Review
, Volume: 9( 1)Sensors Based on Biomimetic Porphyrin Derivatives and their Hybrid Combinations
- *Correspondence:
- Fagadar-Cosma E Institute of Chemistry Timisoara of Romanian Academy, M. Viteazul Ave, No. 24, 300223, Timisoara, Romania
Tel: 40256491818; Fax: 40256491824; E-mail: efagadar@yahoo.com
Received: December 21, 2017; Accepted: January 05, 2018; Published: January 09, 2018
Citation: Eugenia Fagadar-Cosma. Sensors Based on Biomimetic Porphyrin Derivatives and their Hybrid Combinations. Res Rev Electrochem. 2018;9 (1):111
Abstract
Porphyrins are usually hydrophobic π-conjugated macrocycles and as a consequence organic building blocks for supramolecular chemistry, providing in this way the required optoelectronic and morphological properties for several types of sensors. Besides, porphyrins possess huge capacity for chemical functionalization by peripheral substitution and by using them in hybrid combination with polymers, photonic, electronic and magnetic compounds. Polymeric membranes incorporating porphyrins and wide band absorption hybrid materials were prepared from different porphyrins and metalloporphyrins and silver or gold nanoparticles with the purpose to develop optical and electrochemical detection of hydrogen peroxide, gases or anions and cations. These hybrids showed their detection potential in early medical diagnosis, in establishing pharmaceutical compounds toxicological limits or in monitoring of technical processes.
Keywords
Porphyrins; Hybrid materials; Silver and gold nanoparticles; Magnetic nanoparticles; Polymers; Sensors; UV-vis spectroscopy; Fluorescence; Electrochemistry
Introduction
What are porphyrins?
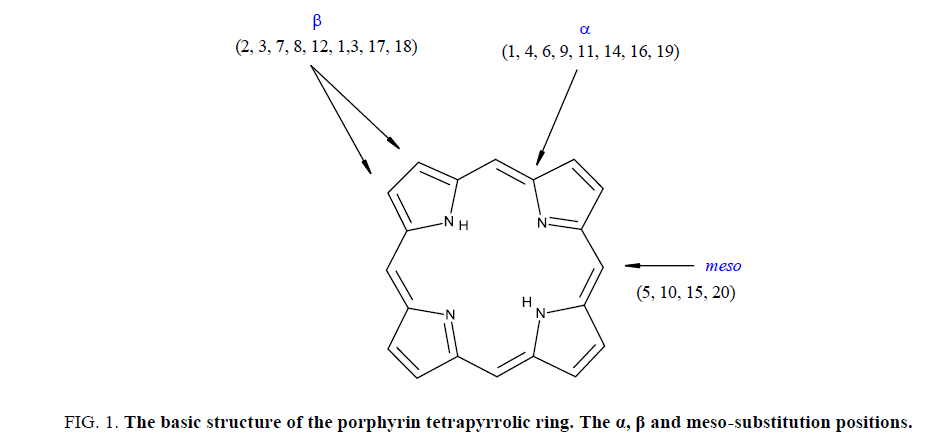
Porphyrins are aromatic macrocyclic structures [1,2] consisting of four pyrrolic rings linked in α position by four methynic groups [FIG. 1]. From the total number of 22 π electrons, 18 of them are involved in extended aromaticity (Hückel’s rule).
FIG 1: The basic structure of the porphyrin tetrapyrrolic ring. The α, β and meso-substitution positions.
Meso-substituted porphyrins derivatives are capable to mimic [3,4] most of the natural porphyrins functions such as optical and electronic modifications and reversible binding of metal ions (porphyrin bases) or anions (metalloporphyrins), volatile organic compounds (VOCs) or other ligands [5].
Porphyrins and their derivatives are of tremendous interest in many crucial processes because they are stable and their electronic properties might be modified by chelating of a metal ion or by different peripheral substitution on the macrocycle.
They are fascinating and important architectures, both because of their complex structure and of their amazing photophysical properties [6] that recommend their applications in photodynamic therapy of cancer (PDT) [7] and photonic applications [8]. Some optoelectronic applications require for self-organization [9] and porphyrins are particularly suitable for supramolecular assembling [10,11].
Supramolecular chemistry [12] allows the precise assembly of randomly oriented molecules into highly ordered supramolecular structures by means of delicate balance of weak non-covalent interactions. Higher efficiency of the charge separation than in the non-linked compounds [13] was obtained by irradiation of a functionalized structure containing a photosensitive porphyrin and possessing also binding sites for silver ions. The irradiation leads to the transfer of one electron from the porphyrin to Ag (as acceptor) and generates a porphyrinium cation of long lifetime. In order to improve the spectroscopic performances of porphyrin derivatives (an absorption that could be more red-shifted to increase its compatibility with in vivo applications) careful tuning of molecular structure revealed that longer lifetime is obtained in environments in which the planarization of the probe is favored [14].
The replacement of the donor groups (deplanarizing) by acceptor groups (planarizing) should increase the push-pull dipole in flipper structures and thus increase the red shift of the emission from the planarized excited state. Such intramolecular competition between planarizing and deplanarizing interactions in the ground state has already been beneficial for polythiophene DNA sensors [15].
Supramolecular chemistry plays a key role in a number of research fields, such as chemical and material science, as well as biology. When reversible, noncovalent enough strong and highly directional interaction took place between molecules, the self-assembly is similar with supramolecular polymerization [16]. The incorporation of nanomaterials into thin films, deposited on different types of surfaces leads to new functionalities. The thin films of porphyrin derivatives (and of their inorganic-, polymer-or silica-hybrid nanomaterials) rose great attention due to their potential uses as catalysts, sensors and actuators. Balancing hydrophobic/hydrophilic properties of porphyrins by grafting different functional groups make them the best candidates for self-assembling [17] by weak Van der Waals forces or hydrophobic effects, hydrogen bonding and π-π stacking interactions that finally produced highly organized nanostructures [18].
Experimental
Sensors: General information
A chemical sensor is a device that transforms certain information such as: presence of an anion or cation, concentration or chemical activity into an analytically measurable signal [19]. The sensors used in chemistry are divided into chemosensors, optical sensors, mass transducers, electrochemical (potentiometric, amperometric and voltammetric) and photoelectrochemical sensors [20].
Electrochemical sensors
Amperometric detection using porphyrins: A dual probe microsensor for detection of the NO released from cultured endothelial cells containing Ni (4-N-tetramethyl) pyridyl porphyrin [21] deposited onto the Pt electrode, was reported and served as amperometric NO sensor. Nitrite anion might be found in waters, foods and physiological processes and due to interaction with amines can form carcinogenic nitrosamines [22]. Excellent sensitivity to nitrite [23] was achieved using an amperometric sensor based on glassy carbon electrode modified with alternated layers of iron (III) tetra-(N-methyl-4-pyridyl)-porphyrin and cobalt (II) tetrasulfonated phthalocyanine. The porphyrin-modified electrode exhibits significant catalytic activity and stability for the nitrite oxidation, decreasing the peak potentials toward less positive values and displaying increased peak currents than those obtained on the bare GC electrode. A linear response in the interval of concentrations 0.2 mol L-1 –8.6 mol L-1 and a detection limit of 0.04 mol L-1 were reported.
Amperometric sensing of bromate (BrO3-) using a Fe-metalloporphyrin and polyelectrolytes together with oxidized multiwall carbon nanotubes (OMWCNTs) was optimized, related to the number of assembled layers, pH of the buffer solution and concentration of Fe-metalloporphyrin immobilized on the electrode surface. A linear response from 100 nM to 2.5 mM of BrO3- and a detection limit of 43 nM were successfully achieved [24].
