Original Article
, Volume: 15( 2)Synthesis and Characterization of Some Schiff Base Derivatives Containing Sydnone as Antimicrobial Agents
- *Correspondence:
- Patel KC , Department of Chemistry, Veer Narmad South Gujarat University, Surat, Gujarat, India, Tel: +91-8141668910; E-mail: ss68910@gmail.com
Received: May 09, 2017; Accepted: May 26, 2017; Published: May 29, 2017
Citation: Savant SS, Patel SM, Gamit EA, et al. Synthesis and characterization of some Schiff base derivatives containing Sydnone as antimicrobial agents. Int J Chem Sci. 2017;15(2):139.
Abstract
The series of Schiff base derivatives incorporating with Mannich base of 3-(3-nitrophenyl) sydnone were synthesized by conventional routes and evaluated for their antimicrobial activities against E. coli, P. aerugenosa, S. aureus, S. pyogenus, C. albicans, A. niger and A. clavatus. Most of the compounds showed moderate to very good biological activity. The structures of synthesized compounds 6a-j were elucidated by C, H, and N analysis, FT-IR, 1H NMR, 13C NMR and Mass Spectrometry.
Keywords
Schiff base; Mannich base; Sydnone; Antibacterial; Antifungal
Introduction
Medicinal chemistry concerns essentially the understanding and explanation of the mechanisms of the drugs. It explains the design and production of compounds that can be used for the prevention, treatment or cure of human and animal diseases. Medicinal chemistry includes the study of already existing drugs, their biological properties and their structure activity relationships. Mesoionic compounds are five membered heterocyclic conjugated betains. At present the most frequently used ‘mesoionic’ structure is of sydnone proposed by Baker and Ollis [1,2]. The sydnone ring bears a fractional positive charge balanced by a corresponding negative charge located on covalently attached oxygen [3]. Due to the unique structure, sydnone possesses both the conjugated and polar character, which makes it sensitive to both electric and magnetic fields. Many sydnone compounds have been found to exhibit pharmacological and biological activities viz, antibacterial [4], antitumor [5,6], antifungal [7], antimalarial [8], anti-inflammatory [9], analgesic, anthelmintic [10], antioxidant [11]. They also show significant response of coronary dilation test, collagen induced platelet aggregation inhibition, local an aesthetic, ant writhing, anticonvulsant, muscle relaxation and moderate cardio tropic activity. A hydrogen atom at the 4th position of the sydnone ring allows substitution with a wide variety of electrophiles, such as bromination, nitration, acylation, and sulfonation. It seems to be possible to substitute the 4th position by electron-releasing groups such as the methylene group by Mannich reaction [12-14].
The compounds containing carbon and nitrogen, which joined together with double bond, include mainly the products of reaction between aldehyde or ketonic components and primary aliphatic or aromatic amines, ammonia, hydrazine, N-phenyl hydrazine, hydroxylamine hydrochloride, semicarbazide, thiosemicarbazide and their substituted derivatives [15]. These compounds are known as Schiff bases to honours Schiff, who first discovered such compounds [16,17]. They are well known intermediates for the preparation of azetidinone, thiazolidinone, formazone, acrylacetamide and many other derivatives. Schiff base have been found to possess pharmacological activities viz, antibacterial [18], antifungal, anti-HIV [19], antiviral [20], anticancer [21], anticonvulsant [22], tuberculostatic [23], anti-inflammatory [24] and antioxidant [25] and DNA interaction [26].
Materials and Methods
Experimental
All the chemicals used were of analytical grade and the solvents were distilled before use. All the melting points reported are uncorrected and were recorded using an electro-thermal melting point apparatus. The structure of synthesized compounds was confirmed by elemental analysis (C, H, N) which was performed on Thermo Scientific FLASH 2000 at G.N.F.C. (Gujarat Narmada Valley Fertilizer Company Ltd., Bharuch). Infrared spectra were recorded with FT-IR Spectrophotometer Perkin Elmer in the frequency range 4,000 cm-1 to 400 cm-1 with samples embedded in KBr disks. Proton nuclear magnetic resonance (1H NMR) spectra of the compound were recorded with a Bruker Advance II 400 Hz NMR and carbon (13C) NMR spectra of the compounds were recorded with a Bruker Avance II 400 NMR spectrometer using DMSO-d6 as a solvent and tetramethylsilane (TMS) as an internal reference at sophisticated analytical instrument facilities (SAIF), Chandigarh. Thin-layer chromatography analysis were performed using aluminium backed Silica-gel plates (Merck 60 F524) and examined under short wave ultraviolet (UV) light.
