into the VTVM. As stated before, these tiny 10 mm×10 mm electrodes are only 10 mm apart. To my amazement the measurement was the same, exactly 300 million Ohm. It should be noted that a variety of analogue and digital multimeters were used in comparison, but none could measure anything like this. Even more surprising was that none of these meters could even be compared with each other. b. The second experiment. To determine if voltages change current rating in the water.
Equipment used
* An AC/DC power supply with rectification and double filtering as a voltage doubler circuit, providing alternatively 30 and 60 volts DC. This power supply unit (PSU) did not incorporate a current control or current limiting circuit.
* A 10 litre capacity acrylic tank filled wait 9.5 litre of distilled water available commercially.
* Two silver electrodes L=150 mm and a diameter of 3.5 mm. Purity, 99.998%.
* An analogue panel meter of 10 mA and a digital multimeter (current range) for verification.
* An AC/AC commercial adaptor with an output of 24 volts AC. This was rectified with a two diode voltage doubling circuit to provide the required 33.6 volts DC, i.e. 24 volts AC full wave rectified times 1.4 equals 33.6 and 67.2 volts under perfect conditions.
Details of the experiment
As expected at such a low voltage potential of either 33.6 or 67.2 volts DC or close to it only a trickle of low value micro ampere flowed, although it was noted that at the higher voltage the current that was flowing was double that of the lower value. At start-up just on 15 to 30 micro amperes respectively was flowing. As any current subjected to a voltage potential of more than 1.23 volts DC, the equilibrium voltage of water, current started increasing marginally and taking literally hours before any significant current in the milli ampere range started to flow until current run-away takes over and eventually destruction of the PSU under some circumstances. Many using such a system in order to produce ionic silver become impatient and add salt to speedy up the slow starting current flow. What is not realised that ionic silver missing an electron shares an electron with chlorine and the bonding creates Silver chloride, a fairly insoluble matter of no benefit other than being considered dangerous to ingest.
Conclusion of these experiments
The reason they have been included in this manuscript is not to be copied or cited, but to point out and provide indisputable proof that many in the industries are not fully aware of what you can and cannot do with water. These are as follows:
1. Water should only be measured on the basis of Ohms, current in ampere or fractions thereof in hours instead of Siemens or fractions thereof per centimeter2. Ohm’s law operates on three factors: Current in ampere/h, resistance in Ohms and voltage potential, i.e. 1 ampere/h will flow through a 1 ohms resistor and subject to a potential of 1 volt. Change anyone of these and another will also change.
2. The concept of the centimetre measure relates to the distance of the electrodes used with conductance meters. This can never be scientifically accurate due to the fact that current through water does not flow in a straight line. Thus the trajectory of direct current between two electrodes 1 centimetre apart could be more like half of an inch (13 mm) or even longer and depending on the purity or contamination of the water. Resistivity/cm as its reciprocal is likewise tainted, since even in a solid metal conductor current flows on the outside of the conducting material but according to the flow of least resistance which is not necessarily the shortest route, i.e. linea recta. One need only observe the unpredictable path of lightning!
3. The volume of water in a container will determine the resistance in Ohm and together with the voltage potential also the current flow. We cannot expect that a 600 ml of water in a glass is representative of a quantity of the same water in a large fish tank.
4. The level of voltage potential will allocate the amount of DC current that will flow. It is obvious from the content of this paper that a controlled and limited current of 500 micro amperes will flow applying 300 volts DC and a doubling of the voltage to 600 volts DC will bring a tripling of the current to 1, 500 micro amperes, obviously a logarithmic response curve.
5. Current flow through water or rather caused by ionic contamination can also be affected by external electrical conditions, such as the tank material itself, any metal on which it is placed and any extraneous conditions. It was having a tank of ionic silver in water, inside a fridge, inside a shielded all metal shed and lose its ionic character during a violent outburst from the sun a few years ago. All of the ions had been converted to neutral silver. Other problems about water purity being ignored. So far too much has been written about testing the water with current, but no time spent on materials in the water that do not carry a charge but are nevertheless of equal importance to notice and identify. These are what is called the organic and inorganic solids immersed in water and which can only be discriminated by physical and optical observation and examination.
