Original Article
, Volume: 15( 2)Optimization of Microbial Fuel Cell for Treating Industrial Wastewater and Simultaneous Power Generation
- *Correspondence:
- Sabarunisha Begum S Department of Biotechnology, Rajalakshmi Engineering College, Chennai, India Tel: +914437181111; E-mail: sabarunisha@gmail.com
Received Date: April 06, 2017 Accepted Date: May 11, 2017 Published Date: May 13, 2017
Citation: Aswin T, Sabarunisha Begum S, Sikkandar Y. Optimization of Microbial Fuel Cell for Treating Industrial Wastewater and Simultaneous Power Generation. Int J Chem Sci. 2017;15(2):132.
Abstract
The dual application of microbial fuel cells (MFC) make them more prolific in research at present. In this study, microbial fuel cell was fabricated in an economical way and the experiment was carried out in two phases. In first phase of experimental run, dairy and leather effluent was used as substrate without any mediators and in second phase, dairy, leather and sewage wastewater were used as substrate with ferroin as mediator. Physiochemical characteristics such as total dissolved solids (TDS), total suspended solids (TSS), chemical oxygen demand (COD), and biochemical oxygen demand (BOD) were determined before and after treating the effluent in MFC using saccharomyces cerevisiae. Heavy metals present in the effluent were also removed during biodegradation process. In first phase of experimental run, COD and BOD removal efficiency was found to be 80.4% and 59.3% for leather effluent, 83.4% and 64.3% for dairy effluent and in second phase, COD and BOD removal efficiency was found to be 80% and 64% for leather effluent, 85.4% and 79% for dairy effluent and 65% and 47% for domestic wastewater. The maximum power generated in first phase during the treatment of dairy and leather wastewater was found to be 385.25 μW and 304.5 μW and in second phase, 1.98 mW, 1.95 mW and 1.28 mW of power was generated during the treatment of dairy, leather and domestic wastewater. In both phases of experimental run, power generated during the treatment of dairy wastewater is more. The results showed that the organic matter has been effectively degraded more in the dairy wastewater and produced more electrons when compared to leather and domestic wastewater.
Keywords
Microbial fuel cell; Wastewater; COD; Biodegradation; Power generation
Introduction
In today’s growing world, we are currently depending upon fossil fuels such as coal, oil, natural gas for our energy requirements. Planet earth is facing an energy crisis owing to an escalation in global demand, continued dependence on fossil based fuels for energy generation and an increase in world population, exceeding seven billion people and rising steadily [1]. Excessive burning of fossil fuels not only depletes natural nonrenewable resource but also leads to carbon dioxide emission which give rise to life threatening conditions such as ozone layer depletion, climatic change and acid rains [2]. This persuades us to find a suiTable alternative energy source. Many research works have been carrying out to find an energy source which is renewable in nature, one such thing is microbial fuel cells.
Microbial fuel cell is a bioreactor that converts chemical energy present in the organic matter into electrical energy by using microorganisms as a catalyst [3]. This catalytic reaction takes place under anaerobic condition and the microorganisms used are termed as electricigens [4]. The advantages of using microbial fuel cell is that they can be used at room temperature itself, easy to handle, not toxic, it can extract 90% of electrons from organic compound, self-sustaining, renewing [5]. The disadvantage is that they cannot produce much current and scale up process is the major problem [6]. Applications are they can be used to produce hydrogen, used in wastewater treatment, used as biosensors, desalination process can be done using microbial fuel cells [7].