Procedure for the Synthesis of the Compounds (6a-j)
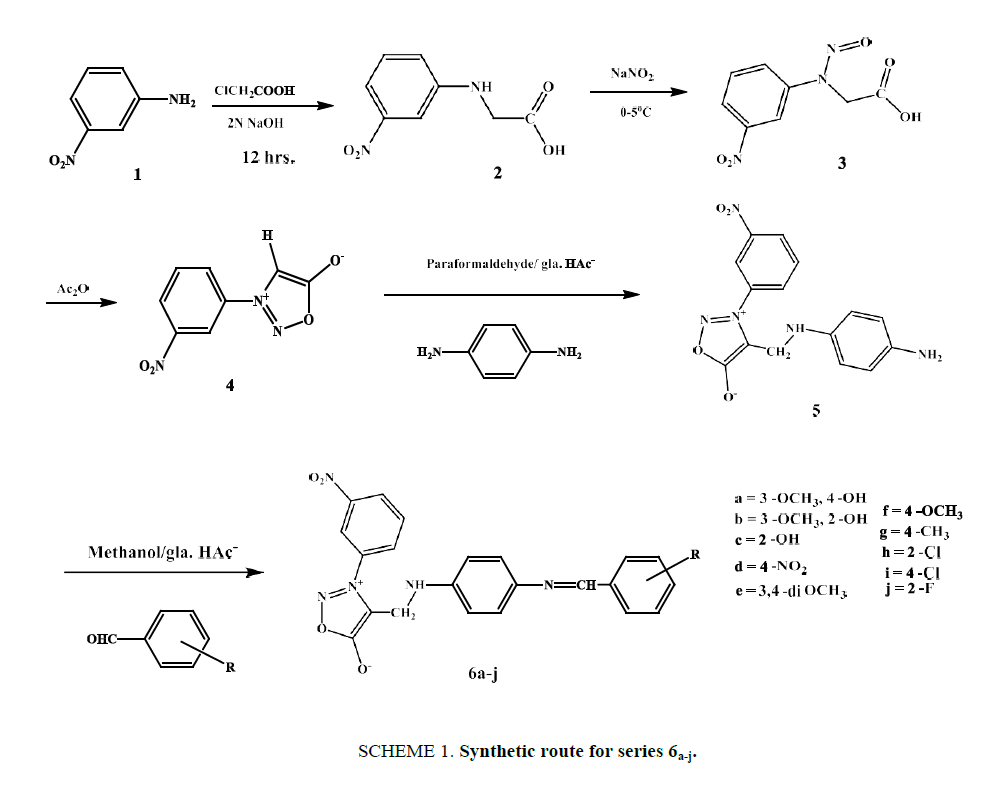
Synthesis of (3-nitrophenyl) glycine (2)
This step, a condensation, involved neutralizing an aqueous solution of chloroacetic acid (0.94 g, 0.01 mol) with an equimolar equivalent of 10% NaOH and adding this solution to an aqueous solution of 3-nitro aniline (1.38 g, 0.01 mol) over a period of 4 h. This reaction mixture was heated for 10 h and the clear liquor was then filtered while hot to remove any decomposition product and refrigerated overnight. The resulting crystals were again filtered to obtain compound 2. Yield 87%, m.p. 145-147ºC. IR: (KBr) v (cm-1): 3465 (OH str. of acid), 1773 (>C=O of acid), 1601, 1507 (C=C of aromatic), 1514 (asym.), 1323 (sym.) (-NO2); 1H NMR (DMSO-d6): δ (ppm): 4.07 (s, 2H,-CH2-), 6.34 (s, 1H,-NH-), 7.25 (d, 1H, Ar-H), 7.37 (t, 1H, Ar-H), 7.59 (d, 1H, Ar-H), 7.66 (s, 1H, Ar-H), 13.12 (s, 1H,-COOH); 13C NMR (DMSO-d6: δ (ppm): 45.91 (-CH2-), 106.45 (Ar-C), 112.29 (Ar-C), 119.57 (Ar-C), 130.38 (Ar-C), 148.54 (Ar-C of C-N), 148.72 (Ar-C of C-N), 171.98 (>C=O of acid).
