Original Article
, Volume: 15( 4)Sono Facilitated Selective Nitration of Naturally Occurring Cardanol Using Phase Transfer Catalyst
- *Correspondence:
- Pandit AB Department of Chemical Engineering, Institute of Chemical Technology, Mumbai, India
Tel: +91-2233611111; E-mail: ab.pandit@ictmumbai.edu.in
Received Date: June 29, 2017 Accepted Date: November 01, 2017 Published Date: November 04, 2017
Citation: Patil BR, Chetta C, Pinjari DV, et al. Sono Facilitated Selective Nitration of Naturally Occurring Cardanol Using Phase Transfer Catalyst. Int J Chem Sci. 2017;15(4):214
Abstract
In the present work, ultrasound facilitated nitration of hydrogenated cardanol was carried out using dilute nitric acid (9 wt%). The nitration was accomplished in the presence of tetrabutylammonium bromide (TBAB), which acts as a phase transfer catalyst. The nitration reaction was carried out in a 50 ml three neck flask, which was placed in an ultrasonic bath operating at a frequency of 24 kHz and with a delivery power of 150 watt. The effects of various parameters viz., agitation speed, temperature, nitric acid concentration, TBAB concentration, were studied on the yield of o-nitro cardanol. Kinetic studies have been performed for the nitration of hydrogenated cardanol, and the activation energy and pre-exponential factor for the reaction has been established. The work has clearly recognized that, the ultrasonic irradiations are superior for the synthesis of ortho nitro cardanol with higher yield as compared to the conventional approach.
Keywords
Hydrogenated cardanol; O-nitro cardanol; Ultrasound; Kinetics; TBAB
Introduction
The nitration of phenols is an important and widely used reaction in the organic synthesis, particularly to produce amino derivatives of the phenols. The importance of nitro phenols in the perspective of industry are significant. They have been used in pesticides, dyes, and pharmaceutics and polymers industries [1,2]. As the nitro derivatives of phenols play a vital role in the synthesis of industrial intermediates, lot of work has been reported on nitration of phenols. In this study, we have reported the nitration of naturally available phenol i.e., cardanol. Cardanol is a naturally occurring unsaturated phenol obtained from cashew nut shell liquid (CNSL), which is a byproduct of cashew industry. Cardanol is a yellow oil obtained after vacuum distillation of CNSL [3-6]. Nitro cardanol is obtained by the nitration of saturated (hydrogenated) cardanol, which was also prepared in our earlier work.
In recent years, large amount of work has been reported on hydrogenated cardanol in the field of chemical transformation. Cardanol is a unique, natural alkyl phenol derivative. It is a meta substitute long chain of unsaturated aliphatic hydrocarbon. Cardanol is commercially available and produced with high purity on a large scale [7]. Cardanol is a relatively cheap, inexpensive natural resource and easily available in the market. It is an entry chemical for various chemical derivatives such as, antioxidants, flame retardants hydrophobic agents etc. These derivatives of hydrogenated cardanol can be a very important renewable resource for developing many green, eco-friendly chemicals starting from a renewable resource. The Nitro and amino derivatives of cardanol are very efficient antioxidant for gasoline, mineral hydrocarbons, petroleum products and lubricating oils. It can also be used as intermediates for the insecticides and fungicides. Nitration of cardanol is very interesting reaction and, not much work has been reported on it so far.
In recent years, large amount of work has been reported on hydrogenated cardanol in the field of chemical transformation. Cardanol is a unique, natural alkyl phenol derivative. It is a meta substitute long chain of unsaturated aliphatic hydrocarbon. Cardanol is commercially available and produced with high purity on a large scale [7]. Cardanol is a relatively cheap, inexpensive natural resource and easily available in the market. It is an entry chemical for various chemical derivatives such as, antioxidants, flame retardants hydrophobic agents etc. These derivatives of hydrogenated cardanol can be a very important renewable resource for developing many green, eco-friendly chemicals starting from a renewable resource. The Nitro and amino derivatives of cardanol are very efficient antioxidant for gasoline, mineral hydrocarbons, petroleum products and lubricating oils. It can also be used as intermediates for the insecticides and fungicides. Nitration of cardanol is very interesting reaction and, not much work has been reported on it so far.
The nitration of cardanol has been reported by Wasserman et al. using concentrated nitric acid. The process they have described is not efficient in yielding the compound selectively [8]. Dawson et al. have also reported and have patented a process of nitration of hydrogenated cardanol [9]. A study of nitration of phenol with ultrasound irradiation using dilute nitric acid (6%), in the presence of phase transfer catalyst (PTC) has also been published [10]. The study of the synthesis to p-nitro cardanol from hydrogenated cardanol by nitration using 65% HNO3 has been published [11]. In recent years, the use of ultrasound has become quite common as it plays an important role in chemical transformations. Ultrasound is known to increase the reaction yield, rate of the reaction, overall conversion, and the selectivity. It also reduces processing time and reduces the formation of by-products in chemical transformation [12,13]. Ultrasound has been used in many chemical transformation or the synthesis reaction in biphasic medium to increase the efficacy of phase transfer catalysts [14,15]. The role of PTC in the chemical synthesis is very vital. The selectivity offered by the catalyst is dependent on its ability to trigger and transport the nucleophile from aqueous to the organic phase. While the use of phase transfer catalyst increases the selectivity and conversion of reaction, it also reduces the reaction time and also facilitates the separation of reaction mass [16].
Considering all the previous work reported above, the present work reports the effect of ultrasound on the nitration of cardanol at different operating conditions. We have studied and synthesized o-nitro cardanol based on regioselectivity from the hydrogenated cardanol at various temperatures, catalyst concentration, nitric acid concentration and a variety of solvents. The synthesis of o-nitro cardanol typically involve L-L two phase system. The nitration of cardanol has been achieved with a lower concentration of nitric acid (9 wt%) in the presence of tetrabutylammonium bromide (TBAB) as a phase transfer catalyst. The kinetics of nitration of cardanol has also been investigated.
