Original Article
, Volume: 17( 1) DOI: 10.37532/0972-768X.2019.17(1).299Properties of Chitosan-based pH-Sensitive Superabsorbent Polymer Modified Oil Well Cement
- *Correspondence:
- Bu Y , Key Laboratory of Unconventional Oil and Gas Development, College of Petroleum Engineering, China University of Petroleum (East China), Qingdao, PR China, E-mail: buyuhuan@163.com
Abstract
Poor zonal isolation due to microcracks in cement sheath is a common problem that affects oil and gas exploration and also impacts the environment. Chitosan-based superabsorbent polymer (CSAP) was synthesized and was used as a self-healing agent for sealing microcracks of cement sheath. CSAP was prepared by solution polymerization. The effect of glutaraldehyde (GA) dosage and chitosan (CS) concentration on the stability of CSAP was studied. It was determined that GA addition should be greater than 0.15% and the CS concentration should be less than 3%. The water absorbing performance of CSAP was pH sensitive and had a pH-sensitive threshold of 10. The pH sensitivity of CSAP ensured that the stability of the cement slurry was less affected, and the properties of cement slurry met the requirements of cementing construction. The CSAP modified oil well cement would guarantee the health, safety and high efficiency in the process of oil and gas production.
Keywords
Oil well cement; Chitosan; Superabsorbent polymer, pH sensitivity; Self-healing cement
Introduction
Cementitious material, as a kind of inorganic gelling materials, has proved to be a suitable material in cementing job of oil fields, because of its easy to use and comparatively low cost. Cement sheath is very important for the normal production of oil wells, including functions of cementing casing into the borehole, preventing casing from corrosion, and isolating fluids and gases between formations. Therefore, the integrity of cement sheath should be maintained during the production life of oil wells. However, the tensile strength of cement stone is very low, compared with the compressive strength. It is easy to form microcracks in cement sheath under internal stress and external load. As a result, fluids and gases channeling through microcracks can cause annulus pressure, interlayer crossflow, casing corrosion, and so on. All of these problems have serious implications for security, environment and production. The usual measure to stop channeling is squeeze cementing, which is expensive and always cannot block, because of the difficulties to determine the location of the microcracks. It seems that repairing these microcracks becomes impossible.
Over recent decades, the concept of self-healing cement has been conducted through numerous scientific studies and has had a lot of achievements [1-5]. Superabsorbent polymers can absorb and retain large amounts of water, increasing their volume [6]. They have applications in various areas, such as medical care [7], agriculture [8-10], and food industry [11], among others. In recent years, they have often been used as self-healing agents in cement-based materials for internal curing [12-14] and microcrack healing. When microcracks form inside cement sheath, Superabsorbent polymers on the crack surface can absorb water that flows through the microcracks and will swell to prevent fluid channeling. In this paper, chitosan-based superabsorbent polymer (CSAP) was synthesized. First, the effect of GA dosage and CS concentration on the stability of CSAP was studied. The pH sensitivity of CSAP was investigated by testing the water absorbing rates in different pH solution. In addition, the properties of CSAP modified oil well cement were evaluated to see if they met the cementing requirements.
Materials and Methods
Materials
Chitosan (CS) was purchased from Shandong Laizhou Haili Biological Products Co., Ltd. Glutaraldehyde (25%), acetic acid (99.5%), ethanol (99.8%), sodium hydroxide (96%) were purchased from Sinopharm Chemical Reagent Co., Ltd., Shanghai, China. Distilled water was used throughout the experiments.
Preparation of chitosan-based superabsorbent polymer (CSAP)
Acetic acid and distilled water were added into a 250 mL flask, and the weighed CS was added in portions. The solution was stirred until the CS was completely dissolved. Subsequently, GA solution (0.25%) was added to the flask. After stirring at room temperature for 1 h, the solution was dried at 70°C for 24 hours to obtain CSAP.
Water absorption performance
The temperature used to test the water absorbing rate of CSAP was set to 75°C. The test procedure was as follows: An 800- mesh nylon net bag was prewetted with the test liquid (distilled water or other solutions). Weighed samples (about 0.1 g) were placed into the bag. The bag and samples were weighed together, and were then immersed in the test liquid at 75°C. After 24 h, the bag was taken out and suspended until droplets ceased dripping. Then, the bag and samples were weighed again. The water absorbing rate of CSAP is given by
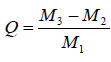
where Q represents the water absorbing rate of CSAP (g/g), M1 represents the weight of dry CSAP samples (g), M2 represents the weight of the nylon bag and dry CSAP samples (g), and M3 represents the weight of the nylon bag and CSAP samples saturated with test liquid (g).
pH sensitivity of CSAP was elucidated by comparing their water absorbing rates in different pH solutions (the pH values were set to 7-13), which were prepared by distilled water and sodium hydroxide. Cement slurry filtrate was prepared through static fluid loss test and was also used to test the water absorbing rate.