Basic principal behind microbial fuel cell is that when the micro-organisms consume a substance such as sugar in aerobic conditions, they produce carbon dioxide and water. However, when oxygen is not present, they produce carbon dioxide, protons, and electrons. These electrons and protons are then carried through external circuit and salt bridge to the cathode respectively. In cathode, electron, proton, and oxygen combined to form water [8]. Whenever electrons flow through a wire it produces electric current, exactly same thing happens in microbial fuel cell as the electrons passes through external circuit, electric current is produced. Electrons are produced by the microorganisms by metabolizing the organic matter present in the sample. With the help of enzyme called cytochrome c that is present in the electron transport chain, the microorganisms transfer the generated electron directly to the anode of the microbial fuel cell. But, not all microorganisms can transfer the electrons directly to the anode as they differ among them [9]. There are mainly three ways by which electrons can be transferred a. through artificial mediators, b. through microorganism’s own mediator, c. through direct transfer of electrons [10]. Most commonly used mediators are methyl blue, thionine, and neutral red [11]. Microorganisms can be used as pure culture or mixed culture. But mixed culture produces more current when compared to pure culture. Some commonly used microorganisms are Shewanella putrefaciens, Geobacteraceae sulferreducens, Escherichia coli, Clostridium butyricum [12].
The main aim of this research work is to find an efficient way to treat wastewater and to increase the electric current by many folds by employing cheap materials. The experiments were carried out in two phases, first without any artificial mediator and then with ferroin indicator as mediator. The system was evaluated before and after running the fabricated setup using different substrate such as diary, leather and domestic wastewater.
Materials and Methods
Collection of wastewater and its characteristics
Dairy wastewater was collected from Government dairy industry, Aavin located at Madhavaram, Chennai, Tamlnadu, India. Leather wastewater was collected from leather industry located at Madhvaram and domestic wastewater was collected at Madhavaram sewage point. The physiochemical characteristics were initially characterized and tabulated.
Five-day biochemical oxygen demand (BOD) test and chemical oxygen demand (COD) were determined by standard method [13]. Total suspended solids (TSS) were measured by filtration of a sample through a pre-weighed filter paper followed by drying at 105°C for 24 hours to constant weight (Table 1). As the experiments were carried out in two phases, the physiochemical characteristics were also analysed separately for each phase.
| Parameters | Leather wastewater | Dairy wastewater |
|---|---|---|
| Colour | Greyish black | Milky white |
| Odour | Pungent | Bad |
| pH | 5.8 | 6.1 |
| BOD (mg/L) | 920 | 562 |
| COD (mg/L) | 2754 | 1711 |
| TDS (mg/L) | 1846 | 2134 |
| TSS (mg/L) | 3010 | 2640 |
Table 1. Physiochemical characteristics of leather and dairy wastewater for phase I study.
Culture and medium
Saccharomyces cerevisiae is used as pure culture along with other organisms which normally present in that specific wastewater. Commercially available active dry yeast is used to metabolize the organic matter. 4 grams of yeast is taken which is in the form of pellets and smashed with help of mortar and pestle (Table 2). Then the yeast is dissolved in 40 mL of warm water by stirring it well. After dissolving it is now transferred to 250 mL closed bottle containing wastewater and kept it in room temperature for 24 hours so that it could adapt to the environment. 20 mL of this solution then transferred to fabricated model to carry out the experiments.
| Parameters | Leather wastewater | Dairy wastewater | Domestic wastewater |
|---|---|---|---|
| Colour | Greyish black | Milky white | Light green |
| Odour | Pungent | Bad | Bad |
| pH | 5.6 | 5.1 | 6.3 |
| BOD (mg/L) | 920 | 522 | 280 |
| COD (mg/L) | 2754 | 1686 | 547 |
| TDS (mg/L) | 1846 | 2050 | 475 |
| TSS (mg/L) | 3010 | 2720 | 262 |
| Sulphate (mg/L) | 340 | 210 | 70 |
| Chloride (mg/L) | 929 | 538 | 538 |
| Copper (mg/L) | - | - | <0.05 |
| Chromium (mg/L) | 29.3 | - | - |
Table 2. Physiochemical characteristics of domestic, leather and dairy wastewater for phase II study.
