Original Article
, Volume: 12( 1)Porous Silica Particles: Synthesis, Physicochemical Characterization and Evaluation of Suspension Stability
- *Correspondence:
- Sushilkumar A Jadhav , Department of Chemistry and NIS Research Centre, University of Torino, Via P Giuria 7, 10125 Torino, Italy, Tel: 390116707650; E-mail: sushil.unige@gmail.com
Received: March 30, 2017; Accepted: May 05, 2017; Published: May 09, 2017
Citation: Jadhav SA, Miletto I, Brunella V, et al. Porous Silica Particles: Synthesis, Physicochemical Characterization and Evaluation of Suspension Stability. Phys Chem Ind J. 2017;S1:102.
Abstract
This work reports about the synthesis and/or physicochemical characterization of five important types of porous silica particles with different characteristic properties. The instrumental characterization includes transmission electron microscopy (TEM) to study particle structure and size, porosimetry (nitrogen adsorption-desorption) to determine specific surface area (SSA), pore diameter and pore volume, dynamic light scattering (DLS) to measure hydrodynamic diameter and zeta potential, thermogravimetric analysis (TGA) to calculate the weight loss percentage upon programmed heating. Aggregation kinetics profiles of bare porous silica particles have been studied with DLS to check their suspension stability. The characterization data presented are critical to compare the properties of synthesized porous silicas with those of reported or newly synthesized porous silica particles and to modulate the performance of mesoporous silica particles according to the applications under study.
Keywords
Porous silica particles; Physicochemical characterization; Aggregation kinetics; Thermal analysis
Introduction
Porous silica particles and related research has noticed a huge growth since last few years and it continues to grow further [1-5]. The increased interest in these porous materials is because of their number of proven and potential applications in various fields. Some of the main applications include their use for removal of pollutants such as heavy metals, organic pollutants and phosphates from water [6], removal of volatile organic carbons [7], as catalysts or supports for catalysts [8], biocatalysis [9], and direct CO2 capture [10]. Porous silica particles are also investigated with great interest for their use in the biomedical field; both in diagnostics and drug delivery applications [11-14]. The list of newer potential technological applications of these materials continues to increase further.
Amongst several types of porous silica particles MCM-41 and SBA-15 are the two most commonly investigated ones [1]. Recently several new, more efficient and modified synthesis techniques for the synthesis of these types of particles have been reported [15-17]. This proves the continuous interest of researchers not only in exploring new applications of porous silica particles but also in inventing, optimizing and scaling-up of simplified synthetic procedures for their preparation. Due to the high demand of this material there is also increased interest in its large scale synthesis and production process optimization to obtain monodisperse porous particles with uniform properties. The properties of porous silica micro or nanoparticles that need to be immediately checked after their synthesis include the particle size and particle size distribution, morphology and orientation of the porous structure, surface area, pore diameters and chemical purity. All these parameters play a crucial role in determining the hydrothermal, colloidal, suspension and dispersion stability of the particles. Although in large scale production of any material in chemical industries slight variations are possible, the variations should be in the limit of acceptance.
For this purpose, a set of standard characterization data of synthetic porous silica particles must be prepared which will serve as reference models for comparison of the properties of the particles produced in large scale with the particles synthesized at standard laboratory scale. In the present work, we report synthesis and characterization and comparative evaluation of different types of porous silica particles including two commercial multimodal porous silica particles. The information provided will serve as a handy document for reproducibility check of synthetic methods and comparative evaluation of porous silica particles. In addition, aggregation kinetics of bare porous silica particles are studied. The completely new characterization data of commercial porous silica particles will help in making their choice or testing of their suitability for the applications under study.
Experimental
Materials
Tetraethyl orthosilicate (TEOS), hexadecyltrimethylammonium bromide (CTAB), 1,3,5-trimethylbenzene (TMB), Pluronic P123 triblock copolymer, ethanol, methanol and sodium hydroxide were purchased from Sigma Aldrich. Porous silica particles with particle size 200 nm and pore size 4 nm were purchased from Sigma-Aldrich, Italy. Commercial porous silica particles of SBA-15 type were purchased from Zecasin S.A., Romania.