Using as sensing material an array of ZnO nanorods coated with Cu (II) and Mn (III) Cl metallo-complexes of 5, 10, 15, 20-tetra (4-sulfonatophenyl)-porphyrin, L-cysteine concentration in water was determined on an amperometric measurement setup [25].
The incorporation of a cobalt ion in porphyrin ligands leads to versatile systems for redox purposes, allowing the exploitation of the redox activity of this metal ion when surrounded by supramolecular porphyrin environment. The studies evidenced the three possible processes, regarding the changes of CoIII–porphyrin to CoII–porphyrin then to CoI–porphyrin and finally to Co0–porphyrin. The Co-metalloporphyrin thin films were realized by matrix assisted pulsed laser evaporation onto silicon wafers, quartz plates and screen-printed electrodes [26]. The deposited layers have been characterized by cyclic voltammetry. Because Co changes its state of oxidation from Co2+ to Co3+ it can act as an effective mediator between dopamine and the CNT substrate.
Potentiometric detection using porphyrins: Porphyrin derivatives imposed themselves as ionophores in potentiometric ion selective membrane electrodes [27]. As a general rule, free base porphyrins, incorporated into polymeric membranes, act as neutral carriers and generate with almost all metallic cations the so-called sitting-a-top complexes [28]. In their turn, metalloporphyrins can readily recognize anions [29] and the anion selectivity can be improved by changing the central metal ion or the macrocycle structure. Metalloporphyrins act in ion selective membrane electrodes as Lewis acids (selective axial coordination) and do not act after Hofmeister selectivity patterns [30].
Nevertheless, exceptions are reported, polymerized porphyrin films containing only one pyrrole group per monomer molecule showed a selectivity pattern similar to the Hofmeister series: SCN>ClO4>NO3>Br>Cl [31a, b, c]. Nowadays, metalloporphyrins were tested as neutral and charged ionophores in solvent polymeric membrane electrodes, for the detection of hydrophilic anions [32].
Reported experience [33] in electronic tongues are distinctively different and are based on a solid electrical contact made from a conductive composite formed by epoxy resin and conducting graphite and the formulated electrodes were used for the determination of alkaline ions.
Sensor arrays based on electropolymerized layers of metalloporphyrins used for electronic noses have also been described [34]. The Mn (III), Fe (III), Co (II), Ni (II) derivatives of 5, 10, 15, 20-tetrakis (3-methoxy-4-hydroxyphenyl) porphyrin and the porphyrin base were tested to the five compounds chosen as representative of the five basic taste sensations, as follows: NaCl for salty, glucose for sweet, HCl for sour, quinine for bitter and monosodium glutamate for umami. The results are different depending on the nature of the metal. The porphyrine base shows the highest sensitivity to glucose and the lower to NaCl, Co-TMHPP has a reversed behavior, meaning a strong sensitivity to NaCl and the less to glucose and both Mn and Ni-metalloporphyrin have a high sensitivity to quinine and a low sensitivity to glucose.
Finally, the use of biosensors of the potentiometric type for certain specific applications, such as for the simultaneous determination of urea, ammonium, potassium and sodium, are to be mentioned [35]. In pharmaceutical analysis manganese (III)-tetraphenylporphyrin chloride incorporated in poly (vinyl) chloride matrices were used for detection of penicillin-G [36] and valproate [37] and manganese tetrakis (3, 5-bis (t-butyl) phenyl) porphyrin chloride showed good selectivity for paracetamol [38].
Starting from this knowledge, some Mn-metalloporphyrins such as: manganese (III) tetraphenylporphyrin chloride and manganese (III)-tetrakis (3-hydroxyphenyl) porphyrin chloride, have been used as ionophores to develop ion selective membrane electrodes for the detection of diclofenac, that is an intensively used analgesic, anti-inflammatory and antipyretic drug [39]. The sensing material was incorporated either in poly (vinyl) chloride or in sol-gel matrices and the best selectivity was achieved for the poly (vinyl) chloride membrane electrode that gave a linear response in the concentration range 3 × 10-6-1 × 10-2 M with a detection limit of 1.5 × 10-6 M. Manganese (III)-tetrakis (3-hydroxyphenyl) porphyrin chloride provided a super-Nernstian slope, explained by its dimerization in the membrane, probably due to the interactions of hydroxyl substituents.
In order to monitor Fe3+ ions from different samples, three A3B porphyrins with mixed functionalization: 5-(4-carboxyphenyl)-10, 15, 20-tris (4-phenoxyphenyl)-porphyrin, -(4-pyridyl)-10, 15, 20-tris (3, 4-dimethoxyphenyl)-porphyrin and 5-(4-pyridyl)-10, 15, 20-tris (4-phenoxy-phenyl)-porphyrin [40] were obtained [41] and used to prepare iron (III)-potentiometric sensors. The influence of the nature of peripheral groups grafted on porphyrin structure on the detection performance of Fe (III) ion was investigated. The best response, with a very good selectivity, was obtained with the porphyrin bearing a carboxyl group, which showed a near Nernstian response in a linear range from 1 × 10-7–1 × 10-1 M.
The detection of Cu (II) ions from recovery solutions from spent lithium batteries was successfully performed using as ionophore a symmetrical porphyrin, tetrakis (4-allyl-phenyl) porphyrin, for construction of a ion selective membrane electrode. The selectivity was tested toward the following ions: Co (II), Li (I), Al (III) and Fe (III) that are usually present in the recovery leach liquors [42]. Due to its chelating properties, this porphyrin was also investigated for the retention of copper from copper solutions and a maximum adsorption capacity of 280 mg/g was achieved.
Voltammetric detection using porphyrins: In volt-amperometric method because of the oxido-reduction processes developed at the electrodes, the current is flowing into the cell and the analyte concentration changes as a result of the oxidized or reduced species formed on the electrode surface. Voltametric sensors are based on the variation of the applied potential. A mercury-free voltametric sensor for detection of copper based on chemical accumulation of the trace metal onto the surface of glassy carbon electrode modified with tetraphenylporphyrin has been reported [43]. The ability of porphyrins and their hybrids to recognize and quantify compounds, such as: thrombin, neurotransmitters, glucose, oxygen, hydrogen peroxide, ascorbic acid, histamine and histidine with significance in early medical diagnosis or in food quality control has a tremendous importance [45-50].
The synergistic activity between gold nanoparticles and manganese (III) meso-tetrakis-(pentafluorophenyl) porphyrin recommend this system for the detection of L-cysteine [44] and for the electrochemical detection of thrombin [45]. MAPLE deposition of (5, 10, 15, 20-tetraphenyl) porphinato manganese (III) chloride thin films onto gold screen-printed electrodes, or on (111) Si substrates [46], were realized and presented globular structures with average diameters decreasing with laser fluence. These Mn (III)-metalloporphyrin thin films were investigated by cyclic voltammetry and proved to act as mediators for dopamine neurotransmitter bio/chemosensing.
Other types of thin films, consisting of asymmetrical functionalized 5-[o-(4-bromine-amyloxy) phenyl]-10, 15, 20-triphenylporphrin, were obtained by electropolymerization on a glassy carbon electrode (GCE) and the electrocatalytic response of thus prepared electrodes to dopamine oxidation were investigated. [47]. The porphyrin modified electrode shows high electrocatalytic activity and selectivity and a detection limit of 6 × 10-8M for dopamine.
In another experiment, glassy carbon electrodes modified with conjugate manganese porphyrin/gold nanoparticle films were obtained and proved to have great potential as electrochemical sensor for determination of hydrogen peroxide [48]. Recently, gold nanoparticles smaller than 20 nm were obtained and functionalized with Co (II) 5, 10, 15, 20-meso-tetra (3-hydroxyphenyl) porphyrin and the hybrid material was exposed to increased amounts of H2O2 and has proven its UV-vis and voltammetric sensing capacity [49]. In order to verify if the range of physiologically-relevant H2O2 concentration levels (5 μM-35 μM) might be detected, three modified glassy carbon (GC) electrodes were realized, by deposition onto their surfaces of nAu and Co-porphyrin (alone and in successive layers) and were tested to evidence the electrochemical response for H2O2. The comparative linear and cyclic voltammetry of the bare and modified GC electrodes evidenced an increased electrocatalytic effect on the reduction of H2O2 onto GC electrode modified with nAu/Co-3OHPP ordered layers.