MFC reactor set up and experimental procedure
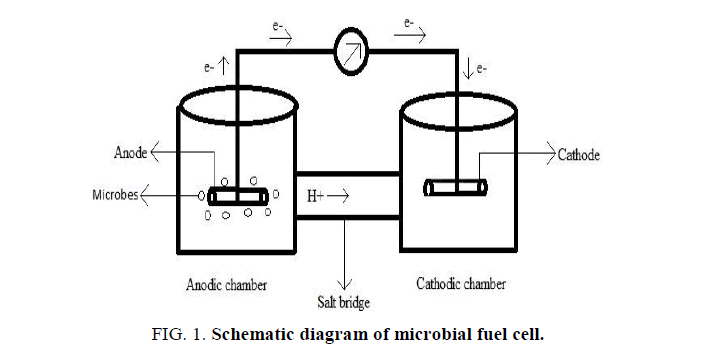
Microbial fuel cell consists of anodic and cathodic chamber of 500 mL capacity. Figures. 1 and 2 shows the schematic representation and photographic picture of MFC set up. The electrode used is a carbon rod. Copper wire is used to hang the carbon rod and it is used to pass the electrons produced from anode to cathode by acting as linker between them. The salt bridge is constructed by using PVC pipe of length 8 cm and diameter of 2.5 cm. Inside the PVC pipe, polymer such as 5% agar is used along with 0.1 M KCl which forms the salt bridge and helps to transfer the proton to anode. The multimeter is connected to the anode and cathode and voltage is noted down.
Figure 1: Schematic diagram of microbial fuel cell.
Figure 2: Photographic picture of the experimental MFC set up.
The experimental runs were carried out in two phases (phase I and phase II) MFC for treating various wastewater such as leather, dairy and domestic sewage water. In the phase I, Microbial fuel cell operated without any artificial mediator and in the phase II, Microbial fuel cell operated with artificial ferroin mediator. During the experimental process, current, power density, current density, COD and BOD were measured as per standard procedure.
Double chamber microbial fuel cell of capacity 500 mL is fabricated and used for the experiment. Figures. 1 and 2 shows the schematic representation and photographic picture of MFC set up. The electrode used is a carbon rod. Copper wire is used to hang the carbon rod and it is used to pass the electrons produced from anode to cathode by acting as linker between them. The salt bridge is constructed by using PVC pipe of length 8 cm and diameter of 2.5 cm. Inside the PVC pipe, polymer such as 5% agar is used along with 0.1 M KCl which forms the salt bridge and helps to transfer the proton to anode. The multimeter is connected to the anode and cathode to measure the voltage and current produced during the process. Three different types of substrate such as sewage, dairy wastewater and leather wastewater were used in this study along with sacchromyces cerevesiae as microorganism that metabolizes the organic matter present in the substrates used. Saccharomyces cerevisiae can transfer small amount of electron to anode of the microbial fuel cell on its own because it is not electrochemically active microorganism [14]. But with the help of mediator, the electron transfer can be enhanced.
The experimental runs were carried out in two phases (phase I and phase II) MFC for treating various wastewater such as leather, dairy and domestic sewage water. In the phase I, microbial fuel cell was operated without any artificial mediator and in the phase II with artificial ferroin mediator. Ferroin indicator is used to increase the electron transfer from sacchromyces cerevisiae to the anode and vice versa through redox reaction. Ferroin has much higher electrode potential of about 1.24 and it is pH independent than other mediators which are used normally in MFC [15].
During the experimental process, the parameters such as chemical oxygen demand (COD), biochemical oxygen demand (BOD), total dissolved solids (TDS), total suspended solids (TSS), heavy metals such as chromium, copper and other chemical compounds like chloride, sulfate, phosphorous were analyzed before and after running the fabricated bioreactor model. Finally, voltage, current density, power density was also measured, compared and analyzed.
Results and Discussions
The experimental results were discussed below in detail for the mediator less (Phase I) and mediator (Phase II) runs in microbial fuel cells.
