Research
, Volume: 19( 7)Synthesis, photocatalytic and antibacterial properties of metal oxide (ZnO, Co3O4 and MnO2) nanoparticles
AR Anusa*
Department of Chemistry, Easwari Engineering College, Chennai, India
*Corresponding author: AR Anusa, Department of Chemistry, Easwari Engineering College, Chennai, India. E-mail: anu.mika1039@gmail.com
Received: July 23, 2021; Accepted: August 06, 2021; Published: August 13, 2021
Abstract
Oxides of metals (ZnO, Co3O4 and MnO2) have been prepared by hydrothermal method and structural information, phase, morphology, optical properties were characterized by XRD, EDAX, FESEM, TEM, UV-Visible absorption spectroscopy. The morphology of the synthesized ZnO nanoparticles was found to be spherical in shape and high crystalline quality with size in the range of 75 nm. The synthesized ZnO nanoparticles have promising optical properties with 2.7 eV of energy gap. The photocatalytic performance of metal oxide was measured with the degradation of methyl orange (MO) at room temperature under UV light irradiation. The experimental results indicated that the well crystalline ZnO nanoparticels exhibited a high photocatalytic activity for the degradation of MO with the apparent rate constant (k) of 4.47 x 102 min-1. A significant antibacterial activity against Gram positive .S aureus and Gram-negative E. coli shown by this catalyst.
Keywords
Metal oxide; UV-light; Photoluminescence; Photocatalyst; E.coli
Introduction
The development of high performance, novel catalytic materials which are active under ambient conditions has gained much importance in recent years. The sight of drug ingredients in aqueous environment has raised escalates concern in recent years. Regular source of the pollutants are pesticides, hospital waste, animal excrements, and liquid industrial sewage. These impurities concentrations are exciting lower compared with other organic pollutions. However, the current development of investigations reported that these drugs have raised concerns due preserve environment and human health. Among various materials, semiconductors materials have shown as excellent photocatalyst because of its rapid recombination, wide band gab, high chemical stability and photocatalytic activity. Metal oxides have been well though out largely due to their fascinating structures and interesting physio-chemical behaviors, as well as their extensive range of application in various fields including photocatalyst [1]. Metal oxide nanoparticles are important materials with several applications of gas sensor, fuel cell, hydrogen storage, antibacterial activities etc.
Nowadays, researchers pointed towards nanosize metal oxide were found to have expressive roles in a distinct range of applications such as photocatalyst and sensing supplications. The great interest in nanomaterials may be attributed to a lot of new physical and chemical properties these nanoparticles exhibit when compared to their bulk materials. Metal oxides have been showing greater interest to synthesis with more attractive morphologies. Since the shape, size and structure of the nanosized matel oxides correlated with its properties, it has been produced with different shapes like nanowire, nanorods, nanobelts, nanospheres, etc. Several reports focused to synthesize of nanosize metal oxides by various chemical and physical methods such as hydrothermal, electrodeposition, microwave solvothermal, sol-gel. It is of greater interest to prepare single digit metal oxide nanostructures in well-defined size and shapes.
In this paper, we prepared and compare metal oxide nanoparticles such as zinc oxide (ZnO), cobalt oxide (Co3O4) and manganese oxide (MnO2) by hydrothermal method. In addition, the synthesized various metal tungstate properties were categorized by different techniques like powder X-ray diffraction (XRD), energy-dispersive X-ray spectroscopy (EDAX), field emission scanning electron microscopy (FESEM), transmission electron microscopy (TEM), UV-Vis absorption spectroscopy. Likewise, the prepared metal oxides were engaged to the degradation of metal orange via photocatalytic reaction under UV light irradiation. We have also studies antibacterial properties of prepared nano powders [2].
Experimental Method
Sample preparation
Transition metal tungstate, namely ZnO, Co3O4 and MnO2, were synthesized by hydrothermal method. All the chemicals were analytic grade reagents without further purification. The respective metal nitrates Zn(NO3)2.6H2O (1 mmol), Co(NO3)2 (1 mmol) and Mn(NO3)2 4H2O (1 mmol) in distilled water (40 mL) was added ammonia solution NH4OH (1 mmol) with constant stirring and the pH of the solutions was adjusted to 6.8 with dilute HCl solution. The resulting suspension was shift into a 100 mL Teflon-linked stainless steel autoclave. The autoclave was heated 180oC for 2 hours, without stirring. Thereafter, the autoclave was stand for cool to room temperature gradually. The collected white nanopowder was washed with double distilled water ethanol for several times in order to remove impurities. The white solid was then annealed 80oC and dried under vacuum for 3 hours. Finally, the nanopowder were sintered at 500°C for 2 hours
Characterization
The morphology of the prepared product were obtained with field emission scanning electron microscopy (FESEM, Hitachi S4800) and transmission electron microscopy. The crystal phase was measured using powder X-ray diffraction (XRD, PANanalytical Pro Diffractometer) with with Cu Kα radiation (λ =0.15406 nm) at 40 Kv and 30 mA. The UV-Visible absorption spectra of the prepared metal tungstate are obtained by a UV-Visible spectrophotometer (Varian, Carry-50 Bio).