The hybrid material formed between Mn (III) tetratolyl-porphyrin chloride and spherical gold colloid has also the capacity to detect ascorbic acid by UV-vis spectroscopy in the range of concentrations 2.6 × 10-6 M to 4.38 × 10-5 M. Modified glassy carbon electrodes were obtained by thin film deposition of Mn-porphyrin [50]. In addition, the catalytic effect of Mn (III) tetratolyl-porphyrin chloride modified glassy carbon electrode on the ascorbic acid oxidation was demonstrated both by the increase of the peak current density in the voltammetric curves and the shifting towards more lower potentials.
The porphyrins with carboxyl functional groups, such as the A3B porphyrin, 5-(4-carboxyphenyl)-10, 15, 20-triphenylporphyrin are appropriate for detection of different amino-compounds. The above-mentioned porphyrin was electrochemically investigated as sensitive thin film for the trace detection of histamine, a chemical compound that is related with meat products quality. The film was deposited by MAPLE technique onto the carbon working electrode. The current density of the oxidations peaks displayed by cyclic voltammetry depends on the concentration of histamine and the detection limit of the sensor is within the range of (0–8) ppm histamine [51].
A sensitive sensor for determination of l-histidine was achieved by modifying gold electrodes with an asymmetrical Fe (III)-porphyrin, substituted with three 2, 6-di-tert-butylphenol groups and one palmitoyl long chain. Two methods were employed to realize the modified electrodes: chemisorption or embedment into dodecanethiol monolayer. Both types of electrodes were tested for detection of l-histidine using square-wave voltammetry and the results were compared. The determination of l-histidine using the electrode modified by embedment technique had higher precision than that obtained by chemisorption and the detection limit was 1nM l-histidine on artificial matrix mimicking human serum samples [52].
Photoelectrochemical detection
Porphyrin is unanimously recognized as a photoactive, light-absorbing chromophore and is used as an effective photosensitizer. Due to this property the porphyrins were firstly interfaced with inorganic matrices to form donor–acceptor complexes for light-harvesting systems [53] and bioinspired photodetectors, even wearable sunlight sensors [54]. The carboxylated graphene oxide based nanocomposites were constructed through π–π conjugation using zinc monoamino porphyrin Zn-5-(4-amino-phenyl)-10, 15, 20-tris-phenylporphyrin loaded with Au nanoparticles. ITO electrodes were modified with this hybrid nanomaterial and provided a good photocurrent response to 4-nitrophenol detection in river water sample matrices, when a potential, -0.1 V was applied. The light irradiation induces photoexcited electrons in porphyrin that are further injected into the conduction band of graphene oxide, followed by transfer to Au-nanoparticles and finally to the ITO support. Based on this synergic conductivity, a novel method for photoelectrochemical detection of 4-nitrophenol was developed with a linear range from 0.1 to 15 nmol/L and detection limit of 0.04 nmol/L [55].
An iron metalloporphyrin, namely: 5, 10, 15, 20-tetrakis (3, 4-dimethoxyphenyl)-porphyrin Fe (III) chloride functionalized with carbon nanotubes, was deposited from solution by Langmuir-Blodgett technique onto a ceramic substrate with interdigital platinum electrodes. The complex film comprised of five monolayers manifest sensitivity to UV radiation due to the fact that an electron is excited from a Fe d orbital into a porphyrin antibonding π orbital while the presence of SWCNT activates the charge carriers [56].
Because, carbon monoxide colorless and odorless gas is responsible for more than half of all fatal poisoning in the world and the symptoms (headache and confusion) cannot be easily discriminated, the well-known affinity of iron porphyrins toward CO is used for the design of small and cheap sensors that prevent both poisoning in familial and industrial environments. A complex sensing device was constructed based on a matrix, consisting in pyridyl-functionalized single walled carbon nanotube and a biomimetic 5, 10, 15, 20-tetraphenylporphyrin iron (III) perchlorate as the sensing element that can be reversed (activated or deactivated) by modifying the window voltage. The limit of detection was 80 ppm of CO in N2, lower than the industrial requirements for CO detectors [57]. The mechanism is attributed to reduction of Fe (III) to Fe (II) associated with a change of Fermi level in the single walled carbon nanotubes.
Recent progress exploits the photoactive nanomaterials [58] wide application potential [59]. Photostable porphyrin photosensitizers 5, 10, 15, 20-tetraphenylporphyrin and 5, 10, 15, 20-tetrakis-(N-methylpyridinium-4yl) porphyrin with high quantum yields were encapsulated in polystyrene nanoparticles and the hybrid nanomaterials were tested for monitoring of oxygen concentrations in situ in aqueous media in the broad region of oxygen concentrations from anaerobic conditions to oxygen saturated media [60]. The effect of temperature on the photophysical behavior must be thoroughly investigated [61] because the lifetime decreased with increasing of temperature.
Porphyrin sensitized transparent thin TiO2 films have been reported to be useful in a wide range of applications. The water soluble 5, 10, 15, 20-tetrakis (1-methyl-4-pyridinio) porphyrin tetra (p-toluene-sulfonate)/TiO2 coated optical fibers were tested into solutions of differing concentrations of ammonia in water [62] and the sensing system respond to concentrations of ammonia in water as low as 0.1 ppm, with a fast response time of less than 30 s.
The high toxicity of most organophosphorus derivatives is known, so that the detection of triphenylphosphine oxide was performed using Mn (III)-5, 10, 15, 20-tetratolyl-21H, 23H porphyrin chloride, as sensitive material. The change produced in UV-vis spectra of porphyrin, that is the decrease in the intensity of the Soret band, by increasing the concentration of triphenylphosphine oxide was investigated. The two isosbestic points at 483 nm and 487 nm respectively confirm the existence of two equilibrium processes due to interaction between the porphyrin and the phosphorus derivative, caused by the strong affinity of manganese to both oxygen and phosphorus atoms. The detection limit is 2.5 μg/mL [63].
Polydimethylsiloxane matrices are regarded as the ideal sensory support for porphyrins [64]. Optical oxygen sensors performing with fast response and high accuracy have been intensively developed, especially those based on the fluorescence quenching principle. A hybrid material based on Pt-5-(4-methoxy-carbonylphenyl)-10, 15, 20-triphenylporphyrin and 3-(trimethoxy-silyl) propyl methacrylate has a limit of detection of 4.7μmol/L for the determination of dissolved oxygen.
Results and Discussion
Optical, fluorescent and colorimetric sensors based on porphyrins
Fluorescent sensors: Fluorescent detection comprising steady-state fluorescence, time-resolved fluorescence and fluorescence dynamics developed with an amazing rate in the last years and is based on the monitoring of changes in fluorescence intensity, fluorescent lifetime and shape of fluorescence spectra.
A fast novel naked-eye and turn-on fluorescence sensor was proposed based on well-known fluorescent water soluble 5, 10, 15, 20-(4-sulphonatophenyl) porphyrin to selectively recognize and detect glutathione in real samples. The fluorescence of 5, 10, 15, 20-(4-sulphonatophenyl) porphyrin was quenched by Hg2+ in a one electron transfer process. The detection limit was 0.43 nM [65]
A highly sensitive and selective fluorescent sensor for the detection of sulfide anion was constructed based on the benefits of steric hindrance effect that lead to efficiently quenching of the fluorescence of fluorescein [68]. The recognition unit consists of dinitrobenzene sulfonate ester group grafted on aromatic ortho-position in the 5-(2-hydroxylphenyl)-10, 15, 20-triphenylporphyrin. Alzheimer's disease, Down's syndrome and diabetes are only a few diseases that might be produced due to the exposure to high levels of sulfide that were proved to act as neuromodulator in the brain [69]. The detection limit was estimated to be 59 nM which is convincingly less than the maximum recommended sulfide concentration (around 15 μM) in drinking water by World Health Organization [70].