Back in the early 1900s, physical observation was well established on two fronts: A. Checking out liquids like water for obscurity, matter in the water that would block the light. The equipment for this was usually referred to as densitometers. They operated by shining incident light through a square glass cell, containing the liquid and having compared the level of obscurity of pure water earlier with a photo sensor and a suitable read-out, could now establish the obscurity of the liquid to be tested. Some incorporated both a cell containing pure water and a second cell for the sample to be tested. In order to get some comparison in the obscurity levels of both cells, a lever was available to shift from one to the other. This could be done several times, just to make sure. The advantage of this instrument was a single optical path and photo detector and read-out being the same for both [19].
B. The so-called Nephelometer: ‘Nephos’ from the Greek, meaning cloud. Most likely the use of the concept of nephelometer is meant for looking at light scattering. Whilst the densitometer performs well in quantifying the level of obscurity, it is not able to identify what the actual obscurity is. To accomplish that as well, light scattering and in particular light scattering via linear cross polarised filters and analysers has a great advantage. Linear cross polarisation enables materials with a refractive index at visible wavelengths to be distinguished from metals that do not. Adding a suitable UV/VIS diffraction grating, also individual metals can be identified by their individual absorption characteristics, i.e. silver will absorb at 420 nm and also disappear from view when at that level. Most other metals however absorb wavelengths in the Ultraviolet. A definite shame however is the departure of the full surface clear glass walls of cells and cuvettes. Instead most are now obscured partially by an amount of mottling or roughness on two opposing surfaces to avoid slipping out from between the fingers holding them. This effectively removes the opportunity of 90 degree (right angle) observation of light scattering or any other angle as well. Light scattering from a variety of angles, either one by one or simultaneously can detect variations in symmetry. In particularly, light scattering at ever shorter wavelengths will enable ever smaller objects in the water to be seen and identified. Using long wavelengths of light may be just too long to be absorbed by items in the quantum realm, below 10 nm in size [20]. To enable maximum efficiency in instrumentation some prototype observation instruments will incorporate the characteristics of a densitometer AND a nephelometer AND the linear cross polarisation facility all-in-one. It requires noting, that these instruments will all be based on analogue principles since what we are measuring are analogue events as well.
Important information concerning light scattering
Light scattering is used more and more and often at a wide choice of wavelengths, well into the ultraviolet. Whilst long wavelengths of light such as red to green will scatter light from of large particles, particles in the low nanometre size will only scatter the dark blue colours such as indigo, violet and ultraviolet. The reason for using shorter wavelengths is the much smaller size of these particles are unable to absorb longer wavelengths.
The most valid form of light scattering is that form that employs scattering at 90 degrees (right angle). This requires a rectangular or square (thin walled) glass cuvette or cell that is clear on all four surfaces. This is to not only observe and analyse at 90 degrees, but also at other angles as well as measure any back-scatter. In addition, light reflected from metals such as silver organic material, plastics and silica based products scatter light as well. The problem with that is a scattering that is quite indiscriminate and to some extent providing useless information. For that reason, it augmented light scattering with linear cross polarisation. Using such a technique will enable materials that have no refractive index at visible wavelengths, i.e. most metals, from being distinguished from materials that do. Silver and Gold are a few exceptions with silver absorbing light at around 420 nm and gold absorbing light at around 550 nm respectively, wavelengths still in the visible range. Using linear cross polarisation filters and analysers and depending which particular wavelength of light is used, optical separation is assured. When using such techniques, it is recommended to use a variable UV/VIS diffraction grating. When both the polariser and analyser are aligned, i.e. maximum light, all matter able to scatter, will scatter. However, if either the polariser OR the analyser is placed at right angle to the other (cross polarised), only materials that do not possess a refractive index at that frequency/wavelength of light can still be seen to scatter, a very useful tool indeed.