Photocatalytic activity
To evaluate the effect of the photocatalytic experiment was carried out of the prepared metal oxide catalyst against MO. Aqueous phase photo degradation of MO was investigate under UV light exposure. The aqueous medium of 10 ppm stock solution of MO was prepared [3]. Reaction mixture is obtained by adding, 30 mg of metal oxide catalyst was suspended in 100 mL of 100 ppm dye solution by ultrasonication for 10 minutes. The catalyst/MO solution was placed in the dark environment for 5 hrs to reach adsorption equilibrium. Subsequently, the solution was exposed to UV irradiation (40 W, Philips) in ambient conditions with constant stirring. The distance between the lamp and beaker was maintained at 100 mm. At a given time, interval a 1.5 mL sample was taken from the beaker and centrifuged to separate the catalyst. The quantitative determination of MO was performed by measuring absorption spectra with UV-Vis. spectrophotometer.
Antibacterial activity
In the present experiment, initially, the antibacterial properties metal oxide nanoparticles were evaluvated by disc diffusion method. To examine the antibacterial susceptibility, pure bacterial cultures Gram positive and Gram negative pathogens strains were spotted in the Muller-Hinton agar plates and 100 μL of the cells were aseptically in the sterile phosphate buffers and inoculate on the Mueller-Hinton agar plates using sterile swab. It ensured an even dense lawn of culture following incubation. Different concentration (5 mg/mL and 10 mg/mL) of ZnO, Co3O4 and MnO2 of nanoparticles were dissolved in DMSO and stand for 1 h for the prepared nanoparticles suffuse. The plates were further incubated at 37°C for 24 h. The antibacterial activity of the prepared nanoparticles was measured by zone of diameter (in mm). In this method DMSO was used as a negative control, while streptomycin served as positive control. The test was performed in triplicate.
Results and discussion
Structural features
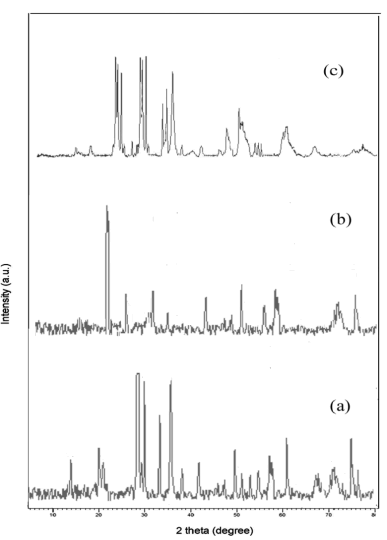
The lattice parameters, structure and crystallite size of the prepared metal oxide were recognized by powder XRD pattern. The XRD pattern of all synthesized metal tungstates was exposed in (Figure 1).
The obtained XRD data were entirely indexed via PAN analytical Pro Diffractometer. The individual indexed data of each metal tungstate were briefly described below: the diffraction peaks of ZnO nanoparticles were seemed 2θ at 15.9, 19.6, 24.5, 30.7, 36.6, 38.6, 42.5, 44.9, 46.3, 49.2, 50.4, 53.2, 54.9. and their resultant hkl planes were predictable such as (010), (100), (011), (110), (111), (021), (200), (121), (112), (211), (022), (220), (130), (202) respectively. It can clearly be seen that all diffraction peaks can be indexed to those of the wolframite ZnO (JCPDS file no 36-1451) The Co3O4 nanoparticles indicated tetragonal structure and their corresponding diffraction peaks (2θ) were obtained at 26.8, 30.1, 33.6,45.3, 46.5, 51.9, 55.7, 56.2, 69.3,72.4, 74.9 (JCPDS card No. 74-1656) parallel to (112), (004), (200), (204), (220), (110), (312), (224), (400), (316), (332), (420). On the other hand, MnO2 nanoparticles revel monoclinic crystalline with distinctive peaks centered 29.02, 29.58, 30.49, 32.31, 35.34, 35.7, 47.7 (JCPDS card No. 44-0141) that matches with (110), (-111), (111), (020), (002), (220) planes. The lattice parameters and crystalline size of the metal tungstates were displayed in (Table 1).
