Original Article
, Volume: 15( 2)Structural Study on Propylamide by FT-IR Spectrometry Using Chemometrics Applications
- *Correspondence:
- Zeyede Aregahegn , Ethiopian Institute of Agricultural Researches, P.O. Box 2003, Addis Ababa, Ethiopia, Tel: +251973123790; E-mail: zeyedearegahegn@gmail.com
Received: May 26, 2017; Accepted: June 08, 2017; Published: June 15, 2017
Citation: Pandit DN, Kumari R, Kumari V. Factors Affecting Acute Toxicity Dose of Lead Nitrate in Certain Indian Air-Breathing Fishes. Int J Chem Sci. 2017;15(2):143.
Abstract
Solvent effects and sample concentration effects on the structural changes of propylamide have been investigated by FT-IR measurement using principal component analysis. The absorption peaks observed at higher frequency and higher intensity when using chloroform and dichloromethane compared to benzene. The concentration study reveals that propylamide form a high degree of association in benzene. Shifting the composition of the solvent from benzene to chloroform or dichloromethane, the intensity that comes from the association of molecule decreases since association is more favored in non-polar solvents. In spectra of propylamide in mixtures of benzene and dichloromethane an increment in the intensity of bands as well as a solvatochromic effect (blue shift) is observed upon increasing the polarity of the medium (solvatochromic effect).
Keywords
Propylamide; Solvent effect; FT-IR spectrometry; Chemometrics; Principal component analysis
Introduction
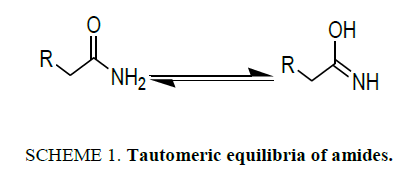
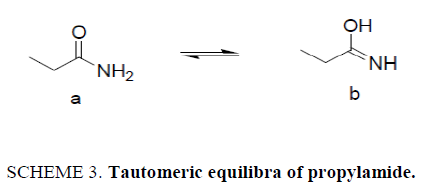
The amide functionality is a common feature in small or complex synthetic or natural molecules existing everywhere in life [1]. The reactivity of amides is related to their structure and their tautomeric equilibria (Scheme 1). Therefore, it can be useful to determine their spectral behavior in different conditions in order to study their tautomeric distribution [2]. Propylamide can form amide imidol tautomer [3,4]. Although enols are thermodynamically unstable species in comparison with their carbonyl isomers, some of them have a long enough lifetime so as to be detected by some instrumental methods [5,6].
There are some reports in the literature in regard to keto-enol tautomerism, for example, Reichardt and Welton [7,8], explained that dissolution of β-dicarbonyl compounds in solvents of low polarity, the percentage of the cis-enolic form increases, whereas polar solvents displace the equilibrium towards the diketo form. According to Reichardt and Welton [8], this decrease in enol content when increasing solvent polarity is due to intermolecular forces, and the enol form is the least polar of the two tautomers because intramolecular hydrogen bonding reduces the dipole-dipole repulsion and this makes stable the enol form, but the dipole-dipole repulsion is unreduced in the diketo form. In regard to amides, few publications are found for the investigation of amide-imidol tautomerism.
Tautomers are capable of changing phase from an apparently established structure to another and then back again when the original conditions are restored disconcerting. A change in structure means changes in properties. Intramolecular hydrogen bonding is the main factor that governs the structure of keto-enol tautomerism in solution and in β-ketoamides, internal hydrogen bonding is possible to be established in several tautomeric forms [5].
Infrared spectroscopy is one of the oldest and well established experimental techniques for the analysis of secondary structure of polypeptides and proteins and the method is convenient, non-destructive, requires less sample preparation, and can be used under a wide variety of conditions [4]. Fourier-transform infrared (FT-IR) spectroscopy is a fast, sensitive and accurate method which is used to determine protein content in food [9], and has been used as universal instrument to analyze varieties of samples due to its ability to identify functional group of chemical compounds, such as hydroxyl, carbonyl, amine and ester. Fourier-transform infrared (FT-IR) spectroscopy has dramatically improved the quality of infrared spectra and minimized the time required to obtain data [10].