Particle synthesis
Mesoporous silica nanoparticles of MCM-41 type [16], pore extended MCM-41 type [17] and SBA-15 type [18] used for characterization in this study were synthesized according to the sol-gel process reported in the references mentioned.
In a typical reaction for synthesis of MCM-41 type mesoporous silica nanoparticles, CTAB (1 g) was dissolved in distilled water (480 mL); the solution was heated at 80°C and then NaOH (3.5 mL, 2 M) was added, the mixture was stirred for 30 min. TEOS (5 mL) was added drop wise. The mixture was then stirred for 2 h at 80oC. After cooling to room temperature (RT), the product was recovered by filtration and washed with distilled water (750 mL) and methanol (500 mL). The product was dried in air for 24 h. The particles were then calcined at 550°C for 7 h in air (2°C/min from room temperature to 550°C in nitrogen and then isotherm at 550°C in air).
The synthesis of mesoporous silica nanoparticles with pore diameters 4.5 nm was carried in same way by using TMB as the micelle core swelling agent. For the synthesis of SBA-15 type porous silica particles CTAB was replaced with Pluronic triblock copolymer. The list of samples synthesized and commercial samples analysed is shown in Table 1.
| Notation | Description |
|---|---|
| A | Mesoporous silica nanoparticles of MCM-41 type |
| B | Mesoporous silica nanoparticles pore extended |
| C | Commercial porous silica particles by Sigma Aldrich |
| D | Mesoporous silica nanoparticles of SBA-15 type |
| E | Commercial porous silica of SBA-15 type by Zekasin |
Table 1: List of the porous silica samples.
Transmission electron microscopy
High resolution transmission electron microscopy (HRTEM) images were obtained with a JEOL 2010 instrument (300 kV) equipped with a LaB6 filament. For specimen preparation, powdery samples were supported onto holed carbon coated copper grids by dry deposition.
Dynamic light scattering
Dynamic light scattering (DLS) measurements were carried out by using Malvern ZS 90 Zetasizer instrument. For the measurement of size app. 0.01% suspensions of nanoparticles were prepared in deionized water and these suspensions were sonicated for 20 minutes before the analysis. Depending upon the aggregation tendency these suspensions were further diluted to obtain stable suspensions. Zeta potential measurements were carried out on slightly concentrated suspensions of the particles. Both for size and zeta potential 5 measurements on each sample were carried out and the mean values are reported.
Gas-volumetric analysis
Gas-volumetric analysis, specific surface area (SSA), pore volume and size were measured by N2 adsorption-desorption isotherms at 77 K using an ASAP 2020 (Micromeritics) gas-volumetric analyzer. SSA was calculated using the Brunauer-Emmett-Teller (BET) method; average pore size and volume were calculated on the adsorption branch of the isotherms according to the Barrett-Joyner-Halenda (BJH) method (Kruk-Jaroniec-Sayari equations). Samples were outgassed at RT overnight before analyses.
Thermogravimetric analysis (TGA)
Thermogravimetric analyses (TGA) were carried out on a TA Q500 model from TA Instruments by programmed heating of samples contained in alumina pans at a rate of 10°C min-1 from 50°C to 600°C in nitrogen flow and from 600°C to 800°C in air. The switching of gas was purposely done to flush out or completely burn any organic residue that may remain with the sample and interfere with the weight loss determination.
Results and Discussion
Synthesis of porous silica
Synthetic porous silica particles mentioned in the work were prepared by sol-gel method. Hydrolysis condensation reaction of silica source tetraethyl alkosysilane (TEOS) on the micelles formed by templates yielded the porous particles [19-21]. Cetyltrimethylammonium bromide (CTAB) was used as the template for obtaining MCM-41 type mesoporous silicas with pore diameters around 3.5 nm and 4.9 nm [16]. Micelle core swelling agent, trimethylbenzene was used to increase the pore diameters from 3.5 nm to 4.9 nm [17]. Pluronic P123 triblock copolymer was used as the templating agent to obtain SBA-15 type mesoporous silica particles with pore diameter of 7.5 nm [22,23]. Calcination of the particles with programmed heating was carried out to remove the templating agents. The post synthesis thermal treatment gave porous silica particles with ordered porosity. Formation of the ordered porous structure in the particles was checked with TEM analysis while the chemical purity of the particles was checked by elemental analysis.