Experimental Section
Material
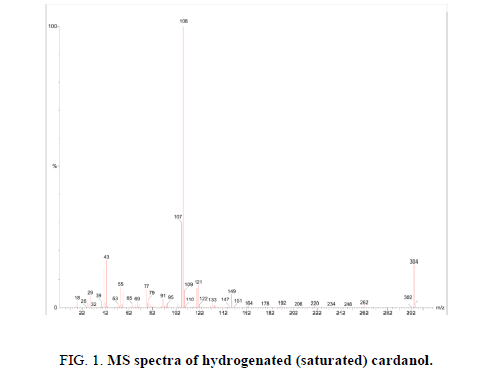
The cardanol was hydrogenated in the lab to get saturated cardanol (purity was 99%). Figure 1 shows the mass spectra of saturated cardanol with m/z=304. Tetra butyl ammonium bromide (TBAB) (extra pure 99%) was procured from S.D. Fine Chemicals Pvt. Ltd. Mumbai. 1, 2 Dichloroethane (LR) and Nitric acid (70%) were obtained from Thomas Baker Chem. Pvt. Ltd Mumbai.
Experimental setup
The hydrogenation of cardanol was carried out in a 250 ml SS-316 high pressure batch reactor (provided by Amar equipment Pvt. Ltd., Mumbai, India). While nitration of hydrogenated cardanol experiments were performed in 50 ml three neck round bottom flask in an ultrasonic bath provided by M/s Dakshin Pvt. Ltd. Mumbai. India. The operating frequency of sonication bath was 24 kHz with a rated power 150 W. The bath was of rectangular design (tank) having internal dimensions of 300 × 150 × 150 mm with four transducers at the bottom. The reaction flask was equipped with variable speed agitator with half-moon Teflon blade impeller, and had a temperature sensor pocket. A temperature sensor was also immersed into the ultrasound bath to measure the water temperature. The temperature of the ultrasound bath having water and the reaction flask having reactants were maintained by continuously changing the water in the bath.
Experimental procedure
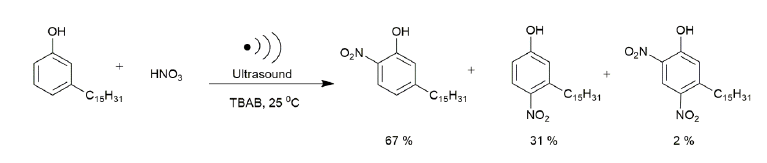
Prior to the nitration of hydrogenated cardanol, hydrogenation of cardanol to get saturated cardanol i.e., saturation at the meta position of the aliphatic hydrocarbon chain was carried out (data reported elsewhere). The iodine value of 245 of the hydrogenated cardanol indicated the 100% saturation of cardanol. The typical nitration reaction of hydrogenated cardanol was carried out in a biphasic L−L system. The aqueous phase consisted of TBAB and HNO3, whereas the organic phase contained hydrogenated cardanol (limiting substrate) in 1, 2 dichloroethane (Solvent). All the reactions were carried out by charging hydrogenated Cardanol 0.76 gm (2.5 mmol) in 20 ml of solvent, taken as the limiting reactant, 70% nitric acid of 0.45 gm (5 mmol) (100% excess of limiting substrate and diluted as per requirement of experiment) and TBAB catalyst 0.0403 gm (15 mol%) of the limiting substrate in the reaction vessel. The reaction proceeded towards complete conversion of hydrogenated cardanol to nitro derivatives i.e. ortho, para and di. The progress of the nitration reaction was monitored on the basis of the disappearance of cardanol from the organic phase. The reaction scheme for the selective ortho nitration of cardanol with HNO3 using a phase transfer catalyst (TBAB) is shown in Table 1. The reactions were carried out in a round bottom flask of 50 ml capacity equipped with a mechanical agitator and the flask was suspended at the center of the ultrasonic bath. In a typical reaction of nitration of hydrogenated cardanol, the reaction mixture was sonicated at various temperatures, i.e., 20°C, 25°C, 30°C and 40°C. We have estimated the actual power dissipated through the ultrasound bath calorimetrically and it was found to be 48 W with 41% calorimetric efficiency. The progress of the reaction was monitored by HPLC (Agilent). After the completion of the reaction, the organic layer was separated from the aqueous layer by separating funnel. The organic layer containing o-Nitro cardanol and other by-products (Para and Di Nitro cardanol) was washed thrice with 30 ml distilled water to remove the traces of the water-soluble catalyst and nitric acid. Then the organic layer was dried over sodium sulphate. The anhydrous (dried) organic layer was subjected to HPLC. For the isolation of o-nitro cardanol the organic layer was concentrated by vacuum distillation, subsequently crude dark black mass was subjected to column chromatography (silica gel 120-200 mesh) using pet ether-chloroform (9:1) as an eluent.
| S. No | Cardanol (mmol) |
HNO3 (Wt.%) |
Time (min) |
Conversion (%) |
Selectivity (%) | ||
|---|---|---|---|---|---|---|---|
| O-Nitro | P-nitro | Di-nitro | |||||
| 1 | 2.5 | 6 | 60 | 94 | 61 | 32 | 1 |
| 2 | 2.5 | 9 | 45 | 100 | 67 | 31 | 2 |
| 3 | 2.5 | 12 | 40 | 100 | 58 | 37 | 5 |
| 4 | 2.5 | 15 | 40 | 100 | 45 | 46 | 9 |
| 5 | 2.5 | 18 | 35 | 100 | 38 | 52 | 10 |
(Reaction condition: Cardanol (2.5 mmol), nitric acid 70% (5 mmol) calculated amount of water to make a particular concentration of nitric acid to 6, 9, 12, 15 and 18 (wt%), TBAB (15 mole%), 1, 2-dichloroethane (20 cm3); agitation speed 350 RPM; temp 25°C.)