Conclusion
There has been insufficient research into all of the water purity testing procedures and even less criticism on such procedures that can now be considered flawed and unscientific, especially such as using terms like Siemens and resistivity created exclusively with solid metal conductors in mind. This questionable water testing procedure is strictly based on purely hypothetical calculations without any ‘real world’ verification or proof of this hypothesis. Another ‘sore point’ is the true character of the so-called Zeta potential, created between water and itself as an interfacial electric charge. Presently, it is not convinced that the current ‘testing” evidence presented. Then there remains the confusion of one group of scientists holding onto water not accommodating an electrical resistance higher than about 18 million Ohm and another group claiming a maximum level of at least 100 million Ohm for ultra-pure water. From my perspective neither are correct. However it depends entirely on how water is measured. It is hoped that the content of this manuscript will bring about changes toward a better understanding of this science and technology (Table 1).
| Complete water purity testing – Including the use of optical observation |
| Terms such as Ohm’s law, conductivity/conductance, resistivity, reciprocal, electrical current, voltage potential and resistance have absolutely nothing to do with water. These terms are directly related to the use of solid metallic electrical conductors such as rods and cables used throughout the world for transporting electrical power to homes and buildings as well as standby power supplies, batteries and electricity generators. |
| 1. Ohm’s law – The well-established relationship between voltage potential, current and resistance, by referring to these as E for voltage potential, I for current in ampere and R for Ohmic resistance. It has been designed to allow calculations to be done on how much current will flow through a known resistance when a particular voltage is applied on the following basis: a current of 1 ampere will flow through a 1 Ohm resistor when a potential of 1 volt is applied. |
| 2. Conductance/conductivity always has a direct relationship with ohmic resistance in such a way, that when resistance is increased, current is reduced and when resistance is reduced, current is increased. When however these parameters are to be measured over some distance, i.e. 1 metre, 1 decimeter, 1 centimeter or 1 millimeter, the term Resistivity is used. Normally resistivity is applied to current flowing between two opposing surfaces of a cube or rectangular block of metal. |
| 3. Reciprocal or inversely proportional is related to the interaction of Ohmic resistance and electrical current as stated in section 2, if resistance is increased, current is reduced and if resistance is reduced, current is increased, i.e. 1 Ohm equals 1 Siemens or 1 Million Ohm equals 1/millionth or 1 micro Siemens. |
| 4. Electrical current is the flow of electrons when a voltage potential is applied. Generally the higher the voltage potential, the lower the current (a build-up of resistance) and the reverse when current is high, the voltage potential is low (a low resistance). |
| 5. Siemens or fractions thereof. The term Siemens originates from a certain Werner von Siemens, an inventor in the late 1800s that created a standard of approximately 0.95 Ohm resistance by designing a column of mercury a metre long in order to establish a standard for a length of copper telegraph cable. |
| Comment: The entire aforementioned are related to metallic electrical conductors. According to the definition by Merriam Webster, the Siemens is a unit of conductance in the ‘meter-kilogram-second system’ equivalent to one ampere per volt. It is remarkable that when using a conductance or conductivity meter the voltage potential is never taken into account. Not even the equilibrium voltage of water at 1.23 volt direct current. Exceeding this level will polarise the ionic content in water by breaking up the water molecules into hydrogen and oxygen. The conclusion, the entire mechanism of determining the resistance and/or the resistivity of water is flawed and not considered scientifically accurate. |
| 6. The claim and the basis of conductance/conductivity measurement that the maximum resistance of water cannot be any higher than around 18 million Ohm at a pH of 7 at a temperature of 25 degrees Centigrade. This cannot possibly be right, especially when the value of 0.0549 micro Siemens is a hypothetical calculation by dividing 1 by 18 or dividing 1 by 0.0549 and realising that these value do not relate to the real world such as Ohms law does. |
| Note! Water is a dielectric and an insulator and just on its own does not allow current to flow and most likely is of some infinite Ohmic resistance, certainly in the range of Tera Ohm when water is extremely pure and not containing contaminants such as carbon dioxide. |
| Remedy |
| A. Everyone concerned with water testing to acquaint themselves with Ohm’s law and the threesome R for resistance, I for current and E or V for voltage potential and understand their relationship as an analogue direct current and not as AC. |
| B. To consider designing instrumentation in two separate areas: Ionic content measurement and optical observation and recording. The ionic content measurement WILL require the use of resistance values at a 1011 ohm (Giga range) and current in values of 10-11 ampere (pico range). The optical instrumentation for the detection of organic and inorganic uncharged contamination by obscurity levels and light scattering techniques enhanced with linear cross polarisation. |
Table 1: Technical table on water testing.