A NIR fluorescent selective sensor for the detection of sulfide anion was based on a different approach [71]. A traping group, 2, 4-dinitrobenzenesulfonate ester, was integrated into the fluorescent porphyrin 5-hydroxy-10, 15, 20 triphenyl porphyrin, decreasing its fluorescence. By increasing the concentration of the sulfide anion, the group 2, 4-dinitrobenzenesulfonate ester is cleaved and this generates the recovery of the fluorescent properties of the non-grafted porphyrin.
Another chemosensor with outstanding potential for medical areas, suitable for detecting Cd2+ in living cells, was designed based on further functionalization of a cis derivative, namely: 5, 10-bis (4-aminophenyl)-15, 20-diphenylporphyrin [72]. The Cd2+ ions generate the increase of emission intensity at 611 nm and accompanied by a decrease of emission intensity at 653 nm. The other physiologically significant metal ions acting in living cells, such as Na+, K+, Ca2+ and Mg2+ do not changed the emission spectrum. The detection limit of the sensor was 0.032 μM.
Ratiometric fluorescence measurements [73] have only recently been conducted to detect metal ions. This method involves monitoring of changes in the emission at two distinctive wavelengths, improving in this way, the selectivity and sensitivity of the detection.
Considering the biological importance of zinc, the development of novel improved ratiometric fluorescent Zn2+ sensors become a demand [74]. The proposed fluorescent material is based on a porphyrin grafted in one of phenyls in the meso-position with a strong chelator bearing three nitrogen atoms, namely 5-[4-(aminoethylene) amino]-10, 15, 20-triphenylporphyrin. This sensitive material is changing its fluorescence band from 650 nm to 610 nm during Zn2+ addition and is highly selective for zinc in the physiological pH range having a detection limit of 1.8 mM.
Two symmetrical porphyrin compounds meso-tetrakis (2-hydroxynaphthyl) porphyrin and meso-tetra (2-thiophene) porphyrin were comparatively investigated regarding their fluorescent response to Hg2+. An efficient fluorimetric membrane based on the fluorescence quenching of meso-tetra (2-thiophene) porphyrin in the presence of Hg2+ ion was successfully achieved. The membrane containing meso-tetra (2-thiophene) porphyrin reversibly responded to Hg2+ in a range of concentrations from 5.0 × 10-9 M to 1.25 × 10-5 M. This fluorescent sensor is highly selective and was used to the determination of mercury content in biofenac medicine, recommended for eye treatment [75].
Another comparative fluorimetric determination of mercury ions was reported using 5, 10, 15, 20-tetra (p-sulfonatophenyl) porphyrin that is a water soluble porphyrin and by using porphyrin doped sol-gel films. Optimization of complexation parameters resulted in the determination of mercury ions with a detection limit of 1.4 μg/1 Hg (II), but the sol-gel hybrids prepared in acid catalysis were not sensitive to Hg (II) [76].
Sensing of oxygen is a great demand because human beings can live maximally 10 min without supply of oxygen (the average need is 200 g/day) [77]. Besides, for thinking personalized PDT treatment of cancer molecular oxygen in irradiated tissues has to be strictly controlled [78].
A fluorescence complex sensing system comprises two units: an ionophore, with function of linking and a fluorophore that is capable to change emission characteristics. A novel conjugate fluorescent system for detection of low O2 concentrations in the range of 40-90 μM was formed between 5, 10, 15, 20-tetrakis (3, 4-dimethoxyphenyl)-porphyrin Fe (III) chloride with binding properties and the highly fluorescent (5, 10, 15, 20-tetraphenylporphinato) dichlorophosphorus (V) chloride [79]. It was established that a concentration of H2O2 higher than 50 μM is cytotoxic for life, so that this system might be relevant for medical diagnosis of many diseases such as Alzheimer, Parkinson, Huntington [80] even cancer [7].
A mesoporous silica-Mn-porphyrin hybrid nanomaterial, obtained by sol-gel method in acid-base catalysis and its application for fluorescent detection of H2O2 was studied at the emission wavelength of 655 nm. The exposure of the silica-Mn-porphyrin hybrid to H2O2 led to major changes regarding the optical, textural and morphological properties of the material. The high sensitivity for hydrogen peroxide in wet media noticed for this hybrid, accompanied by changes in color, justifies the using this material [81].
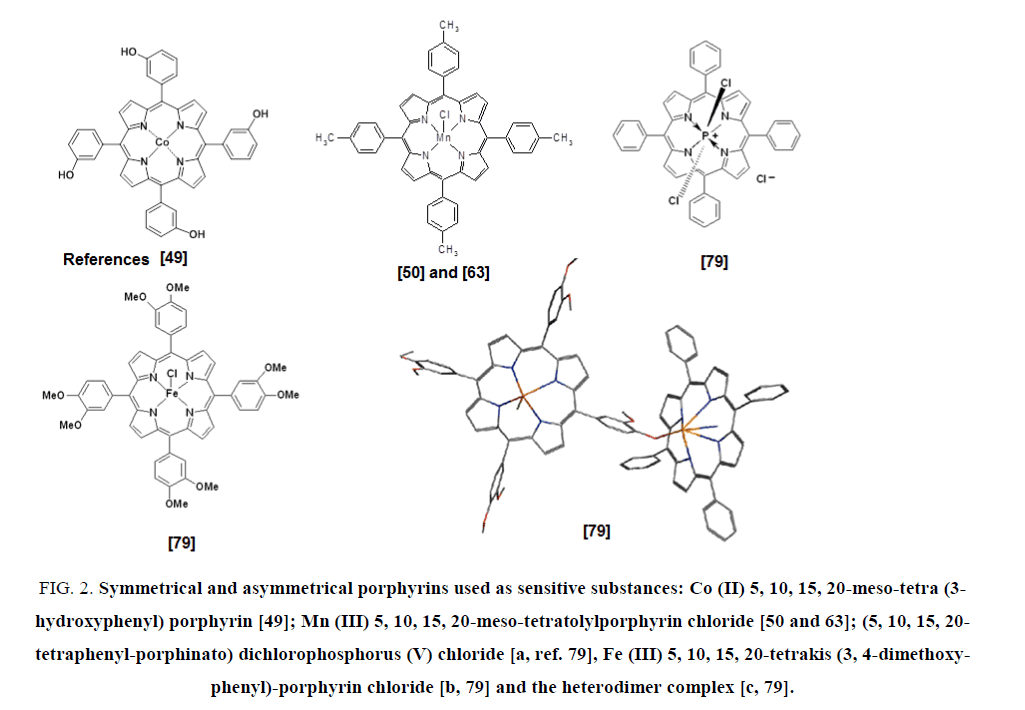
FIG. 2 illustrates a few structures of porphyrins and metalloporphyrins used for the formulation of sensors.
FIG 2: Symmetrical and asymmetrical porphyrins used as sensitive substances: Co (II) 5, 10, 15, 20-meso-tetra (3-hydroxyphenyl) porphyrin [49]; Mn (III) 5, 10, 15, 20-meso-tetratolylporphyrin chloride [50 and 63]; (5, 10, 15, 20-tetraphenyl-porphinato) dichlorophosphorus (V) chloride [a, ref. 79], Fe (III) 5, 10, 15, 20-tetrakis (3, 4-dimethoxy-phenyl)-porphyrin chloride [b, 79] and the heterodimer complex [c, 79].