TEM
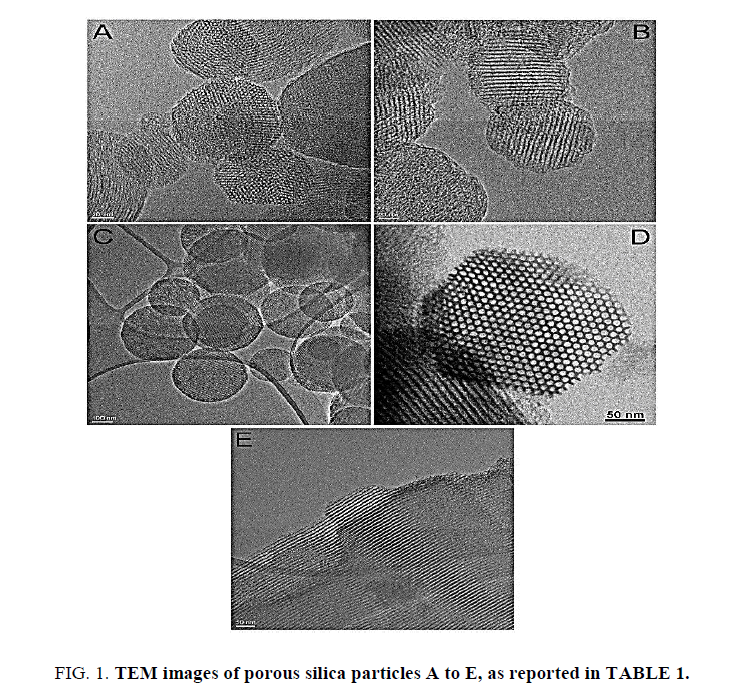
TEM images (Figure. 1) of the porous micro or nanoparticles were recorded to determine the average particle size, presence of porous structure, orientation of multimodal porous channel zones and morphology. The particles were quasi-spherical in shape with rough surface. Average diameters of all porous silica particles were measured and comparatively analysed with their respective synthetic techniques.
Ordered hexagonal pore structure in all porous silicas in the TEM images can be clearly seen. The pore wall thickness varied depending upon the type of porous silica. The pore channels in all particles extend throughout the body of the particles which indicated presence of defect-free particles. The particle diameter varied from 80 nm to 1 μm. For MCM-41 type porous silica, increase in SSA from 901 m2/g to 1228 m2/g was observed with decrease in particle size. This observation pointed out the straightforward relation between particle size and the surface area, also in porous materials. The multimodal porous channel structure in samples C and E was further confirmed by BET analysis which showed the presence of micro and mesopores on the particles.
Elemental analysis
The purity of the particles was checked by elemental analysis. The results of elemental analyses are reported in Table 2. It was observed that the percentage of hydrogen varied due to the presence of a different number of surface hydroxyl groups and water molecules adsorbed on the particles. It is the presence of silanol (-Si-OH) groups that makes the surface of the particles reactive.
| Sample | %C | %H | %N | %S |
|---|---|---|---|---|
| A | 0.93 | 0.68 | 0 | 0 |
| B | 0.98 | 0.51 | 0 | 0 |
| C | 0.40 | 0.28 | 0 | 0 |
| D | 0.77 | 0.40 | 0 | 0 |
| E | 0.87 | 0.48 | 0 | 0 |
a-Significant using Independent t-test @<0.05 level.
Table 2: Elemental analysis data of porous silica particles.
The reactivity of these groups is exploited in the post synthesis surface modification of particles to graft various functional molecules and polymers. The surface silanols also play an important role in determining the suspension stability as a high number of surface silanols can influence greatly the particle-particle interactions. The presence of surface silanol groups also influences the total charge on the particles which is reflected in the zeta potential values.