Acknowledgement
I offer my sincere apologies for following the first rule of scientific progress, by questioning everything that needs questioning. The testing of water purity certainly requires intense and thorough scientifically questioning until current problems and uncertainties are rectified.
References
- Bilenko Y, Shturm I, Bilenko O, et al. New UV technology for point-of-use water disinfection. Clean Technology. 2010.
- Jortner J, Noyes RM. Some Thermodynamic Properties of the Hydrated Electrons. 1968.
- Chaplin M. Water Structure and Science Series. London, UK.
- Laroo H. The Fragile and flawed existence of questionable water purity as used in wet chemistry and in particular where such water is destined for medical applications such as vaccines. IJVV. 2017;4(4):00087.
- Handbook of Industrial Water Conditioning. BETZ Laboratory, Trevosa, PA, 1980.
- Laroo H. Testing of metal derived nanometre sized particle, using analogue methodologies. OAT. 2017.
- Pollack GH. The fourth phase of water, beyond solid, liquid and vapor5. Ebner and Sons, Seattle WA, USA 2013.
- Lower S. A gentle Introduction to Water and its structure. Canada.
- Shields RM, Temelso B, Archer KA, et al. Accurate predictions of water cluster formation, (H2O). J Phys Chem. 2010;114(43):11725-37.
- Shreiner RH, Pratt KW. Standard reference materials: Primary standards and standard reference materials for electrolytic conductivity. NIST 2004;260-142.
- Malmberg CG, Maryott AA. Dielectric Constant of Water from 0°C to 100°C. J Res Natl Bur Stand. 1956;56(1):2641.
- Reiter GF, Kolesnikov AI, Paddison SJ, et al. Evidence of a new quantum state of nano-confined water. Cond Mat Mes Hall. 2011.
- Jortner J, Noyes RM. Some thermodynamic properties of the hydrated electron. J Phys Chem. 1966;70(3):770-4.
- Pope RM, Fry ES. Absorption Spectrum (380-700 nm) of pure water. II. Integrating cavity measurements. Appl Opt. 1997;36(33):8710.
- Santos LP, Ducati RD, Balestrin BS, et al. Water with excess electric charge. J Phys Chem C. 2011;115(22):11226-32.
- Pérez C, Muckle MT, Zaleski DP, et al. Structures of cage, prism and book isomers of water hexamer from broadband rotational spectroscopy. Science. 2012;336(6083):897-901.
- Fry ES. Visible and near-ultraviolet absorption spectrum of liquid water: Comment. Appl Opt. 2000;39(16):2743-4.
- Gorshunov BP, Zhukova ES, Torgashev VI, et al. Quantum behaviour of water molecules confined to nano-cavities in gemstones. J Phys Chem Lett. 2013;4(12):2015-20.
- Laboratory Manual for Queensland Sugar Mills. Division of Mill Technology. Bureau of Sugar Experiment Stations. Queensland Australia. 1939.
- Temmel S, Kern W, Luxbacher T. The ZETA Guide, Principles of the streaming potential technique. Progress in Colloid and Polymer Science. 54-61.