Table 1. Reaction scheme for the selective ortho nitration of cardanol with HNO3 using a phase transfer catalyst (TBAB).
Analysis techniques
The concentration of reactants and products (hydrogenated cardanol and nitro cardanol respectively) were estimated using high performance liquid chromatography (HPLC) technique (Agilent Technologies). The Chromatographic separation was achieved on an ODS-Hypersil C18 column with a mobile phase, which was a mixture of methanol/water in the volumetric ratio of 30:70. Ortho phosphoric acid equivalent to 0.1% of the total mobile phase was used. The chromatogram was obtained using a UV detector set at 278 nm of wavelength. The reaction conversion was estimated with the disappearance of cardanol present in the organic phase. All the results were based on HPLC analysis using authentic standard.
Reaction Scheme
Results and Discussion
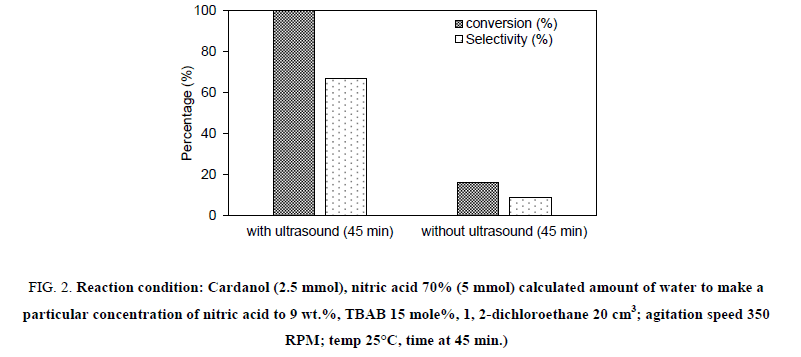
Initially, all experiments were carried out by charging hydrogenated cardanol 0.76 g in ethylene dichloride (20 ml) in quantity. The aqueous layer consisted of 10ml to 15 ml of a known concentration of nitric acid and 0.0403 g of the TBAB catalyst. The basic reaction mechanism in biphasic medium is that the nitric acid reacts with the TBAB catalyst in the aqueous layer to generate the nitronium ion species. The PTC transfers these nitronium species to the organic layer. This is discussed later. For checking the effect of ultrasound on the nitration of hydrogenated cardanol, we have carried out experiment at 350 RPM agitation speed with and without ultrasound. The other parameters were kept constant. Figure 2 shown the obtained results. It can be seen that in the absence of ultrasound, the reaction reached 16% conversion in 45 min, whereas with ultrasound the reaction reached 100% conversion in just 45 min. Furthermore, the selectivity to ortho nitro cardanol was 67% in the presence of ultrasound while in the absence of ultrasound it was only 9%. While we have performed one experiment in the absence of ultrasound and mechanical agitation only 5% conversion to nitro cardanol was obtained using 9% dil. HNO3 in 24 h.
Figure 2: Reaction condition: Cardanol (2.5 mmol), nitric acid 70% (5 mmol) calculated amount of water to make a particular concentration of nitric acid to 9 wt.%, TBAB 15 mole%, 1, 2-dichloroethane 20 cm3; agitation speed 350 RPM; temp 25°C, time at 45 min.)
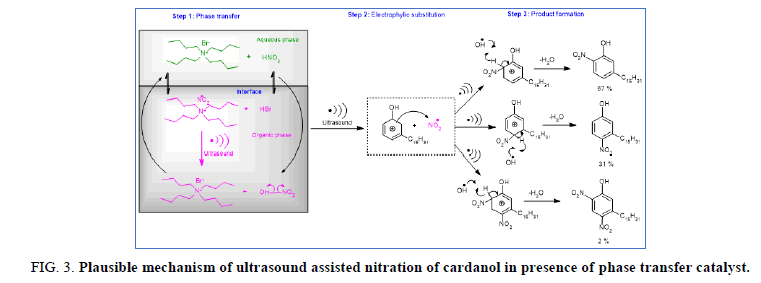
Figure 3 shows a plausible mechanistic pathway of phase transfer catalyst of nitration of hydrogenated cardanol. In the first step there is formation of tetra-n-butyl ammonium nitrate (TBANO-3) and hydrogen bromide (HBr) in the aqueous phase of exchanging bromide and nitrate ion. Then TBANO-3 is transferred to organic phase through the interface because of microlevel turbulence generated by ultrasound at the interfacial region. In the organic phase TBANO-3 reacts with bromide (HBr present in the organic phase) and reforms TBAB and active NO2 radical species. TBAB returns to the aqueous phase. While in the second step the active NO2 radical species react with hydrogenated cardanol by free radical mechanisms. The free radical is expected to be produced during acoustic cavitation created by ultrasound irradiation. In the third step the desired product is formed by free radical mechanisms. Ultrasound or agitation assists and increases the rate of mass transfer of the nitronium ion across the interface. While the cavitation effect plays a predominant role in solublization of the reactants in an L-L system which maximize the mass transfer [17] otherwise it’s very difficult to achieve it under silent condition.
Figure 3: Plausible mechanism of ultrasound assisted nitration of cardanol in presence of phase transfer catalyst.