There are different chemometrics methods which are used for analyzing chemical data. Principal component analysis (PCA) is a data reduction method used to re-express multivariate data with fewer dimensions and the goal is re-orient the data so that a multitude of original variables can be summarized with relatively few factors or components that capture the maximum possible information or variation from the original variables. PCA is one of the most important and powerful methods in chemometrics as well as in the knowledge of other areas [11]. PCA is probably the most widespread multivariate chemometrics technique, and because of the importance of multivariate measurements in chemistry, it is regarded by many as the technique that most significantly changed the chemist’s view of data analysis [2].
For spectral data measurement, PCA can perform mathematical decomposition of the spectral data that reduce the data dimensions of a highly complex chemical system to a smaller number of scores and principal components (PCs) or loadings that effectively carries all the important information of the spectra [7].
Experimental
Chemicals and reagents
For the study of the structure of propylamide by FT-IR spectroscopy, propylamide (98%, Alfa Aesar, Great Britain) was used as a sample throughout all the experiments for the FT-IR measurement. Benzene (99%, BDH Chemicals Limited, Poole, England), chloroform (99% to 99.4%, Sigma-Aldrich, Germany) and dichloromethane (99.9%, Sigma-Aldrich, Germany) were used as a solvent for the FT-IR measurement without any further purification.
Apparatus
Beakers (100 mL), volumetric flasks (100 mL) and balance (precision 0.0001 g) were used for sample preparation. IR spectra of the propylamide were recorded on a Perkin Elmer spectrum 65 FTIR Spectrometer with spectral resolution 1 cm-1 and in a spectral range 3350 cm-1 to 3550 cm-1.
Procedures
In order to study the effect of the solvent polarity on the structure of the propylamide (PA), two strategies were followed namely concentration effect (effect of changing the concentration of PA in a pure solvent) and solvent polarity effect (effect of changing the composition of a binary mixture of two solvents).
Concentration effect: Two sets of data were measured, using chloroform and benzene as solvents. Eight samples were prepared for each set by dilution of 0.005, 0.01, 0.02, 0.03, 0.04, 0.05, 0.06 and 0.07 g of PA dissolved in 10 mL of solvent (which corresponds to 0.5, 1, 2, 3, 4, 5, 6 and 7 mg/mL PA, respectively). The spectral data was measured on the FT-IR instrument taking the pure solvent as background.
Solvent polarity effect: Two set of samples of 4 mg/mL PA in binary mixtures of two solvents (benzene (Bz) + chloroform (CF) and benzene + dicloromethane (DCM)) were prepared in order to analyze the polarity effect in the spectra. In these samples, the percentage of dichloromethane or chloroform to that of benzene ranged between 0% to 100% Bz (w/w) with increments of 10%. 20 g of the mixture of solvents at the desired proportion were prepared by weight. 10 mL were pipetted and mixed with 40 mg PA in sealed bottles. The bottles were stirred and warmed up to 40°C to assure complete dissolution. The FT-IR spectral measurement of each sample was obtained using the remaining solution to take the background.