BET and DLS analysis
Physicochemical characterization data of all porous silica particles obtained from TEM, porosimetry and DLS analysis is collectively reported in Table 3.
| Sample | Diameter (TEM) nm |
S.S.A.1 m2/g |
Pore diameter (Å) | Pore volume (cm3/g) | Average H.D.2 nm |
P.D.I.3 | Zeta potential mV |
pH ± 0.2 |
||
|---|---|---|---|---|---|---|---|---|---|---|
| Micro | Meso | Micro | Meso | |||||||
| A | 80 ± 20 | 1228 | - | 35 | - | 1.45 | 122 ± 10 | 0.3-0.7 | -35.2 ± 1.0 | 6.97 |
| B | 100 ± 16 | 1017 | - | 49 | - | 2.42 | 141 ± 19 | 0.2-0.6 | -39.5 ± 0.7 | 7.21 |
| C | 200 ± 11 | 901 | 13 | 40 | 0.16 | 1.26 | 220 ± 9 | 0.6-0.9 | -17.3 ± 0.3 | 7.01 |
| D | 280 ± 21 | 620 | - | 75 | - | 2.75 | 331 ± 17 | 0.4-0.5 | -32.6 ± 0.5 | 7.10 |
| E | 900 ± 37 | 816 | 20 | 56 | 0.052 | 1.14 | 1090 ± 21 | 0.4-1.0 | -30.5 ± 0.6 | 6.90 |
1Specific surface area, 2 Hydrodynamic diameter, 3 Polydispersity index
Table 3: Demonstrates the correlation values.
The particle diameters were in the range from 80 nm to approximately 1 μm. The pore diameters measured by BJH method were from 3.5 nm to 7.5 nm. These values were in very good agreement with the estimation made by analysis of high resolution TEM images (Figure. 1D and 1E). This confirms the validity of the employed BJH equations (Kruk-Jaroniec-Sayari thickness curve and BJH corrections), which are optimal for this kind of mesoporous materials. Indeed, default Halsey and Faals equations were found to give an underestimation of pore size with respect to what measured by TEM on this class of materials. As expected the SSA was found to decrease with increase in the diameter of the pores in both (MCM-41 and SBA-15) types of particles. Samples C and E, which are commercial porous silica particles, showed the presence of micropores together with mesopores. In the case of the MCM-41 type commercial porous silica sample (C) the micro and mesopore diameters were 13 Å and 40 Å respectively. While in the case of commercial SBA-15 sample (E) they were 20 Å and 56 Å respectively. The determination of composition of micro and mesopores is of importance as it can help to modify or tune the performance of the porous silica particles in their use for delivery of various molecules in different environments.
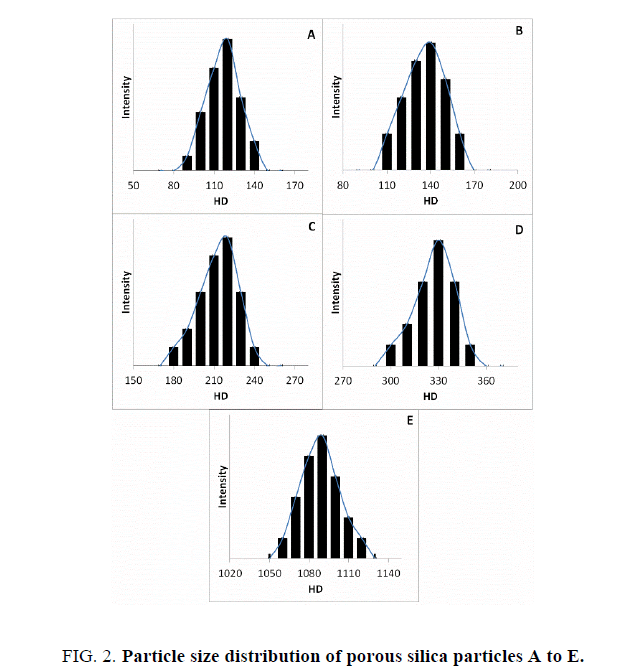
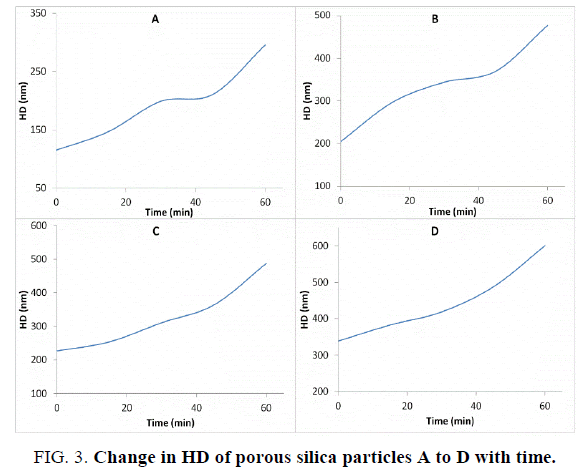
The particle size distributions for the most stable clusters of the particles are shown in Figure. 2, while the change in hydrodynamic diameter with time (aggregation kinetics) of all porous silica particles are shown in Figure. 3.