Colorimetric sensors
A terpyrrolic analog of dipyrrolylquinoxaline (DPQ) that contains two pyrrole anion recognition groups acts as an improved colorimetric and fluorescent sensor for halide and dihydrogen phosphate anions in organic media [82].
A facile method to prepare the 5, 10, 15, 20-tetrakis (4-carboxyl phenyl) porphyrin functionalized ZnS nanoparticles [83] of 5 nm–8 nm in size, was reported and the material was proved to catalyze the colorimetric oxidation of 3, 3′, 5, 5′-tetramethylbenzidine with H2O2 that suffer decomposition to ·OH radicals. Finally, a sensitive colorimetric assay to detect H2O2 in the range of concentration from 0.01 to 0.06 mM was achieved.
Hydrogen peroxide detection is important in biology because its involvement in many enzymatic reactions. Nanoparticles of porphyrin functionalized ceria was used as a colorimetric sensing material for H2O2 detection, due to intrinsic peroxidase activity toward a classical substrate, 3, 3′, 5, 5′-tetramethylbenzidine. If this material is coupled with glucose oxidase, glucose can be also detected. A detection limit of 1.9 × 10-2 mM glucose was reported [84].
Polymeric solid-state CO2 sensors based on a long-decay phosphorescent Pt-porphyrin and a pH indicator dye, α-naphtholphthalein [85,86] were optimized related to CO2 sensitivity, response and recovery times. The application is destined for food packaging industry. A water-soluble polymer-dye hybrid material, with sensing properties, was prepared by incorporating of a mixed substituted A3B porphyrin 5-(4-pyridyl)-10, 15, 20-tris (4-phenoxy-phenyl) porphyrin into the biocompatible polyvinylpyrrolidone polymer [87]. The UV-vis spectrophotometric response of the hybrid material to increasing CO2 amounts, revealed a continuous decrease of the intensity of the Soret absorption band. The color changed from yellow to red in the presence of trace amounts of gas. AFM and TEM microscopy illustrated morphologic changes of the hybrid material surface which lead to the conclusion that the mechanism of CO2 recognition is based on chemisorption phenomena instead of changes in the acidity of the environment, as previously expected.
The awareness of the importance of gold nanoparticles and of their hybrids formed with biomimetic porphyrins for sensing, conducted to obtaining of a hybrid between gold and 5-(4-pyridyl)-10, 15, 20-tris (4-phenoxyphenyl)-porphyrin destined for the detection of CO2. The complex showed wide absorption in the whole UV-vis spectrum, the hybrid band was bathochromically shifted in comparison to gold plasmonic band and proved to be efficient for the CO2 detection in wet environment. The hybrid has double functionality because during its synthesis it can be considered as optical sensor for trace amounts of gold [88].
The same group of researchers, obtained and characterized another A3B mixed substituted porphyrin, namely: 5-(4-pyridyl)-10, 15, 20-tris (3, 4-dimethoxyphenyl) porphyrin and used it to develop novel CO2 sensitive nanomaterials. The presence of pyridyl group in the meso position of the macrocycle led to some distortions of the planar shape and increased the self-assembling properties, proving to be useful for the sensing performances [89].
Multifunctional materials based on 5-(4-carboxyphenyl)-5, 10, 15-tris (4-phenoxyphenyl)-porphyrin, iron oxide magnetic nanoparticles, a silica linker and a polysaccharide, k-carrageenan, were developed. All synthesized materials were tested as catalysts for the Knoevenagel condensation of aldehydes at room temperature. The best performing porphyrin-based catalyst has also the capacity to optically detect CO2 in the range of 35-190 μM of gas concentrations [90].
A fluorescent and reversibly naked-eye colorimetric sensor destined for hydrogen chloride detection was developed in a facile design. The 5, 10, 15, 20-tetraphenylporphyrin was entrapped in a polystyrene membrane. Exposure to HCl gas turns the color from pink to yellow-green. The detection limit is in the range of very low concentrations, 46 ppb and the response time is prompt, in only 5 s [91].
Multianalyte detection by a single porphyrin derivative represent the new trend to discriminate between toxic ions [92 ] different gas compounds [93] and relevant compounds for medicine [94]. β-Dicyanovinyl substituted porphyrinogen acts as a selective and reversible material for fast colorimetric recognition of picric acid and other aromatic nitro substituted being able to detect picric acid with a detection limit of 1.12 ppm (4.99 μM) [95].
A new structure, 10-bromo-15-phenyl-5-(pyridin-2-yl) porphyrin was reported to show good thermochromic reversibility in solution accompanied by a color change from yellow to red. A good solvatochromic behavior of this porphyrin to the axial ligation of coordinating solvents was observed. The axial coordination studies suggested that CN-and F-ions were detected colorimetrically and optically [96].
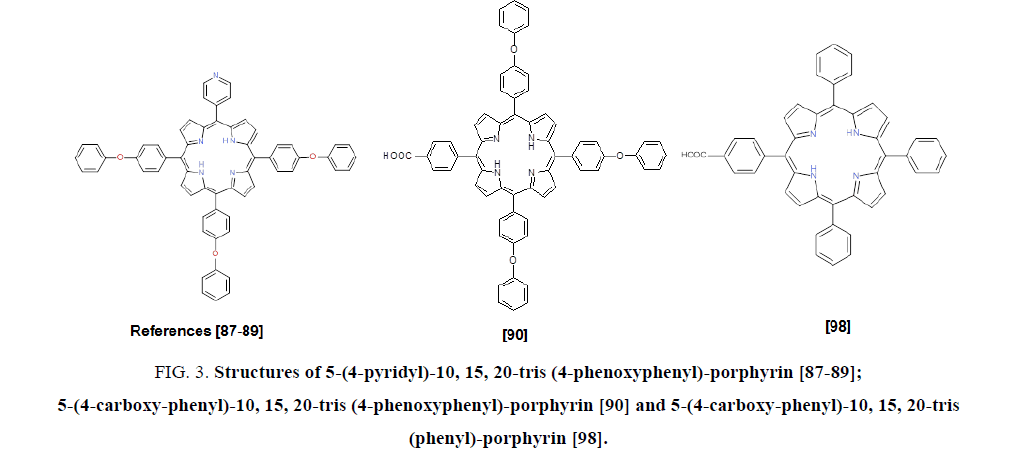
FIG. 3 presents three asymmetric porphyrins used for the design of sensors [87-90,98].
Optical pH sensors
A porphyrin-polymer hybrid material was obtained by the nucleophilic reaction to chlorobenzyl groups of a polysiloxane performed by an asymmetrical hydroxy-functionalized porphyrin, 5-(4-hydroxyphenyl)-10, 15, 20-triphenyl-porphyrin. Both the pure porphyrin and the porphyrin modified polymer proved to be fluorescence pH sensors in acid domain, between 3 and 5.5 [97].
A novel fluorescent pH sensor, in acid media, pH from 1.5 to 5.5, based on 5-(4-carboxy-phenyl)-10, 15, 20-tris (phenyl)-porphyrin, as sensing agent, has been developed. The proposed fluorescent sensor can measure pH in the presence of several metal ions that recommend its use in leaching solutions of the recyclable processes of valuable metals [98]. The potential of a water soluble metalloporphyrin, 5, 10, 15, 20-tetrakis (N-methyl-4-pyridyl) porphyrin-Zn (II) tetrachloride to act as an optical pH sensor in the 5.5-10.5 domain was also proved [99].
Conclusions and Future Perspectives
The well-known physical-chemical versatility of porphyrins and their metalloporphyrins was exploited to design a large variety of sensors capable to selectively detect heavy metals, anions, volatile organic compounds, pharmaceutical and toxic compounds and relevant molecules for medical diagnosis.