Results and Discussion
Concentration effect
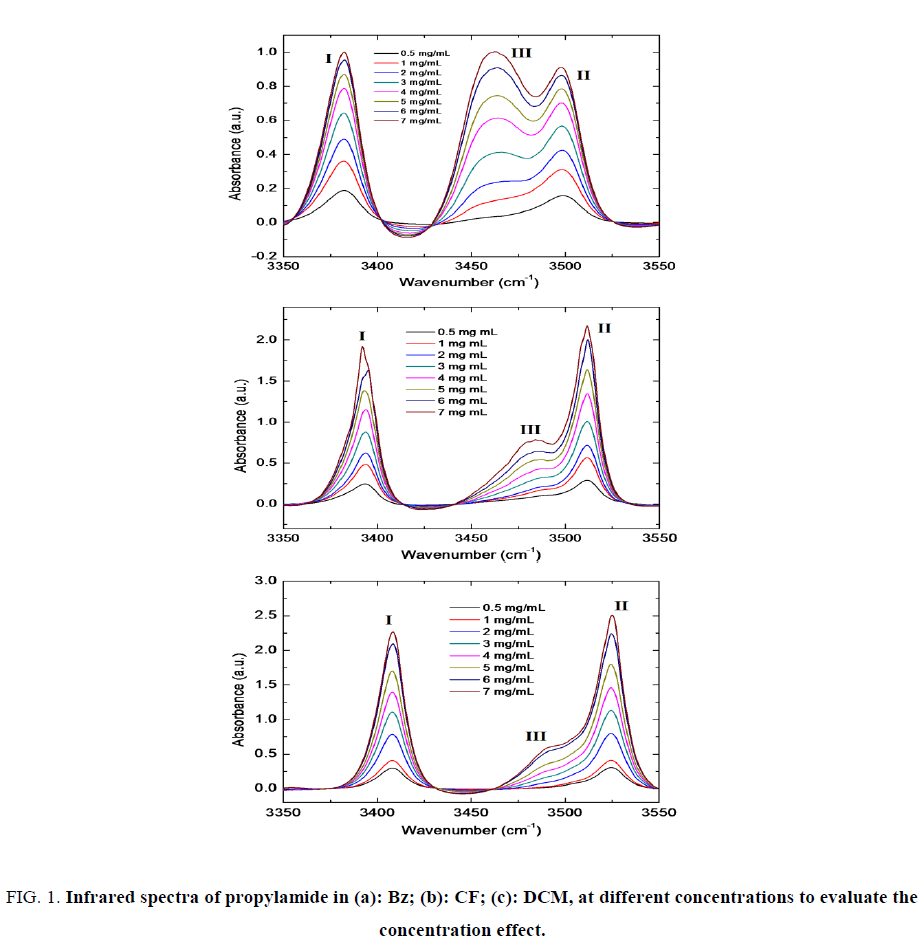
Figure. 1 shows the IR spectra of PA in different pure solvents (benzene, Bz; chloroform, CF and dichloromethane, DCM) varying the concentration of propylamide. The spectral region for the analysis of concentration effect was from 3350 cm-1 to 3550 cm-1, which corresponds to the N-H stretching vibration modes.
Figure 1: Infrared spectra of propylamide in (a): Bz; (b): CF; (c): DCM, at different concentrations to evaluate the concentration effect.
In all spectra, three bands can be distinguished: one band at lower frequencies (Band I), a band at higher frequencies (Band II) and finally a third band close to Band II and displaced to lower frequencies (Band III). The latter band becomes a shoulder in CF and DCM or at concentrations low enough. Typically, Band I and II are assigned to N-H symmetrical and asymmetrical stretching, respectively. Band III is also assigned to association structures of amide by hydrogen bonds. This is consistent with our results since the relative intensity of band III respect band I or II depends greatly on PA concentration. Maxima for the different bands observed are shown in Table 1.
| Solvent | Band I | Band II | Band III |
|---|---|---|---|
| Bz | 3382 | 3498 | 3463 |
| CF | 3393 | 3511 | 3484-3487 |
| DCM | 3408 | 3525 | Shoulder |
Table 1: Maximum stretching frequencies for the spectra of propylamide in the three solvents.
When increasing the concentration of the propanamide, the absorption band of all bands increases. The observed increase of both bands at higher concentration was expected.
When we see the maximum absorbances of propylamide in the three solvents, less absorbance in benzene was observed. Propylamide in chloroform absorbs higher than propylamide in benzene but less than propylamide in dichloromethane (absorbance of propylamide in DCM > in CF > in Bz) for each absorption band.