Properties of CSAP modified cement slurry
The formulations of cement slurry are shown in TABLE 1. The basic water to cement ratio (W/C) was set to 0.44 based on standard ISO 10426-1:2009. The mixing processes, thickening time tests, fluid loss tests, and compressive strength tests of cement slurry/stones were all conducted based on standard ISO 10426-2:2003.
| No. | Water-cement ratio | Retarder | Fluid Loss Agent | Dispersing Agent | CSAP |
|---|---|---|---|---|---|
| C0 | 0.44 | 0.5% | 5% | 1% | 0 |
| C1 | 0.44 | 0.5% | 5% | 1% | 1% |
| C2 | 0.44 | 0.5% | 5% | 1% | 3% |
| C3 | 0.44 | 0.5% | 5% | 1% | 5% |
Table 1: Mixing proportions of cement slurry.
Results and Discussion
Effect of GA addition and CS concentration on water absorption stability of CSAP
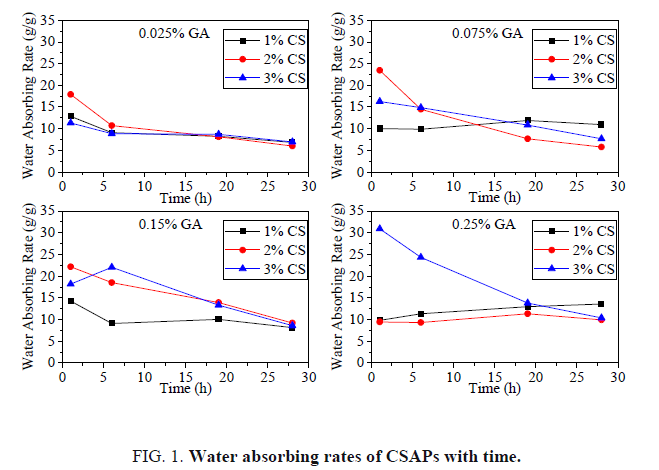
FIG. 1 shows the water absorbing rates of CSAPs. The concentrations of CS solution were 1%, 2% and 3%, respectively. The GA additions were 0.025%, 0.075%, 0.15% and 0.25% of CS, respectively. When the GA additions are 0.025%, 0.075% and 0.15%, the water absorbing rate of the CSAPs gradually decreases with time, indicating that the water absorbing performance of the CSAPs is unstable when GA addition is low. When the GA addition is 0.25%, the water absorbing rates of the CSAPs prepared by 1% and 2% CS solution gradually increase with time. However, the water absorbing rate of the CSAPs prepared by 3% CS solution decreases with time. This difference indicates that the water absorbing performance of the CSAPs is stable when the CS concentration is low, and the water absorbing performance of the CSAPs is unstable when the CS concentration is high. It can be seen from the above experimental results that the GA addition and the CS concentration were the main factors affecting the water absorption stability of the CSAPs. When the CS concentration was low, the CS molecular chains were well stretched in the acetic acid solution, so that they were sufficiently crosslinked by GA. When the CS concentration was increased, the stretch of CS molecular chains was limited. They were in a collapse state in solution, limiting the cross-linking effect of GA. The high concentration caused a partial uncross linking of the CSAP. During the water absorption performance test, a part of the CS molecular chains was dissolved in the solution, so that the water absorbing rates of the CSAP decreased with time.
It can be seen from the above experimental results that the GA addition and the CS concentration were the main factors affecting the water absorption stability of the CSAPs. When the CS concentration was low, the CS molecular chains were well stretched in the acetic acid solution, so that they were sufficiently crosslinked by GA. When the CS concentration was increased, the stretch of CS molecular chains was limited. They were in a collapse state in solution, limiting the cross-linking effect of GA. The high concentration caused a partial to uncross linking of the CSAP. During the water absorption performance test, a part of the CS molecular chains was dissolved in the solution, so that the water absorbing rates of the CSAP decreased with time. Similarly, when the GA concentration was low, the CS molecular chains were not well cross-linked, and some CS molecular chains were also dissolved during the water absorption performance test, resulting in the water absorbing rate of CSAP decreased with time. Therefore, in order to prepare a stable CSAP, the GA addition should be greater than 0.15% and the CS concentration should be less than 3%.