Synthesis of N-(3-nitrophenyl)-N-nitrosoglycine (3)
To an ice-cooled solution of 2 (1.96 g, 0.01 mol) in 40 ml of water, a solution of sodium nitrite (0.69 g, 0.01 mol) in 5 ml of water was added drop by drop with stirring. After stirring for another 2 h and leaving the solution to stand overnight, the reaction mixture was filtered through a Buckner funnel, and the nitroso compound was precipitated by adding concentrated hydrochloric acid to the filtrate. Yellowish needles were obtained as product, yield 87 %, m.p. 154°C to 157°C. IR: (KBr) v (cm-1): 3461 (O-H of acid), 1774 (>C=O of acid), 1616, 1513 (C=C of aromatic), 1597 (N=O str.), 1524 (asym.), 1335 (sym.) (-NO2); 1H NMR (DMSO-d6): δ (ppm): 4.05 (s, 2H,-CH2-), 6.85 (d, 1H, Ar-H), 7.39 (t, 1H, Ar-H), 7.55 (d, 1H, Ar-H), 7.62 (s, 1H, Ar-H), 13.08 (s, 1H, COOH); 13C NMR (DMSO-d66): δ (ppm): 62.25 (-CH2-), 113.11 (Ar-C), 122.49 (Ar-C), 129.52 (Ar-C), 130.42 (Ar-C), 143.33 (Ar-C of-C-N-), 148.75 (Ar-C of C-N), 173.08 (>C=O of acid).
Synthesis
Synthesis of 3-(3-nitrophenyl) sydnone (4)
A mixture of 3 (2.835 g, 0.0126 mol) and acetic anhydride (15 ml) was stirred at room temperature for 12 h in the dark. The solution was poured slowly into cold water which was very well stirred. The pH of the content was adjusted to 7.0 with 10% Sodium bicarbonate solution. The crude sydnone obtained was washed well with water, dried and recrystallized from 95% ethanol afforded a yield of 92% of light yellow needles, m.p. 147°C-149°C. IR: (KBr) v (cm-1): 3108 (C-H of sydnone), 1752 (>C=O of sydnone), 1622, 1517 (C=C of aromatic), 1518 (asym.), 1327 (sym.) (˗NO2); 1H NMR (DMSO-d6): δ (ppm): 4.05 (s, 1H,-CH-of sydnone), 7.59 (t, 1H, Ar-H), 8.19 (d, 1H, Ar-H), 8.25 (s, 1H, Ar-H); 13C NMR (DMSO-d6): δ (ppm): 114.71 (Ar-C), 123.43 (C4 of sydnone), 126.69 (Ar-C), 132.31 (Ar-C), 136.55 (Ar-C), 139.44 (Ar-C of C-N), 147.93 (Ar-C of C-N), 169.18 (C5 of sydnone).
Synthesis of 4-(((4-aminophenyl) amino) methyl)-3-(3-nitrophenyl) sydnone (5)
The mixture of compound 3-(3-nitrophenyl) sydnone (2.07 g, 0.01 mol), paraformaldehyde (0.25 g, 0.00833 mol) and p-phenylenediamine (1.296 g, 0.012 mol) were added to 10 ml of acetic acid and 10 ml ethanol and whole mixture was heated at 70°C for 3 h. After cooling ethanol was distilled off, 20 ml of water was added and neutralized with aqueous sodium bicarbonate to afford the crude product. Recrystallization from 95% ethanol yielded 96% of title compound as crystalline solid. M.P. 207-209°C. IR: (KBr) v (cm-1): 3269 (-NH-), 2932, 2860, (-CH2-of Mannich base), 1749 (>C=O of sydnone), 1628, 1508 (C=C of aromatic), 1525 (asym.), 1332 (sym.) (-NO2); 1H NMR (DMSO-d6): δ (ppm): 4.29 (s, 2H,-CH2-of Mannich base), 4.59 (s, 2H,-NH2), 6.08 (d, 2H, Ar-H), 6.54 (d, 2H, Ar-H), 6.76 (s, 1H,-NH-), 7.59 (t, 1H, Ar-H), 8.22 (d, 2H, Ar-H), 8.27 (s, 1H, Ar-H); 13C NMR (DMSO-d6): δ (ppm): 49.03 (-CH2-of Mannich base), 114.72 (Ar-C), 117.11 (Ar-C), 118.53 (Ar-C), 126.71 (Ar-C), 132.33 (Ar-C), 136.48 (Ar-C),136.83 (Ar-C of C-N), 139.41 (Ar-C), 142.4 (C4 of sydnone), 147.91 (Ar-C of C-N), 168.75 (C5 of sydnone).