The relative intensity of Band III with respect Bands I and II increases as concentration of PA increases. This is a clear sign that Band III corresponds to an aggregated structure (dimmer) that is favored when the amount of PA molecules in the medium is higher. As seen in Figure. 1a the aggregation band (Band III) is even higher than Band II at 7 mg/mL which decreases progressively with decreasing concentration until it is a small shoulder at 0.5 mg/mL. This indicates a high degree of association in this solvent. However, as it can be seen in Figure. 1b and Figure. 1c, B and III is significantly weaker. This is clearly showed that the formation of aggregation between the amide molecules is very weak when using CF or DCM solvents as compared to using Bz. The formation of aggregation becomes higher when using more concentrated PA. This is because the association becomes higher when using high concentrated PA sample. The association becomes higher when using Bz than the other two solvents. This is due to that the chlorine in CF and DCM resists forming association with the other polar molecule (PA) because of the position of Cl. The absorption peaks also towards at higher frequency when using chloroform compared to benzene. This shows that the absorption of propylamide in benzene solvent is towards lower energy (red shift) and it is towards at higher energy (blue shift) in chloroform that is a more polar solvent.
Solvent polarity effect
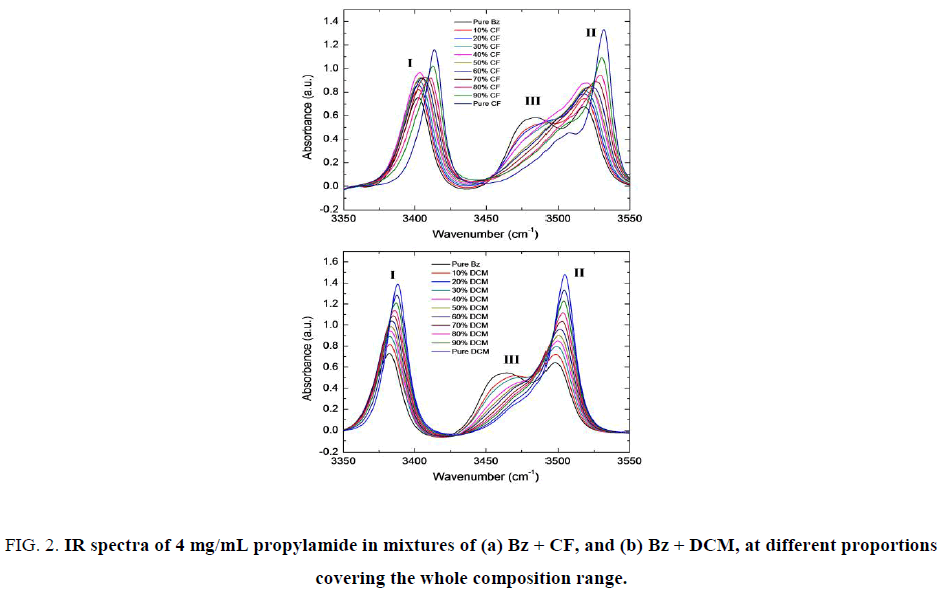
The spectral measurements were recorded in a range from 3350 cm-1 to 3550 cm-1 for a solution of 4 mg/mL of PA in two binary mixtures of solvents: Bz + CF and Bz + DCM, at compositions ranging the whole interval. The results are presented in Figure. 2.
Figure 2: IR spectra of 4 mg/mL propylamide in mixtures of (a) Bz + CF, and (b) Bz + DCM, at different proportions covering the whole composition range.
The spectrum of PA in pure benzene is, as expected, formed by Band I and II from symmetric and asymmetric stretching of NH2 and Band III, from association by hydrogen bond. Upon shifting the composition of the solvent from Bz to CF or DCM, the intensity of Band III decreases since association is more favored in non-polar solvents.
Additionally, for the spectra of PA in mixtures of Bz + DCM an increment of the intensity of Bands I and II as well as a solvatochromic effect (blue shift) is observed which starts from 10% DCM and the shift becomes more upon increasing the polarity of the medium. The situation for the spectra of PA in mixtures of BZ + CF is somewhat different. No such solvatochromic effect is observed up to 60% CF, but the shift between 60% and 80% CF is significantly larger to be moderated again between 80% and 100% CF.