Effect of temperature
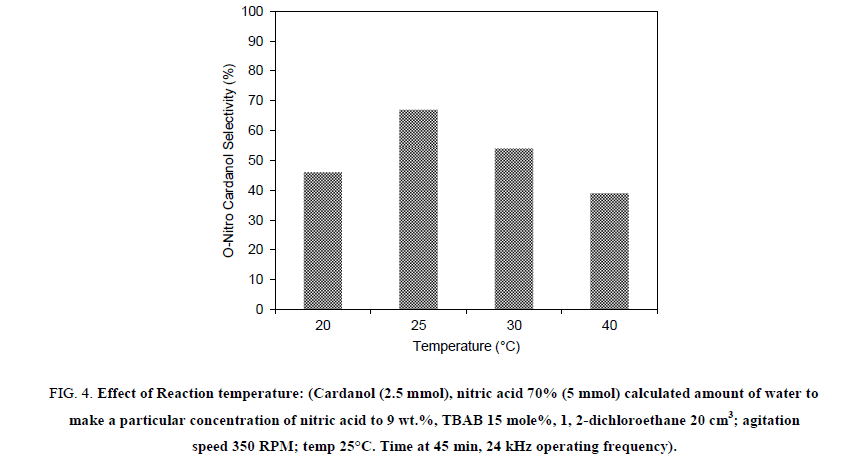
In order to study the effect of the operating temperature on the initial rate of nitration of the hydrogenated cardanol, reactions were carried out at different operating temperature over a range of 20°C, 25°C, 30°C and 40°C and results for the same are reported in Figure 4. As the temperature of the reaction increased from 20°C to 40°C, selectivity of o-nitro cardanol reduced and the conversion increased from 91% to 100% respectively. While para and di nitro cardanol formation increased as a function of time and with an increase in the temperature. Water was continuously circulated from the water tank to maintain specified reaction temperature. When the reaction was carried at 20°C, the conversion of hydrogenated cardanol to nitro cardanol was found to be 78% in 45 min, with 46% ortho selectivity. When the temperature was increased to 25°C, it was found that, conversion of Cardanol was 100% and Ortho selectivity of nitration also increased to 67% within 45 min. Also, at 30°C and 40°C, complete conversion (100%) of hydrogenated cardanol to nitro cardanol was achieved in 45 min and 40 min, respectively though the ortho selectivity decreased to 54% and 39% respectively.
Figure 4: Effect of Reaction temperature: (Cardanol (2.5 mmol), nitric acid 70% (5 mmol) calculated amount of water to make a particular concentration of nitric acid to 9 wt.%, TBAB 15 mole%, 1, 2-dichloroethane 20 cm3; agitation speed 350 RPM; temp 25°C. Time at 45 min, 24 kHz operating frequency).
The probable reason is the micro agitation (surface instabilities) provided by ultrasonic irradiation. This micro agitation raised the local temperature in the vicinity of the cavity in aqueous layer and at the interfacial. The para selectivity increases as the temperature increases. Micro agitation in interfacial region was dominant and was found to play an important role for the increased yield of di and para nitro cardanol.
Effect of agitation speed
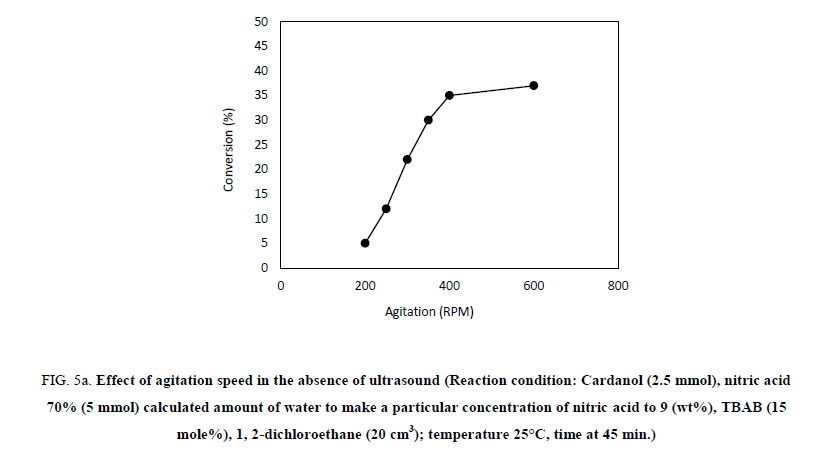
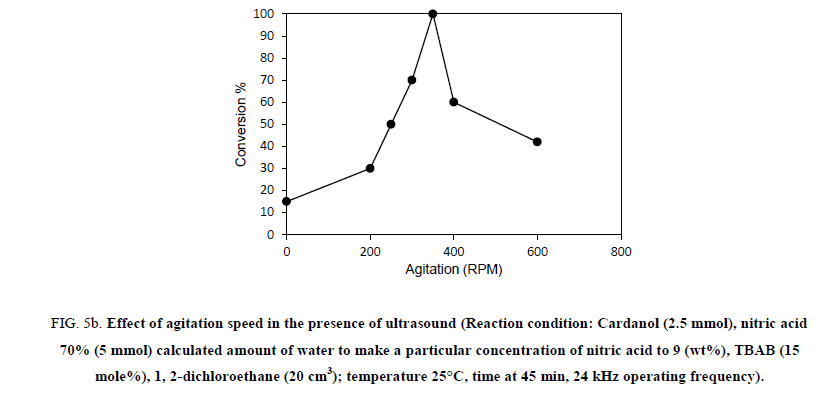
In order to understand the effect of agitation on the rate of nitration of the hydrogenated cardanol, experiments were conducted at different speeds of agitation in the range 200 RPM to 600 RPM. Effect of agitation on the initial rate of the nitration of hydrogenated cardanol in the absence of ultrasound cavitation is shown in Figure 5a. Two sets of experiments were carried out, with and without ultrasound at different agitation speed. When the experiment was carried out without ultrasound with 200 RPM agitation speed, conversion of hydrogenated cardanol to nitro cardanol was obtained as 5% in 45 min. While at 250 RPM, 300 RPM, 350 RPM, 400 RPM and 600 RPM in the absence of ultrasound the conversion to nitrated cardanol was 12%, 22%, 30%, 35% and 37% respectively in 45 min. It indicated that, as RPM increases the conversion increases. This is because as the agitation increased the interfacial area between the organic and the aqueous layer, resulting in the rapid transfer of reactive species, which accelerated the overall reaction rates. Other hand the Figure 5b shown the nitration reaction to agitation speed at 0 RPM, 200 RPM, 250 RPM, 300 RPM, 350 RPM, 400 RPM and 600 RPM in presence of ultrasonic irradiation resulted in 15%, 30%, 50%, 75%, 100%, 60% and 42% conversion to nitrated cardanol within 45 min.