The polydispersity index values for each sample are also reported in Table 3, and were clearly found to depend upon the zeta potential (total charge at almost neutral pH) values and composition of the samples. Negative zeta potentials were observed for all porous silica nanoparticle samples as reported previously [24-27]. The values of zeta potentials were between -39.5 mV and -17.3 mV.
All the porous silica nanoparticles analyzed were bare particles hence stability of the suspensions prepared for DLS analysis was low due to relatively fast aggregation of particles mediated by particle-particle interactions. Higher values of the polydispersity index were observed for samples with less negative zeta potentials. The aggregation profiles of particles in the form of change of hydrodynamic diameter with time up to 60 minutes are reported in Figure. 3. As expected all the samples have shown relatively fast aggregation tendencies. In the case of sample E, which are the commercial SBA-15 type porous silica particles, it was impossible to construct an aggregation profile for two reasons: 1. The hydrodynamic diameter of the particles was almost 1 μ which is at the higher limit of DLS instrument. Hence immediately after aggregation clusters with sizes more than 1 micron were formed which were difficult to analyze 2. Upon aggregation clusters of aggregated particles due to their big size were found to quickly sediment during the analysis.
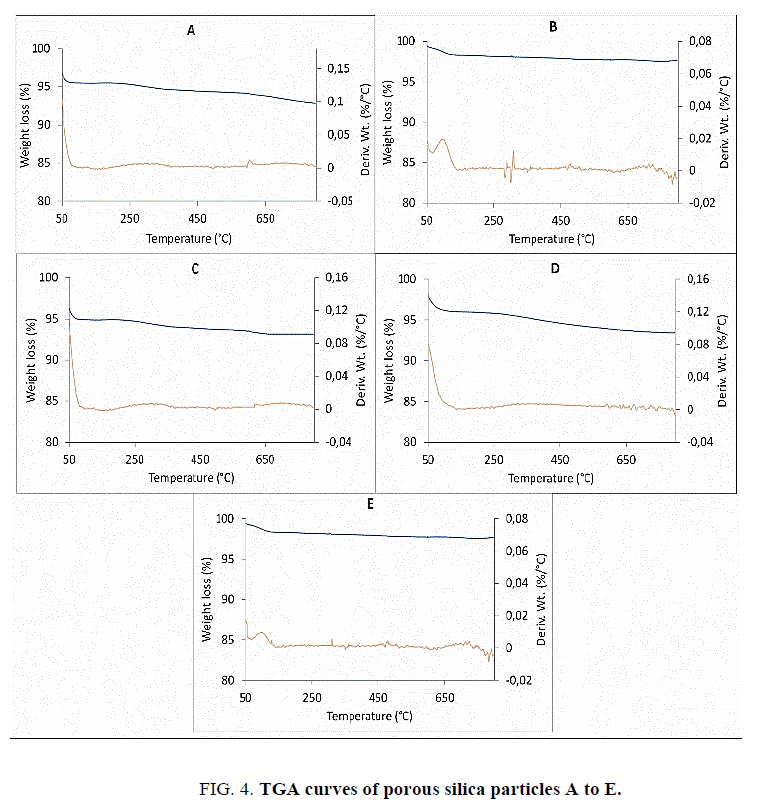
Thermal analysis
Thermal stability is one of the main requirements of the porous materials for their use in various applications [28,29]. Especially, testing of their thermal stability is of importance for their use in catalytic or as catalyst support applications which employ high temperatures. The thermal stability of porous silica particles was tested by programmed heating of them up to 800°C in nitrogen and air atmosphere. The TGA curves reporting the percentage of weight loss with increase in temperature and the corresponding first derivatives calculated by weight loss percentage divided by the temperature are reported in Figure. 4. All the porous silica particles have shown good thermal stability as there was no considerable weight loss observed up to 800°C. The initial weight loss up to 150°C was due to the presence of adsorbed water and it varied for each type of porous silica particles. The gradual and small weight loss at higher temperature is related to surface dehydroxylation, and can be used for a rough estimation of silanol surface density [30]. The amount of adsorbed water was slightly higher for samples A, B and D, which can be linked to the zeta potential values indicating presence of higher surface silanols in these samples. Here, higher the number of surface silanols on the particles greater is the possibility of adsorption of water molecules which form a strongly physisorbed monolayer of water on the silica surface. The TGA data also indicated the difference in the surface properties of porous silicas and ability to adsorb or trap humidity.