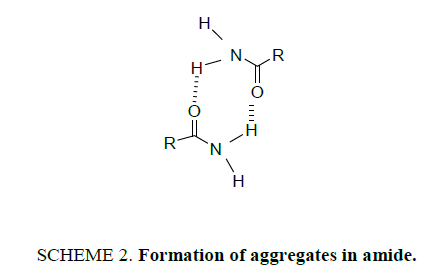
It can be seen that around 3408 cm-1 symmetric stretching bands for NH2 and asymmetric stretching around 3525 cm-1. At high percentage of benzene, a new band appears at lower frequencies than Band II that can be explained by formation of aggregates according to Scheme 2.
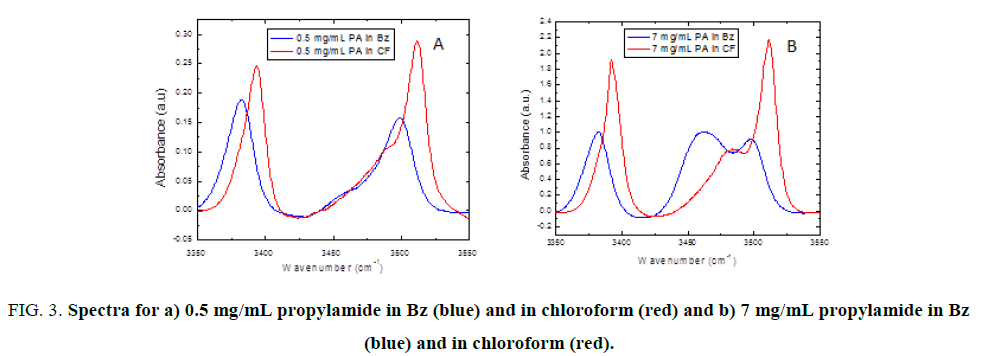
One may think that in non-polar solvents, such as benzene, the formation of hydrogen bonding within propylamide molecule is favored than when working with a polar solvent such as chloroform. Shapes of the spectra are the same but there are certain differences: Figure. 3a, shows together the first spectra (0.5 mg/mL) for PA in benzene (blue) and in chloroform (red) and Figure. 3b, shows together the first spectra (7 mg/mL) for PA in benzene (blue) and in chloroform (red). The bands of propylamide in chloroform appear at higher intensity and at higher frequencies than propylamide in benzene. This is an indicative that in chloroform association is lower, then one can explain that the intensity of the band around 3484 cm-1 is lower (Figure. 3b) in CF than in Bz.
Figure 3: Spectra for a) 0.5 mg/mL propylamide in Bz (blue) and in chloroform (red) and b) 7 mg/mL propylamide in Bz (blue) and in chloroform (red).
The absorption band of propylamide in pure chloroform is very intense and strong as compared to other spectra obtained from mixture of solvents and from propylamide in pure benzene. This difference in the absorption peak intensity is may be due to the association difference when using different solvents that differs in polarity. In this case the association within amide molecule may be weak when using chloroform solvent as compared to using pure benzene and mixtures of benzene with chloroform. Since there are some differences in the magnitude of the absorption peak, this may be an indication of the fact that the shift is originated from different isomers.
Similar absorption peak position was observed in both cases, i.e., in mixture of Bz with CF and Bz with DCM. At higher percentage of dichloromethane, the absorption peak is strong and intense. This situation is similar to the absorption spectra of PA in mixture of Bz and CF. This may be due to the reason that the two solvents, chloroform and dichloromethane have comparable polarities. The possible existence of tautomeric equilibrium is seen in Scheme 3.
The main problem faced when analyzing Figure. 1 and 2 is that it is difficult to identify the effects on the IR spectra of the association and tautomerism separately. The Band III present in all the spectra masks some details that can point to the existence of tautomeric equilibrium. Also, the reason why the shape of the association bands is different in different solvents is still unclear and the nature of the bonds is not known, as well as the origin of the different solvatochromic effects. In order to try to find answers, the strategy to follow will be to carry out a statistical analysis based on chemometrics techniques.