Figure 5a: Effect of agitation speed in the absence of ultrasound (Reaction condition: Cardanol (2.5 mmol), nitric acid 70% (5 mmol) calculated amount of water to make a particular concentration of nitric acid to 9 (wt%), TBAB (15 mole%), 1, 2-dichloroethane (20 cm3); temperature 25°C, time at 45 min.)
Figure 5b: Effect of agitation speed in the presence of ultrasound (Reaction condition: Cardanol (2.5 mmol), nitric acid 70% (5 mmol) calculated amount of water to make a particular concentration of nitric acid to 9 (wt%), TBAB (15 mole%), 1, 2-dichloroethane (20 cm3); temperature 25°C, time at 45 min, 24 kHz operating frequency).
Overall, we can see that, in the absence of ultrasound and mechanical agitation only 0.5% conversion to nitro cardanol was obtained using 9% dil. HNO3 in 45 min at 25°C temperature. When we conducted the reaction under only mechanical agitation (in absence of ultrasound) at 350 RPM, the reaction reached 30% conversion with 17% yield of ortho nitro cardanol in 45 min, while, at 350 RPM in presence of ultrasound 100% conversion with 67% yield of ortho nitro cardanol in 45 min. The conversion obtained under the mechanical agitation (in absence of ultrasound) was 30% more (30% vs. 0.5% in 45 min.) than in the absence of ultrasound and mechanical agitation. It means that, there is the vital role of mechanical agitation (macromixing) to increase the % yield of ortho nitro cardanol. As the reaction is in biphasic medium the mechanical agitation increases the interfacial area of contact between the two phases. However, this contact between the two phases happened at an only macro level, not at the micro level. So, when the RPM was increased up to 600 RPM the in the presence of ultrasound conversion was found to be around 42% in 45 min. At higher agitation speed in presence of ultrasound the conversion is low, indicating limitation of macro level mixing [15] and so that it restricted the formation of active NO2 radicals due to microlevel mass transfer (interfacial instability created by ultrasound) by ultrasound [18]. So, we get lower conversion of nitrated cardanol at such high agitation (beyond 400 RPM). This indicates that, ultrasound cavitation and certain agitation playing speed gives combined synergistic effect with ultrasound the major role in the biphasic L-L system. This also could be due to the different time scale involved in mass transfer (fastest) and reaction rates (slower kinetics).
These results are clearly indicating that, in the presence of ultrasonic irradiation the product (ortho nitro cardanol) synthesis can be achieved much more effectively. It is only because of a uniform mixing at the microscopic level which improved selectivity and % yield of the ortho nitro cardanol as compared to only mechanical agitation. It is also important to note that, 3 orders of magnitude higher power with ultrasound for the reaction was put into liquid as compared to the use of mechanical agitation alone. We have estimated the power consumed in both the cases (Appendix I). The power utilized in the sonication is 1.56 × 10-2 W/ml, while at 350 RPM agitation speed it is only 2.318 × 10-5 W/ml.
Effect of nitric acid concentration
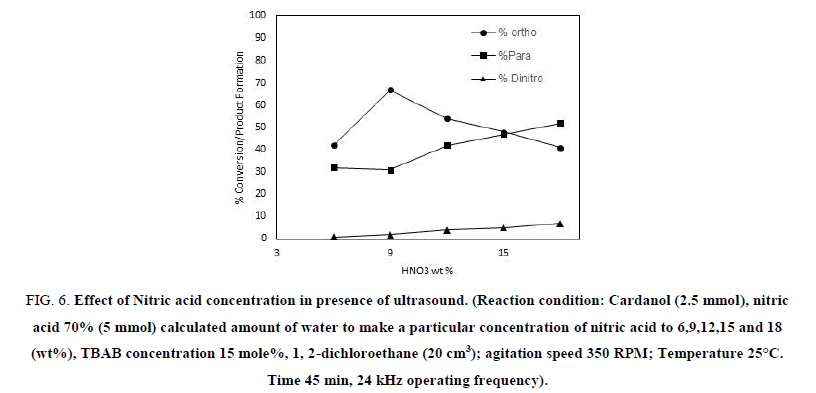
To study the effect of Nitric acid concentration on the rate of nitration of cardanol, experiments were carried out at 25°C. Experiments were carried out by varying the nitric acid concentration from 6% to 18% by wt. Figure 6 suggests that, as the nitric acid concentration increases, the ortho selectivity decreases and the percentage yield of other products (para and di nitro cardanol) increases over the same time period. At 6 wt% of nitric acid concentration the conversion was 75% in 45 min and ortho selectivity was 42%. Whereas, with 9 wt% of nitric acid concentration 100% conversion was obtained in 45 min while ortho selectivity increased to 67%. It can be seen from Figure 6 that the nitration rate of cardanol increases with further increase in the concentration of nitric acid over the range studied, however the ortho selectivity decreases. The higher concentration of nitric acid increased the concentration of active NO2 radicals in the presence of ultrasound, reducing the selectivity to ortho nitro cardanol and also results in the formation of some Di-nitro cardanol product.