TABLE 1. Structural parameters of the synthesized metal oxide nanomaterials.
| Prepared metal tungstate | JCPDS card number | Structure | Crystal size (nm) |
|---|---|---|---|
| ZnO | 36-1451 | Wolframite | 19.5 |
| Co3O4 | 74-1656 | Tetragonal | 29.3 |
| MnO2 | 44-0141 | Monoclinic | 24.7 |
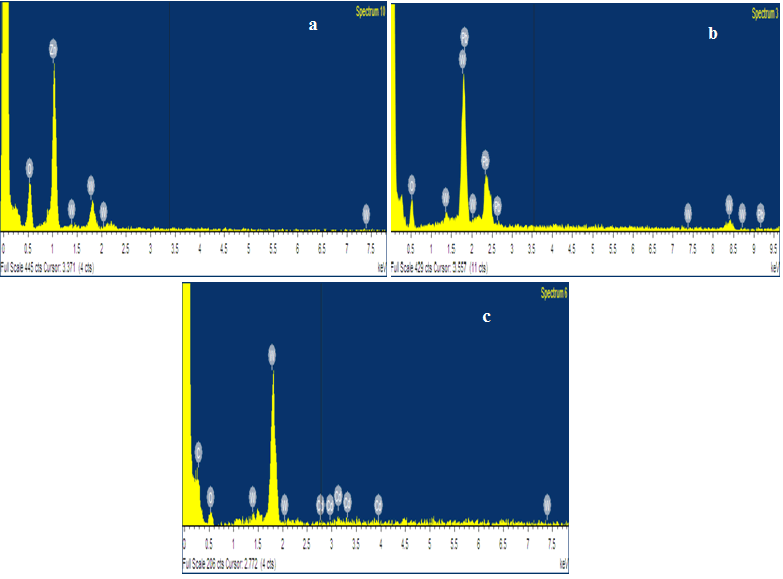
From the XRD results all synthesized metal oxides were pure structure without impurities and their crystallite values are seemed in single digits. The synthesized samples purity is further confirmed from the EDAX spectrum. The EDAX spectrum shows that the prepared metal oxide confirm the composition of O, Zn, Co and Mn elements and absence of impurities in the synthesized metal oxide (Figure 2).
FESEM
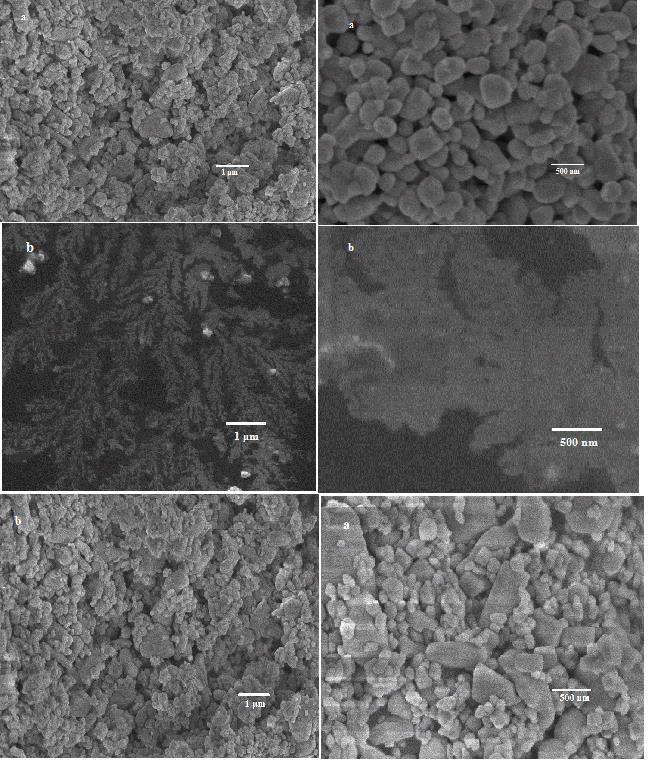
In order to obtain detailed information about the morphology and microstructure of the metal oxide materials both FESEM observation were carried out. The FESEM images of the synthesized metal tungstates were presented. The results illustrated that metal oxide have small size and good dispersion rate. The FESEM images of prepared samples are easy to see a combination and collision of small spherical. Some particles are slightly aggregated but not truly. Actual morphology of each metal tungstate expresses different dimension due to growth and nucleation process. With compare other metal oxide, the FESEM images of ZnO [4]. Illustrate relatively uniform, well connectivity between particles, well dispersed spheres, particles are identical and spherical in shape. At the meantime, the FESEM images of Co3O4 and MnO2 shown striking morphology such as nanoflowers and nanorods which were evidently and c respectively. The variation of metal oxide causes the defect on the lattice structures. Therefore, more defects nobly degenerate the size of particles accordingly variation occurs in grain size and shape of the metal tungstate samples. From the FESEM images of metal oxide indicated dissimilar shape and dimensions (Figure 3).