Statistical analysis
The data was treated by chemometrics tools with MATLAB 7.8 (MATLAB R2009a). Principal component analysis (PCA), carried out with MATLAB 7.8, was mainly used for data reduction to identify a small number of factors that explain most of the variance observed in a much larger number of clearly seen variables. Infrared spectra between 3350 cm-1 and 3550 cm-1, of each sample were exported to be analyzed using MATLAB subroutines. Three spectra data matrices D1, D2, D3 of dimension (8 spectra × 441 wavenumbers) were constructed by arranging the spectra recorded for concentration effect study. And other two data matrices, D4 and D5, of dimension (11 spectra × 441 wavenumbers) were constructed by arranging the spectra recorded for solvent polarity effect study.
In order to analyze the presence of imidol and the formation of aggregates MCR-ALS cannot be used. Since the active groups are the same within the spectra, it is not possible to analyze the presence of imidol by using an analysis of the evolution of bands for -NH and -OH stretching. The strategy followed will be based on principal component analysis. It would be possible to analyze the source of variation to explain the variance in D1, D2, D3. PC1 is done by singular value decomposition (SVD) of the data matrix, D1, D2, D3. Table 2 shows the eigenvalues calculated by SVD and percent variance associated to each PC for each data matrix.
| D1 matrix, PA in benzene |
D2 matrix, PA in chloroform |
D3 matrix, PA in DCM |
||||
|---|---|---|---|---|---|---|
| Singular value (λ) |
(%) Variance | Singular value (λ) |
(%) Variance | Singular value (λ) |
(%) variance | |
| PC1 | 20.99 | 99.30 | 26.13 | 99.58 | 30.74 | 99.78 |
| PC2 | 2.04 | 0.69 | 0.96 | 0.31 | 1.22 | 0.19 |
| PC3 | 0.09 | 0.01 | 0.45 | 0.09 | 0.23 | 0.02 |
| PC4 | 0.05 | 0 | 0.15 | 0.02 | 1.33 | 0.01 |
| Total | 100 | 100 | 100 | |||
Table 2: Eigenvalues calculated by SVD and percent variance for spectral data of propylamide in pure solvents.
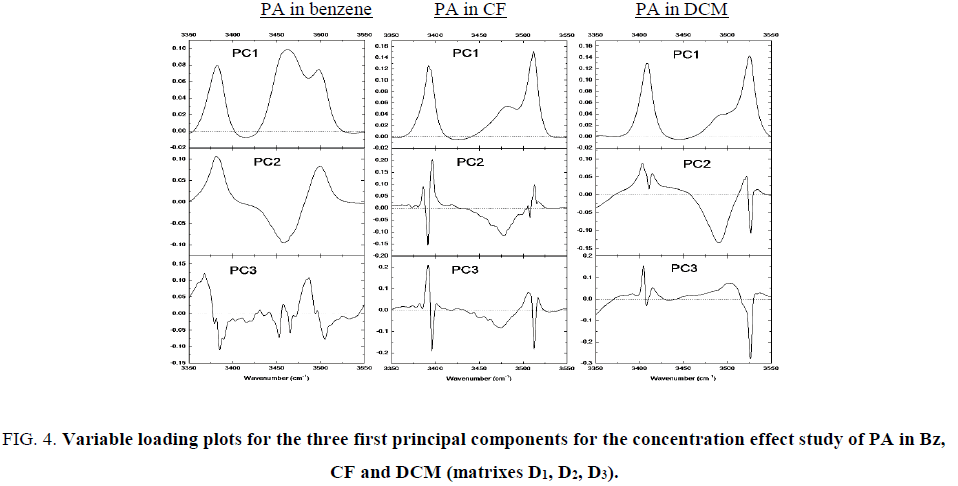
The first PC gathers the highest variability in all situations and it is related to the concentration of propylamide in pure solvents. Subsequent PCs account for much less degree of variance. If the eigenvalue is too small, the factors are related to the experimental noise but sometimes they have important information. We can check it by means of the loading vectors. These results are shown Figure. 4.