Figure 6: Effect of Nitric acid concentration in presence of ultrasound. (Reaction condition: Cardanol (2.5 mmol), nitric acid 70% (5 mmol) calculated amount of water to make a particular concentration of nitric acid to 6,9,12,15 and 18 (wt%), TBAB concentration 15 mole%, 1, 2-dichloroethane (20 cm3); agitation speed 350 RPM; Temperature 25°C. Time 45 min, 24 kHz operating frequency).
Effect of catalyst loading (TBAB)
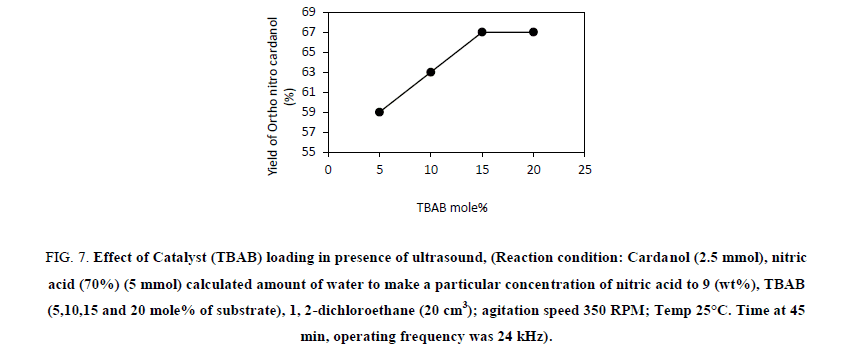
The effect of catalyst loading on the nitration of cardanol was studied at 25°C. For this, the catalyst concentration was kept at 5 mole%, 10 mole%, 15 mole% and 20 mole% by wt. Figure 7 suggests that as the catalyst concentration increases, percentage yield of ortho nitro cardanol increases at optimum reaction conditions. It can be clearly seen from Figure 7 that, at 5 mole% concentration of TBAB 59% conversion achieved in 45 min. with 40% yields of ortho nitro cardanol. At 10 mole% TBAB catalyst concentrations 63% conversion obtained in 45 min. with 42% yield of ortho nitro cardanol. While at 15 mole% of catalyst concentrations 100% conversion is achieved in 45 min with 67% selectivity of ortho nitro cardanol. Beyond 15 mole% catalyst concentration the selectivity to ortho nitro cardanol does not change. It can be seen in Figure 7 that, at 20 mole%, of TBAB, 100% conversion is achieved in 45 min with 67% ortho selectivity. As the amount of TBAB increased from 5-20 mole% the rate of conversion with the selectivity of ortho cardanol increased. An explanation for this can be that, as the amount of TBAB increases the transfer rate of nitronium ion from aqueous to organic layer also increases. Vivekanand et al. have reported that, the rate of reaction increased with an increase in the amount of quaternary ammonium salt until an optimum loading [19]. Also, in the presence of ultrasound the microagitation is generated at the interfacial region due to intense cavity collapse or oscillations of acoustically generated cavities. The role of PTC is also assisted by the creation of an additional interface area for contact between the aqueous and the organic layer.
Figure 7: Effect of Catalyst (TBAB) loading in presence of ultrasound, (Reaction condition: Cardanol (2.5 mmol), nitric acid (70%) (5 mmol) calculated amount of water to make a particular concentration of nitric acid to 9 (wt%), TBAB (5,10,15 and 20 mole% of substrate), 1, 2-dichloroethane (20 cm3); agitation speed 350 RPM; Temp 25°C. Time at 45 min, operating frequency was 24 kHz).
Effect of solvent
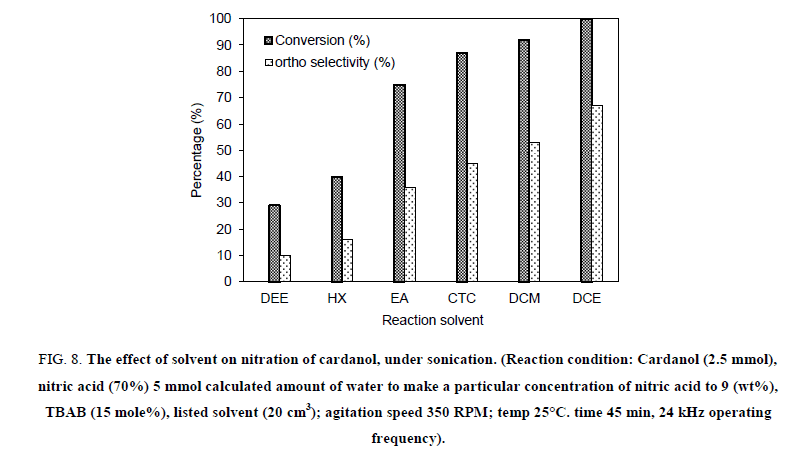
The solvent plays an important key role in the completion of the reaction so we have checked the effect of various solvents on the rate of nitration reaction. We have performed the solvent study using six solvents, namely diethyl ether (DEE), n-hexane (HX), ethyl acetate (EA), carbon tetrachloride (CTC), methylene dichloride (DCM), and ethylene dichloride (DCE). As shown in Figure 8, it has been observed that, the maximum conversion of nitrated cardanol with highest ortho nitro cardanol selectivity is observed in the presence of ethylene dichloride. Whereas the use of other solvents like methylene dichloride, ethyl acetate, diethyl ether, carbon tetrachloride and n-heptane resulted in lower conversion and selectivity as compared to ethylene dichloride. The solvent can affect the PTC reaction in several ways, an increase in the solvent polarity would result in a small decrease in the rate of reaction. As the solvent polarity increases, ionization of tetrabutylammonium nitrate also increases [18]. We also carried out the reaction without the solvent. In this experiment, the reaction occurred and resulted in the formation of polymeric tarry material. While in the presence of ethylene dichloride the reaction proceeds without the formation of polymeric material. Ethylene dichloride was found to be a most effective solvent giving a higher percentage yield of ortho nitro cardanol. The acoustic cavitation created by ultrasonic irradiation is also known to get significantly affected by the physio-chemical properties and the vapour pressure of the solvent. This is also likely to affect the magnitude of interfacial area in biphasic system.