Synthesis of compounds 6a-j
The Schiff base derivatives were prepared by the equimolar reaction between compound 5 and various substituted aldehydes. Each reactant was dissolved in a minimum amount of methanol, then mixed together and followed by addition of catalytic amount of glacial acetic acid. The solution was refluxed for 8 hrs. then cooled to room temperature and poured into ice cold water. The solid product was filtered, dried and recrystallized from ethanol
All the Schiff base derivatives were synthesized by the same procedure. The antimicrobial activity is given in Table 1.
| Compounds | Minimal Inhibition Concentration in mg/ml | ||||||
|---|---|---|---|---|---|---|---|
| Gram-positive | Gram-negative | Fungal strains | |||||
| S. pyogenes | S. aureus | E. coli | P. aeruginosa | C. ablicans | A. niger | A. clavatus | |
| 6a | 250 | 100 | 250 | 250 | 300 | 500 | 1000 |
| 6b | 200 | 250 | 100 | 125 | 500 | 300 | 1000 |
| 6c | 100 | 250 | 250 | 500 | 1000 | 500 | 500 |
| 6d | 100 | 100 | 100 | 200 | 200 | 300 | 500 |
| 6e | 200 | 250 | 200 | 125 | 500 | 1000 | 200 |
| 6f | 250 | 80 | 200 | 100 | 200 | 250 | 500 |
| 6g | 200 | 200 | 100 | 125 | 1000 | 500 | 1000 |
| 6h | 250 | 125 | 60 | 100 | 250 | 1000 | 500 |
| 6i | 200 | 200 | 200 | 200 | 125 | 200 | 250 |
| 6j | 100 | 250 | 100 | 100 | 60 | 250 | 500 |
| Gentamycin | 0.05 | 1 | 0.25 | 0.5 | --- | --- | --- |
| Ampicillin | 100 | 100 | 250 | 100 | --- | --- | --- |
| Chloramphenicol | 50 | 50 | 50 | 50 | --- | --- | --- |
| Ciprofloxacin | 25 | 25 | 50 | 50 | --- | --- | --- |
| Norfloxacin | 10 | 10 | 10 | 10 | --- | --- | --- |
| Nystatin | --- | --- | --- | --- | 100 | 100 | 100 |
| Griseofulvin | --- | --- | --- | --- | 500 | 100 | 100 |
Table 1: Antimicrobial activity of Compounds 6a-j.
Characterization
(6a) IR: (KBr) v (cm-1): 3434 (Ar-OH), 3272 (-NH-), 2928, 2857, (-CH2-of Mannich base), 1752 (>C=O of sydnone), 1651 (-C=N-of Schiff base),1632, 1506 (C=C of aromatic), 1521 (asym.), 1339 (sym.) (-NO2) 1239, 1049 (C-O-C of-OCH3); 1H NMR (DMSO-d6): δ (ppm): 3.85 (s, 3H,-OCH3), 4.28 (s, 2H,-CH2-of Mannich base), 6.45 (d, 2H, Ar-H), 6.88 (d, 1H, Ar-H), 7.32 (d, 1H, Ar-H), 7.36 (s, 1H,-NH-), 7.38 (s, 1H, Ar-H), 7.39 (d, 2H, Ar-H), 7.61 (t, 1H, Ar-H), 8.22 (d, 2H, Ar-H), 8.26 (s, 1H, Ar-H), 8.52 (s, 1H,-CH= of Schiff base), 9.57 (s, 1H,-OH); 13C NMR (DMSO-d6): δ (ppm): 49.14 (-CH2-NH-), 56.11 (C of-OCH3), 112.15 (Ar-C), 114.74 (Ar-C), 114.82 (Ar-C), 117.05 (Ar-C), 122.92 (Ar-C), 123.18 (Ar-C), 126.74 (Ar-C), 130.89 (Ar-C), 132.23 (Ar-C), 136.51 (Ar-C), 139.44 (Ar-C of-C-N), 140.41 (Ar-C of-C-N), 142.41 (C4-sydnone), 147.84 (Ar-C of-C-N), 147.93 (Ar-C of-C-N), 149.33 (Ar-C of-C-O), 151.10 (Ar-C of-C-O), 160.07 (C of-CH=N-), 168.78 (C5-sydnone); MS m/z (rel. int. %): 462.4 (M+1)+.