The sensing properties of porphyrin derivatives can be enhanced by their own supramolecular organization or by tailoring novel hybrid materials based on porphyrins (silica, polymer, plasmonic materials or heterodimers or trimers). A certain property of the matrix (transparency, conductance) will be enhanced by the porphyrin partner (fluorescence, wide absorbance range), so that the materials will find novel applications in sensing. The novel techniques to realize mono-layer or multi-layer films by deposition through LB, LS or PLD and MAPLE methods contribute to the quality of the films. As a conclusion the field of porphyrin applications is infinite both regarding the novel hybrid materials to be obtained and with respect to the targets to be detected.
Some promising trends in porphyrin-based sensors involve their future applications in dual-mode opto-electrochemical systems [31b]. Being able to mimic human receptors, porphyrins will find novel applications in multisensory analysis [31c].
References
- Taniguchi M, Lindsey JS. Handbook of Porphyrin Science. In: Kadish KM, Smith KM, Guilard R, editors. World Scientific: Singapore. Enumeration of Isomers of Substituted Tetrapyrrole Macrocycles: From Classical Problems in Biology to Modern Combinatorial Libraries. 2012;23:1-80.
- Lindsey JS. The Porphyrin Handbook. Kadish KM, Smith KM, Guilard R, editors. Academic Press: San Diego, CA. Synthesis of meso-substituted porphyrins. 2000;1:45-118.
- Chandrashaker V, Ptaszek M, Taniguchi M, et al. Synthesis of diverse acyclic precursors to pyrroles for studies of prebiotic routes to tetrapyrrole macrocycles. New J Chem. 2016;40:8786-808.
- Soares AR, Taniguchi M, Chandrashaker V, et al. Expanded combinatorial formation of porphyrin macrocycles in aqueous solution containing vesicles. A prebiotic model. New J Chem. 2013;37:1073-86.
- Biesaga M, Pyrzynska K, Trojanowicz M. Porphyrins in analytical chemistry. A review. Talanta. 2000;51:209–24.
- Senge MO, Stafford S. Getting it right: 3D cell cultures for the assessment of photosensitizers for photodynamic therapy. Future Med Chem. 2015;7 (15):1957-60.
- Filatov MA, Senge MO. Molecular devices based on reversible singlet oxygen binding in optical and photomedical applications. Mol Syst Des Eng. 2016;1:258-72.
- Mielke J, Hanke F, Peters MV, et al. Adatoms underneath single porphyrin molecules on Au (111). J Am Chem Soc. 2015;137 (5):1844-9.
- Di Natale C, Monti D, Paolesse R. Chemical sensitivity of porphyrin assemblies. Mater Today. 2010;13 (7-8):46-52.
- Făgădar-Cosma E, Făgădar-Cosma G, Vasile M, et al. Synthesis, spectroscopic and self-assembling characterization of novel photoactive mixed aryl-substituted porphyrin. Curr Org Chem. 2012;16 (7):931-41.
- Tarabukina E, Fagadar-Cosma E, Enache C, et al. Molecular properties and aggregation of porphyrin modified polysiloxane in solutions. Journal of Macromolecular Science Part B: Physics. 2013;52 (8):1077-91.
- Lehn J-M. Perspectives in supramolecular chemistry-from molecular recognition towards molecular information processing and self-organization. Angew Chem Int Ed Engl. 1999;29 (11):1304-19.
- Gubelmann M, Harriman A, Lehn J-M, et al. Quenching of porphyrin excited states by silver (I) ions and charge separation in bimolecular systems and in macropolycyclic coreceptors. J Phys Chem. 1990;94 (1):308-15.
- Dal Molin M, Verolet Q, Soleimanpour S, et al. Mechanosensitive membrane probes. Chem Eur J. 2015;21 (16):6012-21.
- Dal Molin M, Verolet Q, Colom A, et al. Fluorescent flippers for mechanosensitive membrane probes. J Am Chem Soc. 2015;137 (2):568-71.
- Aida T, Meijer EW, Stupp SI. Functional supramolecular polymers. Science. 2012;335 (6070):813-77.
- Drain C, Varotto M, Radivojevic I. Self-organized porphyrinic materials. Chem Rev. 2009;10 (5)9:1630-58.
- Birdeanu M, Fagadar-Cosma E. Quantum Nanosystems: Structure, Properties and Interactions. Apple Academics Press, Toronto-New Jersey Chapter 5: The Self-Assembly of Porphyrin Derivatives into 2D and 3D Architectures. 2014;173-206.
- Hulanicki A, Glab S, Ingman F. Chemical sensors: Definitions and classification. Pure Appl Chem. 1991;63 (9):1247-50.
- Paolesse R, Nardis S, Monti D, et al. Porphyrinoids for Chemical Sensor Applications. Chem Rev. 2017;117 (4):2517-83.
- Isik S, Etienne M, Oni J, et al. Dual microelectrodes for distance control and detection of nitric oxide from endothelial cells by means of scanning electrochemical microscope. Anal Chem. 2004;76 (21):6389-94.
- Tricker AR, Preussmann R. Carcinogenic N-nitrosamines in the diet: occurrence, formation, mechanisms and carcinogenic potential. Mutat Res. 1991;259 (3-4):277-89.
- Wilney JR, Santos AL, Sousa RCS, et al. Amperometric sensor for nitrite using a glassy carbon electrode modified with alternating layers of iron (III) tetra-(N-methyl-4-pyridyl)-porphyrin and cobalt (II) tetrasulfonated phthalocyanine. Talanta. 2006;70 (3):588-94.
- Lee Y-G, Lee HJ, Jang A. Amperometric bromate-sensitive sensor via layer-by-layer assembling of metalloporphyrin and polyelectrolytes on carbon nanotubes modified surfaces. Sensors and Actuators B: Chemical. 2017;244:157-66.
- Y, Pudi R, Lvova L, et al. The light modulation of the interaction of L-cysteine with porphyrins coated ZnO nanorods. Sensors and Actuators B: Chemical. 2015;209:613-21.
- Cristescu R, Popescu C, Popescu AC, et al. Functional porphyrin thin films deposited by matrix assisted pulsed laser evaporation. Materials Science and Engineering B. 2010;169 (1-3):106-10.
- Paolesse R, Monti D, Nardis S, et al. The Porphyrin Handbook. Kadish KM, Smith KM, Guilard R, editors. World Scientific Publishing, Singapore Porphyrin based chemical sensors.2011;12:121-226.
- Bobacka J, Ivaska A, Lewenstam A. Potentiometric ion sensors. Chem Rev. 2008;108 (2):329-51.
- Górski Ł, Malinowska E, Parzuchowski P, et al. Recognition of anions using metalloporphyrin-based ion-selective membranes:state-of-the-art. Electroanalysis. 2003;15 (15-16):1229-35.
- Vlascici D, Fagadar-Cosma E, Bizerea-Spiridon O. A new composition for Co (II)-porphyrin-based membranes used in thiocyanate-selective electrodes. Sensors (Basel). 200;6 (8):892-900.
- (a) Lvova L, Mastroianni M, Di Natale C, et al. Towards hyphenated sensors development: Design and application of porphyrin electropolymer materials. Electroanalysis. 2012;24 (4):776-89. (b) Lvova L, Galloni P, Floris B, et al. A ferrocene-porphyrin ligand for multi-transduction chemical sensor development. Sensors. 2013;13 (5):5841-56.
- (c) Lvova L, Di Natale C, Paolesse R. Porphyrin-based chemical sensors and multisensor arrays operating in the liquid phase. Sensors and Actuators B. 2013;179:21-31.