Figure 8: The effect of solvent on nitration of cardanol, under sonication. (Reaction condition: Cardanol (2.5 mmol), nitric acid (70%) 5 mmol calculated amount of water to make a particular concentration of nitric acid to 9 (wt%), TBAB (15 mole%), listed solvent (20 cm3); agitation speed 350 RPM; temp 25°C. time 45 min, 24 kHz operating frequency).
Reaction Kinetics
The system under investigation in this work is the reaction between hydrogenated cardanol in the organic phase with dil. HNO3 and PTC in aqueous phase. The reaction kinetics were estimated on the following assumptions,
(i) Acceleration of single electron transfer
(ii) Improved mass transfer, increased with effect of phase transfer catalysis in the L-L system
(iii) Homolytic fragmentation to radical’s generation of the excited state
(iv) Disrupture of solvent structure, thus alternating solvation of the reactants.
The cavitation creates physical effects include local turbulence and circulation, which favours the mass transfer and intensifies the reaction which is essentially a mass transfer limited. Sonication has also resulted in chemical effects such as the generation of hot spots and reactive species such as free radicals that intensify the chemical processes limited by intrinsic chemical kinetics [20].
Nitration of phenol followed first order reaction kinetics using dil. Nitric acid as reported by Nandurkar et al. [10]. So that we have considered the nitration of cardanol also to follow first order kinetics in biphasic medium. For the L-L system, the reactant A is distributed in the organic phase and at the interface but not in the bulk aqueous phase due to its insolubility. While synthesis of ortho nitro cardanol can be represented as,
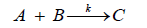
Where A is hydrogenated cardanol, B is nitric acid and C is ortho nitro cardanol. Rate expression for this reaction may be expressed that,
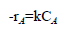 (1)
(1)
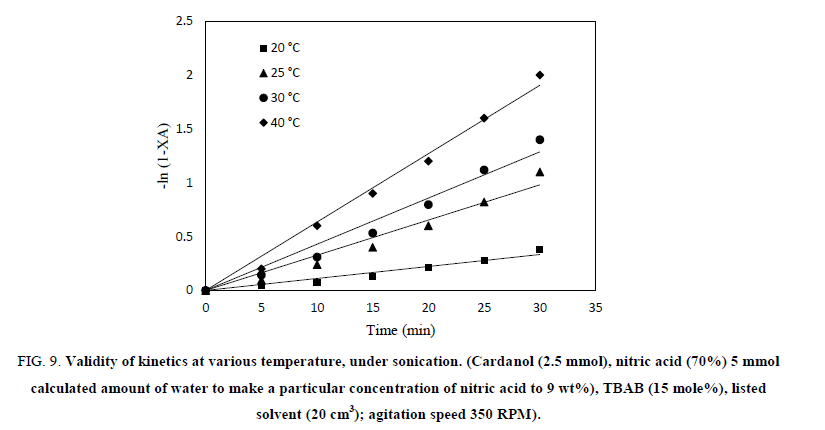
The plot of –ln (1-XA) vs. time has been shown in Figure 9 at various temperature to confirm the assumption of first order kinetics.
Figure 9: Validity of kinetics at various temperature, under sonication. (Cardanol (2.5 mmol), nitric acid (70%) 5 mmol
calculated amount of water to make a particular concentration of nitric acid to 9 wt%), TBAB (15 mole%), listed
solvent (20 cm3); agitation speed 350 RPM).
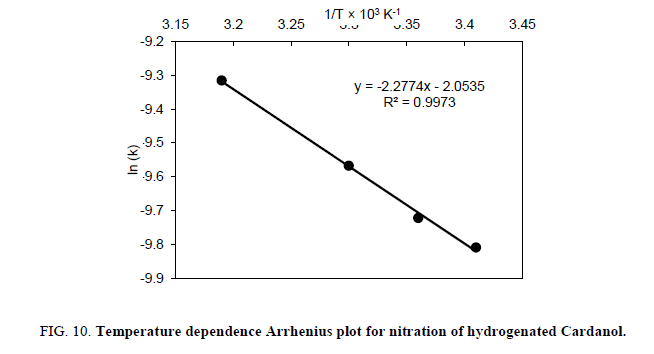
The different rate constant was estimated at various temperatures for the nitration of cardanol. This rate constants were used to estimate the activation energy and the frequency factor (Table 2) by Arrhenius plot. That Arrhenius plot gives the relation between the rate constant (k) at various temperature and energy of activation (E) as follows:
| Activation Energy (kcal/mole) | Frequency factor (min-1) |
|---|---|
| 4.5 | 9 × 105 |
Table 2. Activation energy and frequency factor.
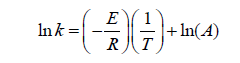
While the Arrhenius plot ln k against 1/T was the straight line obtained to determine the activation energy (E a) is shown in Figure 10. The slop of the line is related to the activation energy of the reaction and the intercept represent the apparent reaction frequency factor. Thus, the frequency factor of the rate constant has increased with ultrasound irradiation. The possible explanation for this is, linked to the absolute rate theory, where the reaction frequency factor is related to the vibratory motion of the reacting molecules [21]. The use of ultrasound may be responsible in generating tremendous local pressure gradient during cavitation which, in turn, may increase the vibratory motion of the reacting molecules. The activation energy of the reaction in the presence of ultrasound was 4.5 kcal/mole, which is significantly lower than the reported literature value 18.0 kcal/mole for a conventional approach [22-25].