(6b) IR: (KBr) v (cm-1): 3425 (Ar-OH), 3266 (-NH-), 2919, 2865, (-CH2-of Mannich base), 1747 (>C=O of sydnone), 1632 (-C=N-of Schiff base), 1621, 1509 (C=C of aromatic), 1525 (asym.), 1345 (sym.) (-NO2) 1252, 1060 (C-O-C of-OCH3); 1H NMR (DMSO-d6): δ (ppm): 3.89 (s, 3H,-OCH3), 4.29 (s, 2H,-CH2-of Mannich base), 6.44 (d, 2H, Ar-H), 6.89 (t, 1H, Ar-H), 7.04 (d, 1H, Ar-H), 7.34 (d, 1H, Ar-H), 7.36 (s, 1H,-NH-), 7.38 (d, 2H, Ar-H), 7.58 (t, 1H, Ar-H), 8.24 (d, 2H, Ar-H), 8.28 (s, 1H, Ar-H), 8.86 (s, 1H,-CH= of Schiff base), 13.79 (s, 1H,-OH); 13C NMR (DMSO-d6): δ (ppm): 49.10 (-CH2-NH-), 56.11 (C of-OCH3), 114.72 (Ar-C), 114.83 (Ar-C), 115.04 (Ar-C), 116.62 (Ar-C), 119.51 (Ar-C), 123.12 (Ar-C), 124.44 (Ar-C), 126.76 (Ar-C), 132.33 (Ar-C), 136.55 (Ar-C), 139.42 (Ar-C), 140.39 (Ar-C of-C-N-), 142.38 (C4-sydnone), 147.79 (Ar-C of-C-N-), 149.03 (Ar-C of-C-O-), 150.09 (Ar-C of-C-O-), 160.05 (C of-CH=N-), 168.77 (C5-sydnone); MS m/z (rel. int. %): 462.4 (M+1)+.
(6c) IR: (KBr) v (cm-1): 3425 (Ar-OH), 3266 (-NH-), 2919, 2865, (-CH2-of Mannich base), 1749 (>C=O of sydnone), 1634 (-C=N-of Schiff base), 1621, 1509 (C=C of aromatic), 1525 (asym.), 1345 (sym.) (-NO2); 1H NMR (DMSO-d6): δ (ppm): 4.31 (s, 2H,-CH2-of Mannich base), 6.47 (d, 2H, Ar-H), 6.91 (d, 1H, Ar-H), 7.17 (t, 1H, Ar-H), 7.33 (t, 1H, Ar-H), 7.35 (s, 1H,-NH-), 7.39 (d, 2H, Ar-H), 7.59 (t, 1H, Ar-H), 7.62 (d, 1H, Ar-H), 8.24 (d, 2H, Ar-H), 8.29 (s, 1H, Ar-H), 8.41 (s, 1H,-CH= of Schiff base), 11.14 (s, 1H,-OH); 13C NMR (DMSO-d6): δ (ppm): 48.08 (-CH2-NH-), 114.71 (Ar-C), 114.78 (Ar-C), 117.81 (Ar-C), 120.56 (Ar-C), 121.40 (Ar-C), 123.11 (Ar-C), 126.67 (Ar-C), 132.12 (Ar-C), 132.33 (Ar-C), 132.41 (Ar-C), 136.54 (Ar-C), 139.41 (Ar-C), 140.45 (Ar-C of-C-N-), 142.46 (C4-sydnone), 147.78 (Ar-C of-C-N-), 160.02 (C of-CH=N-), 161.14 (Ar-C of-C-O-), 168.72 (C5-sydnone); MS m/z (rel. int. %): 432.3 (M+1)+.