- Fagadar-Cosma G, Vlascici D, Fagadar-Cosma E. 5, 10, 15, 20-Tetrakis (4-pyridyl)-21, 23H-porphyrin-Zn (II)- iodide-selective ionophore in formulation of new polymeric membrane electrodes. J Biol Inorg Chem. 2007;12:218.
- Gallardo J, Alegret S, De Roman MA, et al. Determination of ammonium ion employing an electronic tongue based on potentiometric sensors. Anal Lett. 2003;36 (114):2893-908.
- Paolesse R, Di Natale C, Burgio M, et al. Porphyrin based array of cross-selective electrodes for analysis of liquid samples. Sens Actuators B. 2003;95 (1-3):400-5.
- Gutie´rrez M, Alegret S, del Valle M. Potentiometric bioelectronic tongue for the analysis of urea and alkaline ions in clinical samples. Biosens Bioelectron. 2007;22 (9-10):2171-8.
- Santos EMG, Araújo AN, Couto CMCM, et al. Ion selective electrodes for penicillin-G based on Mn (III) TPP-Cl and their application in pharmaceutical formulations control by sequential injection analysis. J Pharm Biomed Anal. 2004;36 (4):701-9.
- Santos EMG, Araújo AN, Couto CMCM, et al. Construction and evaluation of PVC and sol-gel sensor membranes based on Mn (III) TPP-Cl. Application to valproate determination in pharmaceutical preparations. Anal Bioanal Chem. 2006;384 (4):867-75.
- Saraswathyamma B, Pajak M, Radecki J, et al. PVC supported liquid membrane and carbon paste potentiometric sensors incorporating a Mn (III)-porphyrin for the direct determination of undissociated paracetamol. Electroanalysis. 2008;20:2009-15.
- Vlascici D, Pruneanu S, Olenic L, et al. Manganese (III) porphyrin-based potentiometric sensors for diclofenac assay in pharmaceutical preparations. Sensors. 2010;10 (10):8850-64.
- Vlascici D, Fagadar-Cosma E, Popa I, et al. A novel sensor for monitoring of iron (III) ions based on porphyrins. Sensors. 2012;12 (6):8193-203.
- Fagadar-Cosma E, Fagadar-Cosma G, Vasile M, et al. Synthesis, spectroscopic and self-assembling characterization of novel photoactive mixed aryl-substituted porphyrin. Curr Org Chem. 2012;16 (7):931-41.
- Vlascici D, Popa I, Chiriac VA, et al. Potentiometric detection and removal of copper using porphyrins. Chemistry Central Journal. 2013;7:111.
- Frey HH, McNeil CJ, Keay RW, et al. Characterization of a copper detecting amperometric electrode. Electroanalysis. 1998;10:480-5.
- Gallo MC, Pires BM, Toledo KCF, et al. The use of modified electrodes by hybrid systems gold nanoparticles/Mn- porphyrin in electrochemical detection of cysteine. Synthetic Met. 2014;198:335-9.
- Zheng Y, Yuan Y, Chai Y, et al. A label-free electrochemical aptasensor based on the catalysis of manganese porphyrins for detection of thrombin. Biosens Bioelectron. 2015;66:585-9.
- Cristescu R, Popescu C, Popescu AC, et al. MAPLE deposition of Mn (III) metalloporphyrin thin films: Structural, topographical and electrochemical investigations. Appl Surf Sci. 2011;257 (12):5293-7.
- Ye F, Nan J, Wang L, et al. The ultrasonic electropolymerization of an 5-[o-(4-bromine amyloxy) phenyl]-10, 15, 20-triphenylporphrin (o-BrPETPP) film electrode and its electrocatalytic properties to dopamine oxidation in aqueous solution. Electrochimica Acta. 2008;53 (12):4156-60.
- Sebarchievici I, Taranu BO, Birdeanu M, et al. Electrocatalytic behavior and application of manganese porphyrin/gold nanoparticle-surface modified glassy carbon electrodes. Appl Surf Sci. 2016;390:131-40.
- Fagadar-Cosma E, Sebarchievici I, Lascu A, et al. Optical and electrochemical behavior of new nano-sized complexes based on gold-colloid and Co-porphyrin derivative in the presence of H2O2. J Alloys Compd. 2016;686:896-904.
- Sebarchievici I, Lascu A, Fagadar-Cosma G, et al. Optical and electrochemical mediated detection of ascorbic acid using manganese porphyrin and its gold hybrids. Comptes Rendus Chimie. 2017; In press.
- Iordache AM, Cristescu R, Fagadar-Cosma E, et al. Histamine detection using functionalized porphyrin as electrochemical mediator. Comptes Rendus Chimie. 2017; In press.
- Kurzątkowska K, Shpakovsky D, Radecki J, et al. Iron (III) porphyrin bearing 2, 6-di-tert-butylphenol pendants deposited onto gold electrodes for amperometric determination of l-histidine. Talanta. 2009;78 (1):126-31.
- Yella A, Lee HW, Tsao HN, et al. Porphyrin-sensitized solar cells with cobalt (II/III)-based redox electrolyte exceed 12 percent efficiency. Science. 2011;334:629-34.
- Pyo S, Kim W, Jung H-I, et al. Heterogeneous integration of carbon-nanotube–graphene for high-performance, flexible and transparent photodetectors. Small. 2017;13 (27):918.
- Ma X, Wu Y, Devaramani S, et al. Preparation of GO–COOH/AuNPs/ZnAPTPP nanocomposites based on the π–π conjugation: Efficient interface for low-potential photoelectrochemical sensing of 4-nitrophenol. Talanta. 2018;178:962-9.
- Baschir, L, Fagadar-Cosma E, Creanga I, et al. UV sensing effect in Langmuir-Blodgett complex films containing a novel synthesized Fe (III) porphyrin. Dig J Nanomat Bios. 2014;9 (2):847-57.
- Swager TM, Savagatrup S, Schröder V, et al. Bio-inspired carbon monoxide sensors with voltage-activated sensitivity. Angew Chem Int Ed. 2017;56 (45):14066-70.
- Henke P, Lang K, Kubát P, et al. Polystyrene nanofiber materials modified with an externally bound porphyrin photosensitizer. ACS Appl Mater Interfaces. 2013;5 (9):3776-83.
- Vinklarek IS, Scholz M, Dedic R, et al. Singlet oxygen feedback delayed fluorescence of protoporphyrin IX in organic solutions. Photochem Photobiol Sci. 2017;16 (4):507-18.
- Kubát P, Henke P, Berzediová V, et al. Nanoparticles with embedded porphyrin photosensitizers for photooxidation reactions and continuous oxygen sensing. ACS Appl Mater Interfaces. 2017;9 (41):36229-38.
- Suchánek J, Henke P, Mosinger J, et al. Effect of temperature on photophysical properties of polymeric nanofiber materials with porphyrin photosensitizers. J Phys Chem B. 2014;118 (23):6167-74.
- Tiwari D, Mullaney K, Korposh S, et al. An ammonia sensor based on Lossy Mode Resonances on a tapered optical fibre coated with porphyrin-incorporated titanium dioxide. Sensors and Actuators B: Chemical. 2017;242:645-52.
- Palade A, Lascu A, Creanga I, et al. Triphenylphosphine oxide detection in traces using Mn (III)-5, 10, 15, 20- tetratolyl-21H, 23H porphyrin chloride. Dig J Nanomat Bios. 2015;10 (3):729-35.
- Mao Y, Zhao Q, Pan T, et al. Platinum porphyrin/3-(trimethoxysily) propyl-methacrylate functionalized flexible PDMS micropillar arrays as optical oxygen sensors. New J Chem. 2017;41 (13):5429-35.
- Chen J, Ma Q, Hu X, et al. Design of a novel naked-eye and turn-on fluorescence sensor based onthe 5, 10, 15, 20- (4-sulphonatophenyl) porphyrin (TPPS4)-Hg2+system:Monitoring of glutathione (GSH) in real samples and DFT calculation. Sensors and Actuators B. 2018;254:475-482.