As stated earlier porous silica particles are studied with great interest for their use in biomedical applications. Huge numbers of reports highlight the suitability and importance of porous silica micro or nanoparticles as drug delivery vehicles. But by looking at the collective physicochemical characterization data and especially the low suspension stability of the bare particles it can be concluded that the bare porous silica particles may not be a good choice as drug delivery vehicles. To tackle this problem suitable surface modification of the particles with moieties such as organic compounds, polymers, targeting agents such as amino acids or proteins, antibodies or receptors is necessary. Such surface modification will not only improve the efficiency of the particles as delivery vehicles but also will improve their suspension stability which is a prime requirement in biomedical application of nanomaterials. The detailed characterization and comparative evaluation of any low/nano dimensional material as presented in this work is an important step before proposing such materials for their potential applications in various fields.
Conclusion
Porous silica particles with different characteristic features were prepared, characterized and comparatively analyzed with the commercial porous silica samples. Complete physicochemical characterization of the particles was performed and aggregation profiles of the bare particles were constructed. It is shown that surface properties influence the total charges, particle-particle interactions, suspension stability and thermal properties of the porous particles. A set of standard characterization data has been developed in this work which can be used as handy model document for the comparative analysis of further newly synthesized porous silica particles. The new and complete characterization data presented both on well-known laboratory prepared porous silica particles as well as on the commercial particles is helpful in screening their suitability for the target applications under study.
Acknowledgments
The authors thank Dr. Elena Ugazio for providing sample of commercial porous silica particles by Sigma Aldrich.
References
- Jadhav SA. Incredible pace of research on mesoporous silica nanoparticles. Inorg Chem Front. 2014;1(10):735-9.
- ALOthman ZA. A review: Fundamental aspects of silicate mesoporous materials. Materials. 2012;5(12):2874-902.
- Tang F, Li L, Chen D. Mesoporous silica nanoparticles: Synthesis, biocompatibility and drug delivery. Adv Mater. 2012;24(12):1504-34.
- Li Z, Barnes JC, Bosoy A, et al. Mesoporous silica nanoparticles in biomedical applications. Chem Soc Rev. 2012;41(7):2590-605.
- Argyo C, Weiss V, Bra¨uchle C, et al. Multifunctional mesoporous silica nanoparticles as a universal platform for drug delivery. Chem Mater. 2013;26(1):435-51.
- Walcarius A, Mercier L. Mesoporous organosilica adsorbents: Nanoengineered materials for removal of organic and inorganic pollutants. J Mater Chem. 2010;20(22):4478-511.
- Delaney P, Healy RM, Hanrahan JP, et al. Porous silica spheres as indoor air pollutant scavengers. J Environ Monitor. 2010;12(12):2244-51.
- Huirache-Acuña R, Nava R, Peza-Ledesma CL, et al. SBA-15 mesoporous silica as catalytic support for hydrodesulfurization catalysts: Review. Materials. 2013;6(9):4139-67.
- AbdallahNH, Schlumpberger M, Gaffney DA, et al. Comparison of mesoporous silicate supports for the immobilisation and activity of cytochrome c and lipase. J Mol Catal B Enzym. 2014;108:82-8.
- Kumar A, Madden DG, Lusi M, et al. Direct air capture of CO2 by physisorbent materials. Angew Chem Int Ed. 2015;54(48):14372-7.
- McCarthy CA, Ahern RJ, Dontireddy R, et al. Mesoporous silica formulation strategies for drug dissolution enhancement: A review. Expert Opin Drug Deliv. 2016;13(1):93-108.