(6d) IR: (KBr) v (cm-1): 3244 (-NH-), 2926, 2842, (-CH2-of Mannich base), 1759 (>C=O of sydnone), 1649 (-C=N-of Schiff base), 1632, 1515 (C=C of aromatic), 1531 (asym.), 1352 (sym.) (-NO2); 1H NMR (DMSO-d6): δ (ppm): 4.33 (s, 2H,-CH2-of Mannich base), 6.43 (d, 2H, Ar-H), 7.34 (s, 1H,-NH-), 7.40 (d, 2H, Ar-H), 7.58 (t, 1H, Ar-H), 8.17 (d, 2H, Ar-H), 8.23 (d, 2H, Ar-H), 8.28 (s, 1H, Ar-H), 8.35 (d, 2H, Ar-H), 8.87 (s, 1H,-CH= of Schiff base); 13C NMR (DMSO-d6): δ (ppm): 49.03 (-CH2-NH-), 114.71 (Ar-C), 114.81 (Ar-C), 117.80 (Ar-C), 123.18 (Ar-C), 124.09 (Ar-C), 126.67 (Ar-C), 127.79 (Ar-C), 132.33 (Ar-C), 136.56 (Ar-C), 139.44 (Ar-C), 140.41 (Ar-C of-C-N-), 142.39 (C4-sydnone), 142.49 (Ar-C), 147.77 (Ar-C of-C-N-), 147.8.8 (Ar-C of-C-N-), 150.22 (Ar-C of-C-N-), 160.01 (C of-CH=N-), 168.67 (C5-sydnone); MS m/z (rel. int. %): 461.2 (M+1)+.
(6e) IR: (KBr) v (cm-1): 3252 (-NH-), 2916, 2856, (-CH2-of Mannich base), 1755 (>C=O of sydnone), 1649 (-C=N-of Schiff base), 1632, 1515 (C=C of aromatic), 1531 (asym.), 1352 (sym.) (-NO2) 1231, 1029 (C-O-C of methoxy); 1H NMR (DMSO-d6): δ (ppm): 3.83 (s, 3H,-OCH3), 3.85 (s, 3H,-OCH3); 4.33 (s, 2H,-CH2-of Mannich base), 6.43 (d, 2H, Ar-H), 7.05 (d, 2H, Ar-H), 7.34 (s, 1H,-NH-), 7.38 (d, 2H, Ar-H), 7.42 (d, 1H, Ar-H), 7.55 (s, 1H, Ar-H), 7.63 (t, 1H, Ar-H), 8.24 (d, 2H, Ar-H), 8.26 (s, 2H, Ar-H), 8.54 (s, 1H,-CH= of Schiff base); 13C NMR (DMSO-d6): δ (ppm): 49.1 (-CH2-NH-), 56.09 (C of-OCH3), 109.18 (Ar-C), 111.77 (Ar-C), 114.66 (Ar-C), 114.80 (Ar-C), 123.11 (Ar-C), 125.19 (Ar-C), 126.71 (Ar-C), 130.61 (Ar-C), 132.33 (Ar-C), 136.48 (Ar-C), 139.45 (Ar-C), 140.37 (Ar-C of-C-N-), 142.41 (C4-sydnone), 147.78 (Ar-C of-C-N-), 147.87 (Ar-C of-C-N-), 149.89 (Ar-C of-C-O-), 152.14 (Ar-C of-C-O-), 160.02 (C of-CH=N-), 168.71 (C5-sydnone); MS m/z (rel. int. %): 476.4 (M+1)+.
Result and Discussion
Chemistry
The multi-component condensation of a primary amine or secondary amine and enolizable carbonyl compound with the aim to synthesized amino methylated products are referred to as the Mannich Reaction. The synthesis of Schiff base derivatives (6a-j) Mannich base of sydnone is shown in Scheme. 1. We focused on the synthesis of Mannich base by reacting 3-(3-nitrophenyl) sydnone with p-phenylene diamine. Synthesis of 3-(3-nitrophenyl) sydnone [4] comprises of three steps procedure viz, condensation with chloroacetic acid, nitrosation and cyclodehydration. Compound [4] reacted with paraformaldehyde and p-phenylene diamine to give amino methylated compound [5]. This on further condensed with substituted aldehydes in presence of gla. HAc-to give desired Schiff base 6a-j.