Figure 4: Variable loading plots for the three first principal components for the concentration effect study of PA in Bz, CF and DCM (matrixes D1, D2, D3).
For the second PC the percent of variance is very small, but the loading vector is not associated to the noise. They are associated to the variation of the bands because the sign of them sometimes changes. As the concentration of propylamide increases, the variation of intensity of the bands evolutes in opposite direction. This second source of variation can be related to the process of association.
But in the case of CF and DCM solutions, the representative bands of the stretching frequencies are divided in two peaks. Both are correlated negatively in PC2 with the band at 3392 cm-1 and 3497 cm-1 and at 3479 cm-1 and 3513 cm-1 associated to the intermolecular H bonds. The fact suggests that the existence of more than one chemical structure representative of associated amines. It may be explained by the presence of imidol also able of forming hydrogen bonds. PC3 only explains 0.01% of variance for PA in Bz. There are broad bands around 3368 cm-1 and 3487 cm-1 that could be characteristic of intermolecular bonds through OH group. Loading vectors for propylamide in CF show sharp peaks at 3392 cm-1 and 3513 cm-1 which may be the NH stretching spectra of amide. For the PC analysis for the matrix of propylamide in the mixture of benzene with chloroform and in the mixture of benzene with dichloromethane, the eigenvalues are shown in Table 3.
| D3 matrix, PA in benzene + chloroform |
D4 matrix, PA in benzene + dichloromethane |
||
|---|---|---|---|
| Singular value(λ) | (%) variance | Singular value (λ) | (%) variance |
| 28.26 | 87.68 | 29.08 | 94.23 |
| 7.05 | 11.75 | 5.88 | 5.57 |
| 1.67 | 0.35 | 1.44 | 0.11 |
| 0.31 | 0.16 | 0.22 | 0.07 |
| - | 99.94 | - | 99.98 |
Table 3: Eigenvalues calculated by SVD and percent variance for spectral data of propylamide in mixture of solvents.
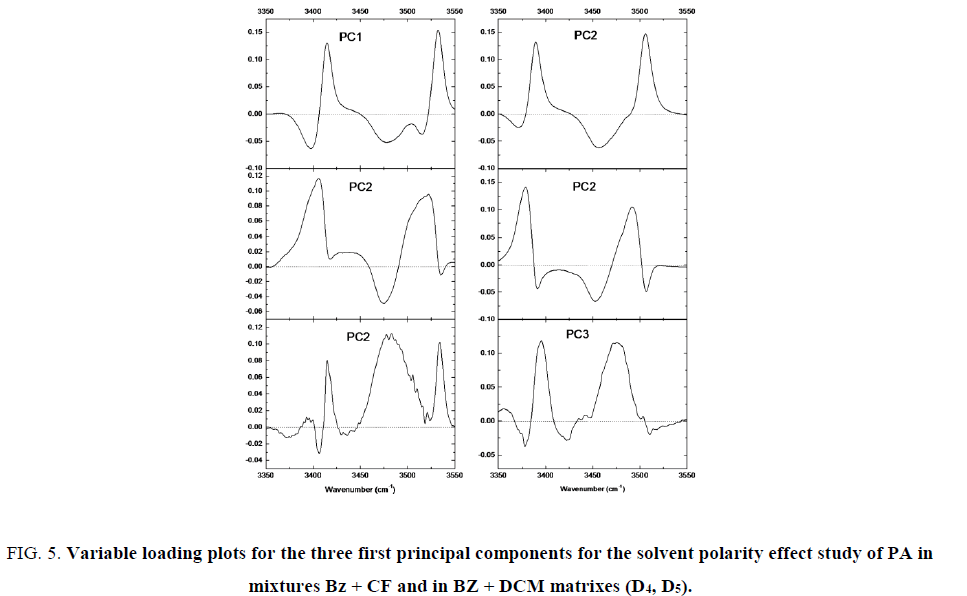
Figure. 5, shows the PCs of 4 mg/mL of propylamide in mixture of Bz with CF (matrix D3) and in mixture of Bz with DCM (matrix D4). Spectral variations are associated to the change of polarity for the medium. According to Figure. 5, the associations are less intense with increasing polarity. PC1 (94.23% of variance), has a loading vector with negative correlation between bands with higher contribution. It can be interpreted as follows. When the polarity increases, the intensity of the band around 3505 cm-1 and 3387 cm-1 increases whereas it decreases around 3442 cm-1 and 3523 cm-1.