- Jadhav SA, Brunella V, Berlier G, et al. Effect of multimodal pore channels on cargo release from mesoporous silica nanoparticles. J Nanomater. 2016;2016:1325174.
- Jadhav SA, Brunella V, Scalarone D, et al. Poly (NIPAM-co-MPS)-grafted multimodal porous silica nanoparticles as reverse thermoresponsive drug delivery system. Asian J Pharm Sci. 2017.
- Jadhav SA, Scalarone D, Brunella V, et al. Thermoresponsive copolymer-grafted SBA-15 porous silica particles for temperature-triggered topical delivery systems. Express Polym Lett. 2017;11(2).
- Beltrán-Osuna ÁA, Perilla JE. Colloidal and spherical mesoporous silica particles: Synthesis and new technologies for delivery applications. J Sol-Gel SciTechnol. 2016;77(2):480-96.
- Jana SK, Mochizuki A, Namba S. Progress in pore-size control of mesoporous MCM-41 molecular sieve using surfactant having different alkyl chain lengths and various organic auxiliary chemicals. Catalsurv Asia. 2004;8(1):1-3.
- Peng J, Liu J, Liu J, et al. Fabrication of core-shell structured mesoporous silica nanospheres with dually oriented mesochannels through pore engineering. J Mater Chem A. 2014;2(21):8118-25.
- Wang X, Zhang Y, Luo W, et al. Synthesis of ordered mesoporous silica with tunable morphologies and pore sizes via a nonpolar solvent-assisted stober method. Chem Mater. 2016;28(7):2356-62.
- Brunella V, Jadhav SA, Miletto I, et al. Hybrid drug carriers with temperature-controlled on-off release: A simple and reliable synthesis of PNIPAM-functionalized mesoporous silica nanoparticles. React FunctPolym. 2016;98:31-7.
- Jadhav SA, Miletto I, Brunella V, et al. Controlled post-synthesis grafting of thermoresponsive poly (N-isopropylacrylamide) on mesoporous silica nanoparticles. Polym Adv Technol. 2015;26(9):1070-5.
- Trewyn BG, Slowing II, Giri S, et al. Synthesis and functionalization of a mesoporous silica nanoparticle based on the sol-gel process and applications in controlled release. Acc chem res. 2007;40(9):846-53.
- Lopes dos Santos SM, Barros Nogueira KA, de Souza Gama M, et al. Synthesis and characterization of ordered mesoporous silica (SBA-15 and SBA-16) for adsorption of biomolecules. MicroporMesopor Mater. 2013;180:284-92.
- Cao L, Kruk M. Synthesis of large-pore SBA-15 silica from tetramethylorthosilicate using triisopropylbenzene as micelle expander. Colloids Surf A Physicochem Eng Asp. 2010;357(1):91-6.
- DeMuth P, Hurley M, Wu C, et al. Mesoscale porous silica as drug delivery vehicles: Synthesis, characterization, and pH-sensitive release profiles. MicroporMesopor Mater. 2011;141(1):128-34.
- Yuan L, Tang Q, Yang D, et al. Preparation of pH-responsive mesoporous silica nanoparticles and their application in controlled drug delivery. J Phys Chem C. 2011;115(20):9926-32.
- Luo GF, Chen WH, Liu Y, et al. Multifunctional enveloped mesoporous silica nanoparticles for subcellular co-delivery of drug and therapeutic peptide. Sci rep. 2014;4:6064.
- Xu W, Riikonen J, Nissinen T, et al. Amine surface modifications and fluorescent labeling of thermally stabilized mesoporous silicon nanoparticles. J Phys Chem C. 2012;116(42):22307-14.
- Gu G, Ong PP, Chu C. Thermal stability of mesoporous silica molecular sieve. J Phys Chem Solids. 1999;60(7):943-7.
- Adeniran B, Mokaya R. On the shelf life and aging stability of mesoporous silica:
- Insights on thermodynamically stable MCM-41 structure from assessment of 12-year-old samples. Chem Mater. 2012;24(22):4450-8.
- Gallas JP, Goupil JM, Vimont A, et al. Quantification of water and silanol species on various silicas by coupling IR spectroscopy and in-situ thermogravimetry. Langmuir. 2009;25(10):5825-34.