In order to perform effective, selective nitration of hydrogenated cardanol using dilute nitric acid at various parameters such as catalyst loading (5%-20 mole%), nitric acid concentration (6%-15% by wt), temperature (20°C-40°C) and agitation (200-600) were studied. The results show that the nitration of cardanol was successfully achieved using 15 mole% PTC, TBAB. Using 9% nitric acid concentration nitration of cardanol, regioselectivity was successfully achieved under sonication condition at 24 kHz frequency with rated power of 150 W. The study revealed that, sonication is an efficient technology to synthesis the nitrated cardanol. The rate of reaction and the selectivity of ortho nitro cardanol formation in the presence of ultrasound is more than with only mechanical agitation. The combination of sonication and the mechanical agitation, are showing some synergistic effect. The use of PTC plays an important role in the synthesis of ortho nitro cardanol. PTC cannot be replaced by ultrasound, it plays an important role only in increasing the efficacy of PTC. The kinetic studies have clearly indicated that, the use of ultrasound has enabled the reduction the activation energy for the process showing some catalytic effects.
Acknowledgement
Authors would like to acknowledge the University Grants Commission (UGC) New Delhi, India for financial support.
References
- Gigante B, Prazeres AO, Marcelo-Curto MJ, et al. Mild and selective nitration by claycop. J Org Chem. 1995;60(11):3445-7.
- Olife IC, Jolaoso MA, Onwualu AP. Cashew processing for economic development in Nigeria. Agri J. 2013;8(1):45-50.
- Kozubek A, Tyman JH. Resorcinolic lipids, the natural non-isoprenoid phenolic amphiphiles and their biological activity. Chem Rev. 1999;99(1):1-26.
- Baroncini EA, Kumar Yadav S, Palmese GR, et al. Recent advances in bio?based epoxy resins and bio?based epoxy curing agents. J App Poly Sci. 2016.
- Mazzetto SE, Lomonaco D, Mele G. Cashew nut oil: Opportunities and challenges in the context of sustainable industrial development. Quimica Nova. 2009;32(3):732-41.
- Mele G, Del Sole R, Vasapollo G, et al. Polycrystalline TiO2 impregnated with cardanol-based porphyrins for the photocatalytic degradation of 4-nitrophenol. Green Chem. 2004;6(12):604-8.
- Minigher A, Benedetti E, De Giacomo O, et al. Synthesis and characterization of novel cardanol based benzoxazines. Natural Product Comm. 2009;4(4):521-8.
- Wasserman D, Dawson CR. Cashew nut shell liquid. VIII. A proof of structure of the mono-nitro and mono-amino derivatives of 3-pentadecylphenol (Hydrocardanol). J Am Chem Soc. 1950;72:4994-7.
- Latham HG, May EL, Mosettig E. Amino-and guanidino-pentadecylphenyl glucosides. J Org Chem. 1951;16:995-8.
- Dawson CR, David W. Inventors; Harvel Corp, assignee. Nitro-hydrogenated cardanols and process for preparing same. United States patent. US. 2,502,708. 1950.
- Nandurkar NS, Bhanushali MJ, Jagtap SR, et al. Ultrasound promoted regioselective nitration of phenols using dilute nitric acid in the presence of phase transfer catalyst. Ultrasonics Sonochemistry. 2007;14:41-5.
- Attanasi OA, Berretta S, Fiani C, et al. Synthesis and reactions of nitro derivatives of hydrogenated cardanol. Tetrahedron. 2006;62(25):6113-20.
- Kelkar MA, Gogate PR, Pandit AB. Process intensification using cavitation: Optimization of oxidation conditions for synthesis of sulfone. Ultrasonics Sonochem. 2006;13:523-8.
- Louis LJ. Synthetic organic sonochemistry. Springer Science & Business Media. 2013.
- Entezari MH, Keshavarzi A. Phase-transfer catalysis and ultrasonic waves II: Saponification of vegetable oil. Ultrasonics Sonochemistry. 2001;8:213-6.
- Mahamuni NN, Gogate PR, Pandit AB. Ultrasonic synthesis of benzaldehyde from benzyl alcohol using H2O2: Role of ultrasound. Indus and Eng Chem Res. 2006;45:98-108.
- Dubey SM, Gogate PR. Ultrasound assisted synthesis of 4-Benzyloxy-3-methoxybenzaldehyde by selective O-alkylation of vanillin with benzyl chloride in the presence of tetrabutylammonium bromide. Indus and Eng Chem Res. 2011;53:7979-85.
- Mason TJ. Large scale sonochemical processing: Aspiration and actuality. Ultrasonics Sonochem. 2000;7:145-9.
- Petrier C, Luche JL. Synthetic organic sonochemistry. Plenum Press, New York. 1998;pp:53-6.
- Nandurkar NS, Bhor MD, Samant SD, et al. Ultrasound-assisted regioselective nitration of phenols using dilute nitric acid in a biphasic medium. Ind and Eng Chem Res. 2007;46:8590-6.
- Vivekanand PA, Wang ML. Sonocatalyzed synthesis of 2-phenylvaleronitrile under controlled reaction conditions: A kinetic study. Ultrasonics Sonochem. 2011;18:1241-8.
- Deshmane VG, Gogate PR, Pandit AB. Process intensification of synthesis process for medium chain glycerides using cavitation. Chem Eng J. 2008;145:351-4.
- Chen J, Kalback JW. Effect of ultrasound on chemical reaction rate. Ind and Eng Chem Fund. 1967;6:175-8.
- Sheats GF, Strachan AN. Rates and activation energies of nitronium ion formation and reaction in the nitration of toluene in ∼78% sulphuric acid. Canadian J Chem. 1978;56:1280-3.
- Ramezanian MS, Padmaja S, Koppenol WH. Nitration and hydroxylation of phenolic compounds by peroxynitrite. Chem Res in Toxicol. 1996;9:232-40.