pH sensitivity of CSAP
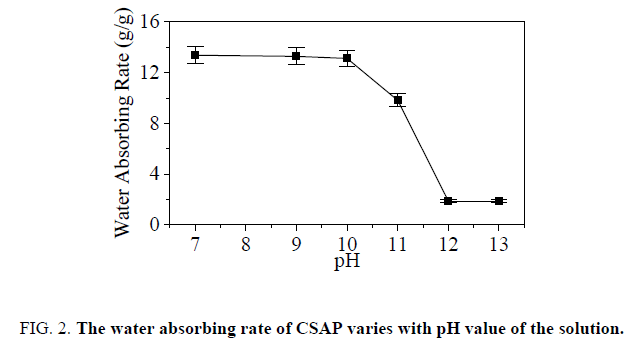
For CSAP prepared when GA addition was 0.25% and CS concentration was 1%, the water absorbing rate were tested in different pH solution. FIG. 2 shows the result. When the pH value of the solution is 7-10, the water absorbing rate of the CSAP remains unchanged, about 13.5 g/g. When the pH value of the solution is greater than 10, the water absorbing rate of the CSAP begins to decrease significantly. The water absorbing rate reaches a minimum, 1.87 g/g, when the pH of the solution is 12-13. The results indicated that the CASP was pH-sensitive, absorbing more water under neutral and weak alkaline solution and less water under strong alkaline solution. The reason why the CSAP was pH-sensitive was determined by the amino groups (-NH2) on the CSAP. When the aqueous solution was neutral or weakly alkaline, -NH2 groups ionized into -NH3+. Due to the repellency of the charged molecular segments, the molecular segments were mutually exclusive and absorbed more water. When the aqueous solution was strongly alkaline, -NH2 groups didn’t ionize. Molecular chain shrinkage greatly reduced the amount of water absorbed by the CSAP. Therefore, it is possible to achieve less water absorption in the cement slurry and more water absorption in the formation water.
Water absorption performance in cement slurry filtrate (CSF)
The pH of the CSF was measured to be 12.6. The water absorbing rate of CSAP was tested in NaOH solution (pH=12.6) and CSF. The results were 1.87 and 1.56 g/g respectively. At the same pH value, the water absorbing rate of the CSAP in the CSF was smaller than that in the NaOH solution. This is because the CSF had calcium ion, which further reduced the ionization of the -NH2 group in the solution and reduces the stretching of the CS molecules in the solution, thereby reducing the water absorbing rate.
Effect of CSAP on the thickening performance of oil well cement slurry
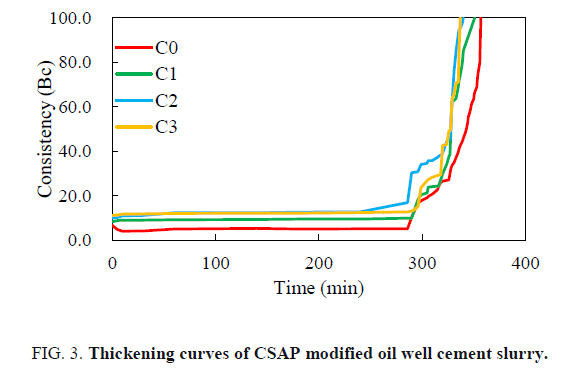
The effect of CSAP on the thickening performance of cement slurry was investigated by comparing the thickening curves obtained with different CSAP addition. The result is shown in FIG. 3. It can be seen that the initial consistency of the cement slurry increases with the increase of the CSAP addition. The addition of the CSAP has little effect on the total thickening time. The curves show obvious right-angle thickening performance. The thickening performance meets the cementing requirements.
Effect of CSAP on the water loss performance of oil well cement slurry
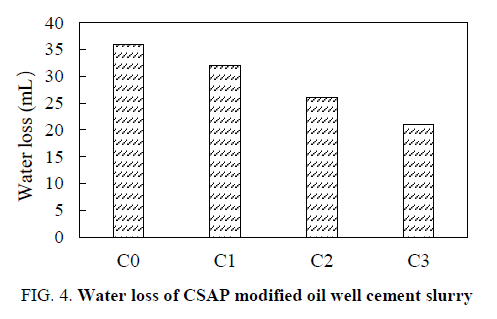
FIG. 4 shows the water loss of CSAP modified oil well cement slurry. The addition of the CSAP reduces the water loss of the cement slurry. The larger the amount of the CSAP, the lower the water loss. This is mainly because the added CSAP absorbed a small amount of water in the cement slurry, and the viscosity of the cement slurry was slightly increased, thereby reducing the water loss of the cement slurry. The water loss of the cement slurry added CSAP meets the cementing requirements.