Elemental Analysis and Spectral data were used to confirm the structures of synthesized compounds. Compound (4) showed two characteristics IR absorption band at 3.108 cm-1 and 1.752 cm-1 due to C-H and >C=O streaching of the sydnone. 1H-NMR (DMSO d6) spectra of compound (4) showed sharp singlet peak at δ 7.42 ppm, characteristics band for active proton at 4th position of sydnone. The absence of this sharp peak in compound (5) confirms the formation of Mannich base. IR spectra of compound (5) showed two characteristics band at 3.269 cm-1 and 2.860 cm-1 due to-CH2and-NH-of Mannich base. 1HNMR (DMSO-d6) spectra of compound (5) showed singlet at δ 4.59 ppm due to-NH2, which was disappeared in compounds 6a-j due to the formation of Schiff base derivatives. The-C=N-streaching of Schiff base in compounds 6a-j found between 1.665 cm-1 to 1.595 cm-1. Some additional peaks appear due to substitution in aromatic ring. 13C-NMR spectra showed characteristics signal for the carbonyl carbon around δ 168.7 ppm, methylene carbon around δ 49 ppm.
Antimicrobial activity
Control of microbial population is necessary to prevent transmission of disease, infection, decomposition, contamination and spoilage caused by them. This was one of the purposes of our present work. The synthesized compounds were screened for their in vitro antibacterial activity against Gram positive and Gram negative bacterial strains, compounds were also screened for their in vitro antifungal activity. Gram positive bacteria viz., Staphylococcus aureus, Streptococcus pyogenes, gram negative bacteria viz., Escherichia coli and Pseudomonas aeruginosa were used in this assay. Gentamycin, Ampicillin, Chloramphenicol, Ciprofloxacin and Norfloxacin were used as standard antimicrobial compounds. The antifungal activity was screened in vitro against pathogenic yeast, Candida albicans, and moulds like Aspergillus niger and Aspergillus clavatus. Antifungal compounds, Nystatin and Griseofulvin, were used as standard. The investigation was carried out by Minimum Inhibitory Concentration (MIC) by Broth Dilution Method.
Compounds 6f (R=4-OCH3) is most active against Gram Positive bacteria S. aureus, Compounds 6h (R=2-Cl) is highly active against Gram negative bacterial strain viz., E. coli. Compound 6j showed excellent antifungal activity against pathogenic yeast C. albicans. All other compounds were showed moderate to good activity and some are inactive against all strains.
Acknowledgement
The authors would like to express their gratitude to the Department of Chemistry, Veer Narmad South Gujarat University, Surat for providing basic research facilities. The authors wish to thank to UGC-BSR-SAP for financial assistance.
References
- Baker W, Ollis WD.Meso-ionic compounds. Quart Rev Chem Soc.1957;11:15-29.
- Ollis WD, Ramsden CA. Advances in heterocyclic chemistry. Academic Press. New York. 1976;19:3-122.
- Brookes P, Walker J.Formation and properties of sydnone imines, a new class of meso-ionic compound, and some sydnones related to natural a-amino-acids. J Chem SocBioorg Med Chem.1957;20:4103-8.
- Thanh ND, Duc HT, Duyen VT, et al. Synthesis and antibacterial and antifungal activities of N-(tetra-O-acetyl-ß-d-glucopyranosyl)thiosemicarbazones of substituted 4-formylsydnones. Chem Cent J.2015;9:60.
- Tegginamath G, Kamble RR, Taj T, et al. Synthesis of novel imidazo[2,1-b][1,3,4]thiadiazoles appended to sydnone as anticancer agents. Med Chem Res.2013;22:4367-75.
- Dunkley CS, Thoman CJ. Synthesis and biological evaluation of a novel phenyl substituted sydnone series as potential antitumor agents. Bioorg Med Chem Lett.2003;13:2899-901.