- Li Y-H, Wang H, Li J-S, et al. Simultaneous intracellular β-d-glucosidase and phosphodiesterase I activities measurements based on a triple-signaling fluorescent probe. Anal Chem. 2011;83 (4):1268-74.
- Yu L, Wang S, Huang K, et al. Fluorescent probes for dual and multi analyte detection. Tetrahedron. 2015;71 (29):4679-706.
- Chen G, Tang M, Fu X, et al. A highly sensitive and selective fluorescent sensor for detection of sulfide anion based on the steric hindrance effect. Journal of Molecular Structure. 2018;1151:230-5.
- Eto K, Asada T, Arima K, et al. Brain hydrogen sulfide is severely decreased in Alzheimer's disease. Biochem Biophys Res Commun. 2002;293 (5):1485-8.
- Fiorucci S, Antonelli E, Mencarelli A, et al. The third gas: H2S regulates perfusion pressure in both the isolated and perfused normal rat liver and in cirrhosis. Hepatology. 2005;42 (3):539-48.
- Cheng FM, Wu XS, Liu ML, et al. A porphyrin-based near-infrared fluorescent sensor for sulfur ion detection and its application in living cells. Sens Actuators B Chem. 2016;228:673-8.
- Huang W-B, Gu W, Huang H-X, et al. A porphyrin-based fluorescent probe for optical detection of toxic Cd2+ ion in aqueous solution and living cells. Dyes and Pigments. 2017;143:427-35.
- Lv Y, Cao M, Li J, et al. A sensitive ratiometric fluorescent sensor for zinc (II) with high selectivity. Sensors. 2013;13 (3):3131-41.
- Li J, Yin C, Huo F. Development of fluorescent zinc chemosensors based on various fluorophores and their applications in zinc recognition. Dyes and Pigments. 2016;131:100-33.
- Shamsipur M, Sadeghi M, Beyzavi MH, et al. Development of a novel fluorimetric bulk optode membrane based on meso-tetrakis (2-hydroxynaphthyl) porphyrin (MTHNP) for highly sensitive and selective monitoring of trace amounts of Hg2+ ions. Materials Science and Engineering: C. 2015;48 (1):424-33.
- Plaschke M, Czolk R, Ache HJ. Fluorimetric determination of mercury with a water-soluble porphyrin and porphyrin-doped sol-gel films. Analytica Chimica Acta. 1995;304 (1):107-13.
- Wang X, Wolfbeis OS. Optical methods for sensing and imaging oxygen: Materials, spectroscopies and applications. Chem Soc Rev. 2014;43:3666-761.
- Zang L, Zhao H, Hua J, et al. Ratiometric oxygen sensing using the tunable ratio of phosphorescence to fluorescence emissions from gadolinium porphyrin and porphyrin. J Luminescence. 2017;183:452-7.
- Fagadar-Cosma E, Badea V, Fagadar-Cosma G, et al. Trace oxygen sensitive material based on two porphyrin derivatives in a heterodimeric complex. Molecules. 2017;22 (10):1787.
- Jeong H, Kim S, Seol H, et al. Determination of hydrogen peroxide on modified glassy carbon electrode by polytetrakis (2-aminophenyl) porphyrin nanowire. Bull Korean Chem Soc. 2009;30 (12):2979-83.
- Lascu A, Palade A, Fagadar-Cosma G, et al. Mesoporous manganese-porphyrin–silica hybrid nanomaterial sensitive to H2O2 fluorescent detection. Mater Res Bull. 2016;74:325-32.
- Shevchuk SV, Lynch VM, Sessler JL. A new terpyrrolic analogue of dipyrrolylquinoxalines: An efficient optical- based sensor for anions in organic media. Tetrahedron. 2004;60 (49):11283-91.
- Liu Q, Chen P, Xu Z, et al. A facile strategy to prepare porphyrin functionalized ZnS nanoparticles and their peroxidase-like catalytic activity for colorimetric sensor of hydrogen peroxide and glucose. Sensors and Actuators B: Chemical. 2017;251:339-48.
- Liu Q, Yang Y, Lv X, et al. One-step synthesis of uniform nanoparticles of porphyrin functionalized ceria with promising peroxidase mimetics for H2O2 and glucose colorimetric detection. Sensors and Actuators B: Chemical. 2017;240:726-34.
- Amao Y, Komori T. Optical CO2 sensor of the combination of colorimetric change of [alpha]-naphtholphthalein in poly (isobutyl methacrylate) and fluorescent, porphyrin in polystyrene. Talanta. 2005;66:976-81.
- Borchert NB, Kerry JP, Papkovsky DB. A CO2 sensor based on Pt-porphyrin dye and FRET scheme for food packaging applications. Sensors and Actuators B. 2013;176:157-65.
- Fagadar-Cosma E, Tarabukina E, Zakharova N, et al. Hybrids formed between polyvinylpyrrolidone and an A3B porphyrin dye: Behaviour in aqueous solutions and chemical response to CO2 presence. Polym Int. 2016;65 (2):200- 9.
- Fagadar-Cosma E, Lascu A, Palade A, et al. Hybrid material based on 5-(4-pyridyl)-10, 15, 20-tris (4- phenoxyphenyl)-porphyrin and gold colloid for CO2 detection. Dig J Nanomat Bios. 2016;11 (2):419-24.
- Fagadar-Cosma E, Vlascici D, Fagadar-Cosma G, et al. A sensitive A3B porphyrin nanomaterial for CO2 detection. Molecules. 2014;19 (12):21239-52.
- Mak CA, Pericas MA, Fagadar-Cosma E. Functionalization of A3B-type porphyrin with Fe3O4 MNPs. Supramolecular assemblies, gas sensor and catalytic applications. Catal Today. 2017; In press.
- Hu M, Kang W, Zhao Y, et al. A fluorescent and colorimetric sensor based on a porphyrin doped polystyrene nanoporous fiber membrane for HCl gas detection. RSC Adv. 2017;7:26849-56.
- Ding Y, Zhu W-H, Xie Y. Development of ion chemosensors based on porphyrin analogues. Chem Rev. 2017;117 (4):2203-56.
- Wagner T, Haffer S, Weinberger C, et al. Mesoporous materials as gas sensors. Chem Soc Rev. 2013;42 (9):4036- 53.
- Jentzsch AV, Hennig A, Mareda J, et al. Synthetic ion transporters that work with anion-π interactions, halogen bonds and anion-macrodipole interactions. Acc Chem Res. 2013;46 (12):2791-800.
- Chahal MK, Sankar M. β-Dicyanovinyl substituted porphyrinogen: Synthesis, reversible sensor for picric acid among explosives and unique sensor for cyanide and fluoride ions by switching between various porphyrinoid states. Dalton Trans. 2017;12;46 (35):11669-78.
- Jeong J, Kumar RS, Naveen M, et al. Synthesis, thermochromic, solvatochromic and axial ligation studies of Zn- porphyrin complex. Inorganica Chimica Acta.2018;469:453-60.
- Grama S, Hurduc N, Fagadar-Cosma E, et al. Novel porphyrin-based polysiloxane micromaterial. Digest Journal of Nanomaterials and Biostructures. 2010;5 (4):959-73.
- Fagadar-Cosma E, Vlascici D, Birdeanu M, et al. G. Novel fluorescent pH sensor based on 5-(4-carboxy-phenyl)-10, 15, 20-tris (phenyl)-porphyrin. Arabian Journal of Chemistry. 2014: In press.
- Fagadar-Cosma E, Creanga I, Maranescu B, et al. Dependence of optical response on pH of a water-soluble Zn (II)- metalloporphyrin. Dig J Nanomater Bios. 2011;6 (1):75-80.