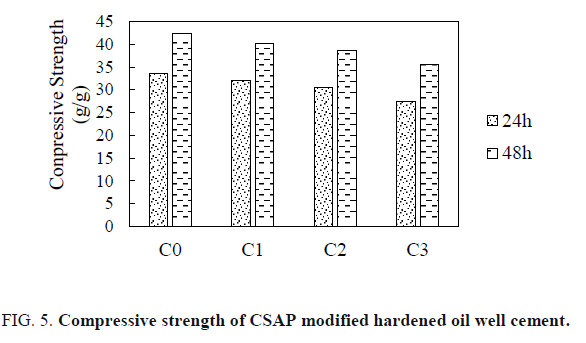
Effect of CSAP on the compressive strength of hardened oil well cement
FIG. 5 shows the results of the compressive strength of CSAP modified hardened oil well cement. Adding CSAP to cement reduces the compressive strength of hardened cement. The larger the CSAP addition, the lower the compressive strength of hardened cement. This is because the CSAP is a non-gelling material, which does not participate in the reaction during the cement solidification process. The strength of the CSAP is much lower than that of the hardened cement, so that the compressive strength of the hardened cement is low. However, the reduced compressive strength still meets the requirements of the API standard RP-10B-2013.
Conclusion
In this paper, CSAP was successfully prepared. The GA addition and the CS concentration were the main factors affecting the water absorption stability of CSAP. In order to prepare a stable CSAP, the GA addition should be greater than 0.15% and the CS concentration should be less than 3%. The water absorbing performance of CSAP was pH-sensitive. When the pH value was 7-10, the water absorbing rate remained unchanged at about 13.5 g/g; When the pH value was greater than 10, the water absorbing rate began to decrease; When the pH value was greater than 12. The water absorbing rate reached the maximum at about 1.87 g/g. At the same pH value, the water absorbing rate of the CSAP in the CSF was smaller than that in the NaOH solution.
The effect of CSAP on thickening performance and water loss performance of cement slurry and compressive strength of hardened cement were tested. The addition of CSAP to the cement slurry increased the initial consistency, had little influence on the thickening time, reduced the water loss and reduced the compressive strength of hardened cement. When the addition of CSAP was less than 5%, the properties of the cement slurry met the cementing requirements.
Acknowledgments
This research work was funded by the National Basic Research Program of China (973 Program) (2015CB251202) and Program for Changjiang Scholars and Innovative Research Team in University (NO.IRT_14R58).
References
- Granger S, Loukili A, Pijaudier-Cabot G, et al. Experimental characterization of the self-healing of cracks in an ultra-high-performance cementitious material: Mechanical tests and acoustic emission analysis. Cement Concrete Res. 2007;37(4):519-27.
- Termkhajornkit P, Nawa T, Yamashiro Y, et al. Self-healing ability of fly ash?cement systems. Cement Concrete Comp. 2009;31(3):195-203.
- Wu M, Johannesson B, Geiker M. A review: Self-healing in cementitious materials and engineered cementitious composite as a self-healing material. Constr Build Mater. 2012;28(1):571-83.
- Huang H, Ye G, Damidot D. Characterization and quantification of self-healing behaviors of microcracks due to further hydration in cement paste. Cement Concrete Res. 2013;52:71-81.
- Wang C, Bu Y, Guo S, et al. Self-healing cement composite: Amine- and ammonium-based pH-sensitive superabsorbent polymers. Cement Concrete Comp. 2019;96:154-62.
- Horie K, Barón M, Fox R, et al. Definitions of terms relating to reactions of polymers and to functional polymeric materials (IUPAC Recommendations 2003). Pure Appl Chem. 2004;76(4):889-906.
- Das A, Kothari V, Makhija S, et al. Development of high-absorbent light-weight sanitary napkin. J Appl Polym Sci. 2008;107(3):1466-70.
- El-Rehim HA, Hegazy ESA, El-Mohdy HA. Radiation synthesis of hydrogels to enhance sandy soils water retention and increase plant performance. J Appl Polym Sci. 2004;93(3):1360-71.
- Liu M, Liang R, Zhan F, et al. Preparation of superabsorbent slow release nitrogen fertilizer by inverse suspension polymerization. Polym Int. 2007;56(6):729-37.
- Wu L, Liu M, Liang R. Preparation and properties of a double-coated slow-release NPK compound fertilizer with superabsorbent and water-retention. Bioresource Technol. 2008;99(3):547-54.
- Lopez-Rubio A, Almenar E, Hernandez-Muñoz P, et al. Overview of active polymer-based packaging technologies for food applications. Food Rev Int. 2004;20(4):357-87.
- Bentz DP, Jensen OM. Mitigation strategies for autogenous shrinkage cracking. Cement Concrete Comp. 2004;26(6):677-85.
- Esteves LP. Superabsorbent polymers: On their interaction with water and pore fluid. Cement Concrete Comp. 2011;33(7):717-24.
- Craeye B, Geirnaert M, De Schutter G. Super absorbing polymers as an internal curing agent for mitigation of early-age cracking of high-performance concrete bridge decks. Constr Build Mater. 2011;25(1):1-13.