Figure 5: Variable loading plots for the three first principal components for the solvent polarity effect study of PA in mixtures Bz + CF and in BZ + DCM matrixes (D4, D5).
Analysis of loading vectors Figure. 5, associated to the other three PCs, PC2 and PC4, explain 11.75% and 0.35% of variance for Bz + CF and, 5.57% and 0.11% for Bz and DCM (Table 1). They show relatively high contribution to the already considered bands and the negative contribution for the others. It can be explained as the presence of imidol groups in equilibrium with amide groups and forming associated structures among them.
Conclusion
Principal component analysis (PCA) was applied to study variation of absorption peaks for FT-IR measurements of propylamide in pure solvents and mixture of solvents by varying concentration of samples and polarities of solvents. Propylamide coexist in equilibrium and their predominance is highly solvent dependent. The formation of aggregation between the propylamide molecules is very weak when using CF or DCM solvents as compared to using Bz. In non-polar solvents, such as benzene, the formation of hydrogen bonding within propylamide molecule is favored than when working with a polar solvent such as chloroform. The spectral intensity and stretching frequencies of propylamide in Bz were less than in the other two solvents (CF and DCM). Upon shifting the composition of the solvent from Bz to CF or DCM, the intensity that comes from the association of molecule decreases and this shows association is more favored in non-polar solvents. PCA is also able to detect a different chemical environment when PA is in polar solvents that can be due to the presence of the imidol tautomer.
Acknowledgement
The author thanks Ethiopian Institute of Agricultural Research, for providing financial support for this research study.
References
- Allegretti PE, Peroncini V, Castro EA, et al. Study of the occurance of tautomeric forms of ureas and thioureas by mass spectrometry. Int J Chem Sci.2003;1:1-2.
- Brereton RG. A short history of chemometrics: A personal view. J Chemometrics.2014;28:749-60.
- Bro R,Smilde AK. Principal component analysis, Analy Meth (RSC). 2015;6:2812-31.
- Kong J, Yu S. Fourier transform infrared spectroscopic analysis of protein secondary structures, Acta Biochimica et BiophysicaSinica. 2007;39:549-59.
- Laurella SL, Sierra MG, Furlong JJP, et al. Substituent, temperature and solvent effects on the keto-enol equilibrium in b-ketoamides: A nuclear magnetic resonance study. OJPC. 2013;3:138-49.
- Montalbetti CA,Falque V. Amide bond formation and peptide coupling. Tetrahedron. 2005;61:10827-52.
- Ong YH, Lim M, Liu Q. Comparison of principal component analysis and biochemical component analysis in Raman spectroscopy for the discrimination of apoptosis and necrosis in K562 leukemia cells, Optical Society of America.2012;20:22158-71.
- Reichardt C,Welton T. Solvents and solvent effects in organic chemistry. Reichardt C and Welton T,editors.4th edition. WILEY-VCH: Weinheim, Germany. 2011.
- Sjahfirdi L, Mayangsari,Nasikin M. Protein identification using Fourier transform infrared (FT-IR). IJRRAS, 2012;10:418-21.
- Stuart B. Infrared spectroscopy: Fundamentals and applications. Stuart B (Ed.), John Wiley & Sons, Ltd. 2004.
- Taylor PJ, Zwan G,Antonov L. Tautomerism: Introduction, history, and recent developments in experimental and theoretical methods. (First ed.), Wiley-VCH Verlag GmbH and Co. KGaA: Antonov L. 2014.