TEM
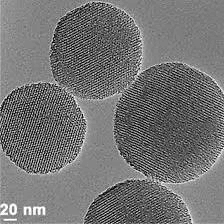
TEM micrograph of prepared ZnO samples by hydrothermal method succeed arising from the interaction of the coating beam electrons from transmission through the coating. The mean size of the ZnO nanoparticles from TEM observation size is 20 nm. From the TEM image confirm that the particle size belong to the nano range with irregular spherical shape with high accumulation (Figure 4).
Band gap analysis
The UV-Visible absorption spectra of the prepared metal oxide nanoparticles. From the strong absorption wavelength and band gab (Eg) values of each metal oxide were sharply identified and tabulated in (Table 2).
TABLE 2. Absorption wavelength, band gap and degradation efficiency of the entire synthesized metal oxidenanomaterials.
| Prepared metal tungstate | Absorption wavelength (λ) | Band gap (Eg) | Degradation of methyl orange (%) |
|---|---|---|---|
| ZnO | 430 | 2.89 | 77 |
| Co3O4 | 375 | 3.28 | 58 |
| MnO2 | 365 | 3.34 | 64 |
The strong absorption of synthesized ZnO, Co3O4 nanoparticles samples appeared in UV region and MnO2, of nanoparticles sample wavelength is in visible region. Therefore in this work, maximum number of prepared materials trust on UV irradiation, so that these characteristics help to engage UV light for irradiation during degradation time.
Photoluminescence
The photoluminescence spectra of the synthesized ZnO nanoparticles by hydrothermal method. With the excited wavelength 325 nm, the emission peaks centered at 280 nm, 317 nm, 400 nm, 450 nm and 570 nm. The photoluminescence spectra are useful technique to survey the separation efficiency of the photogenerated charge carriers in semiconductors, because photoluminescence emission mainly results of recombination from free carriers. Generally a lower PL intensity, the bigger probability the charge carriers separate. The lower PL intensity of the ZnO nanoparticles suggests the enhanced separation efficiency of the phot-generated carriers. In the emission spectrum of ZnO nanoparticles contains various emission bands in the UV and visible regions. The UV emission band centered at 240-317 nm is assigned to the recombination of excitionic centers and the quantum confinement effect. The PL peaks obtained in the visible region around 450-570 nm due to the structural defects and oxygen vacancies formed during sample preparation. The oxygen vacancies are usually referred to as partially incomplete crystallization and nanoparticles with high surface to volume ratio. The green emission, it could be attribute to the recombination of a photo-generated hole with the singly ionized charge state of the intrinsic defects such as oxygen and metal ions [5]. Therefore, the utilization of photo-generated carriers plays the key role in the photoctalytic activity of the metal oxide.
Conclusions
In conclusion, the photocatalytic ability of ZnO, Co3O4 and MnO2 were efficient performance of degradation of methyl orange. Compared with other metal oxide (Co3O4 and MnO2), the photocatalytic activity of ZnO has showed superior degradation rate because of its spherical shape and crystallinity. The optical properties and photocatalytic activity of these metal oxide extremely based on the morphology and the nature of the metal ions. The entire characterization results were assisted to identify the shape, size and chemical combination and band gap of the synthesized metal oxides. From the above results, ZnO, Co3O4 and MnO2 nanoparticles which have promising antibacterial activity against the Gram-negative bacteria and Gram-positive bacteria.
References
- Warsi MF, Shaheen N, Sarwar MI, et al. A comparative study on photocatalytic activities of various transition metal oxides nanoparticles synthesized by wet chemical route. Desal Wat Treat. 2021;211(1):181-195.
- Shaheen I, Ahmad KS. Synthesis of binary metal oxide-doped Co3 O4 nanoparticles by organic template and investigation of its structural, optical and electrochemical properties. Journal of Materials Science: Materials in Electronics. 2020;31:10323-10333.
- Xiong S, Yuan C, Zhang X, et al. Controllable synthesis of mesoporous Co3O4 nanostructures with tunable morphology for application in supercapacitors. Chem Eur J. 2009;15(21):5320-5326.
- Salam MA, AbuKhadra MR, Mohamed AS. Effective oxidation of methyl parathion pesticide in water over recycled glass based-MCM-41 decorated by green Co3O4 Environ Poll. 2020;259(1):113874.
- Zafar N, Madni A, Khalid A, et al. Pharmaceutical and Biomedical Applications of Green Synthesized Metal and Metal Oxide Nanoparticles. Curr Pharm Des. 2020;26(45):5844-5865.