- Bansode S, Kamble R. Synthesis of novel 2-(3?-aryl-sydnon-4?-ylidene)-5?-substituted[1,3,4]-hiadiazolylamines and [1,3,4]-thiadiazol-20-yl-3-oxo[1,2,4]-triazoles as antimicrobial agents. Med Chem Res.2012;21:867-73.
- Nyberg WH, Cheng CC. 3-Piperonylsydnone: A new type of antimalarial agent. J Med Chem.1965;8:531-3.
- Wagner H. Anti-inflammatory sydnones. J Med Chem.1974;17:1337-8.
- Kalluraya B, Rahiman M, Banji D. Sydnone derivatives: Part v-synthesis and pharmacological properties of some novel triazolothiadiazepines. Ind J Chem.2002;41:1712-7.
- Shih MH, Ke FY. Syntheses and evaluation of antioxidant activity of sydnonyl substituted thiazolidinone and thiazoline derivatives. Bioorg Med Chem.2004;12:4633-43.
- Tien HJ, Yeh MY, Chou JT, et al. Studies on the synthesis of sydnone derivatives and their properties. XV. Synthesis of 4-sydnonylmethyl acetates. Bull Chem Soc of Japan.1981;54:947-8.
- Rahiman MA, Kalluraya B. Synthesis, characterization, antimicrobial and anthelmintic activity of some sydnone-N-Mannich Bases. J Ind Council Chem.2002;25:10-4.
- Patel KC, Savaliya PP, Akbari VK, et al. Synthesis, characterization, and antimicrobial screening of some Mannich base sydnone derivatives. Med Chem Res.2013;22:5789-97.
- Kumar S, Saxena PN. Application of metal complexes of Schiff bases: A review. JSc Ind Res. 2009;68:181-7.
- Schiff H. Information from the university laboratory in Pisa: A new series of organic bases. Annls Chem.1864;131:118.
- Kamaria P, Kawathekar N, Chaturvedi P. Microwave assisted synthesis and antimicrobial evaluation of Schiff bases of Indole-3-aldehyde. e-J Chem.2011;8:305-11.
- Kathiravan A, Sundaravel K, Jacob M, et al. Pyrene Schiff Base: Photophysics, aggregation induced emission, and antimicrobial properties. Phys Chem B.2014;118:13573-81.
- Pandeya SN, Sriram D, Nath G, et al. Synthesis, antibacterial, antifungal and anti-HIV activities of Schiff and Mannich bases derived from Isatin derivatives and N-[4-(4'-chlorophenyl)thiazol-2-yl]thiosemicarbazide. Euro J Pharm Sci.1864;9:25-31.
- Kumar KS, Ganguly S, Veerasamy R, et al. Synthesis, antiviral activity and cytotoxicity evaluation of Schiff bases of some 2-phenyl quinazoline-4(3)H-ones.2010;45:5474-9.
- T?ang A, Lien E, Lai MMC. Optimization of the Schiff bases of N-hydroxy-N'-aminoguanidine as anticancer and antiviral agents. Euro J Med Chem.1985;28:1103-6.
- Sridhar SK, Pandeya SN, Stables JP, et al. Anticonvulsant activity of hydrazones, Schiff and Mannich bases of isatin derivatives. Euro J Med Chem.2002;16:129-32.
- Cohen VI, Rist N, Duponchel C. Synthesis and anti-tuberculosis activity of thiocarboxamide derivatives of Schiff?s bases. J Pharma Sci. 1977;66:1332-4.
- Sondhi SM, Singh N, Kumar A, et al. Synthesis, anti-inflammatory, analgesic and kinase (CDK-1, CDK-5 and GSK-3) inhibition activity evaluation of benzimidazole/benzoxazole derivatives and some Schiff?s bases. Bioorg Med Chem.2006;14:3758-65.
- Liu ZC, Wang BD, Yang ZY, et al. Synthesis, crystal structure, DNA interaction and antioxidant activities of two novel water-soluble Cu2+ complexes derivated from 2-oxo-quinoline-3-carbaldehyde Schiff-bases. Euro J Med Chem.2009;44:4477-84.
- Xiao YN, Zhan CX. Studies on the interaction of DNA and water-soluble polymeric Schiff base-nickel complexes. J Appli Poly Sci.2002;84:887-93.