Mediator less MFC (Phase I)
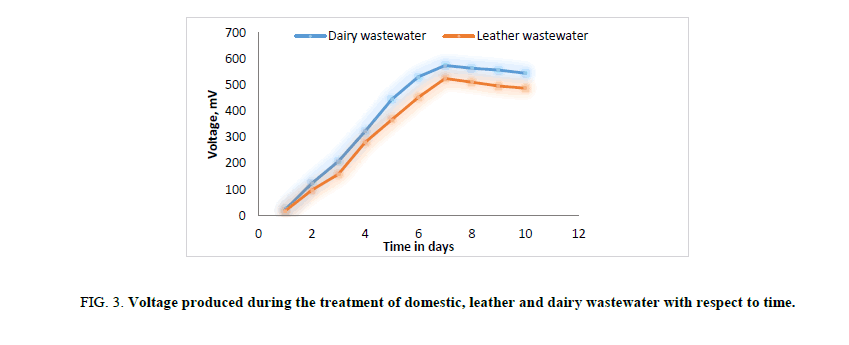
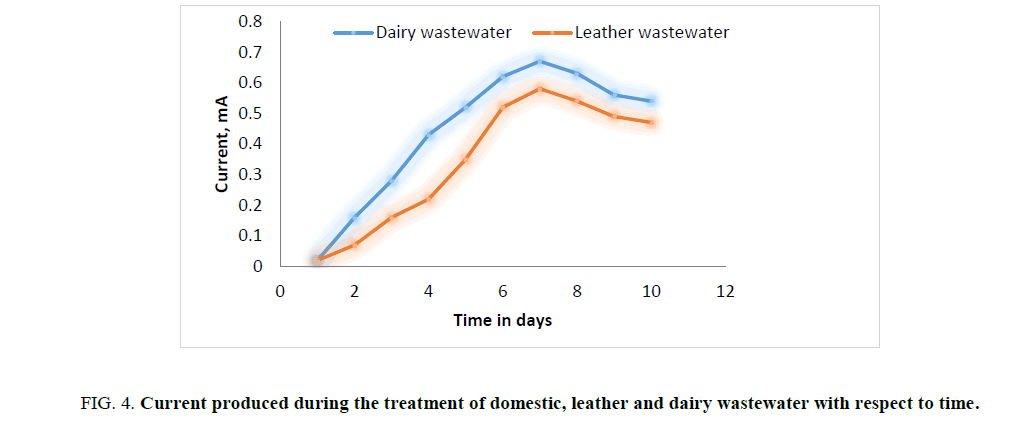
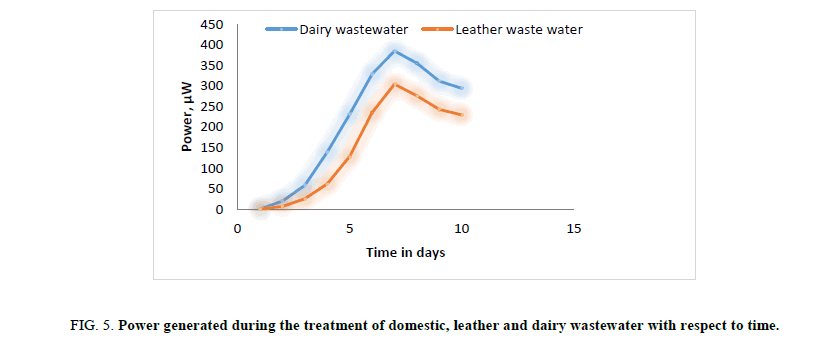
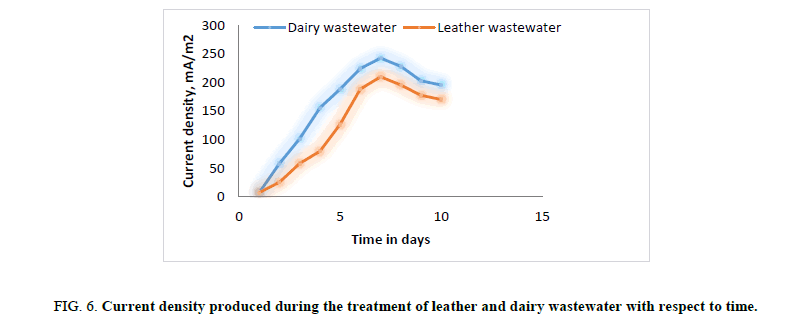
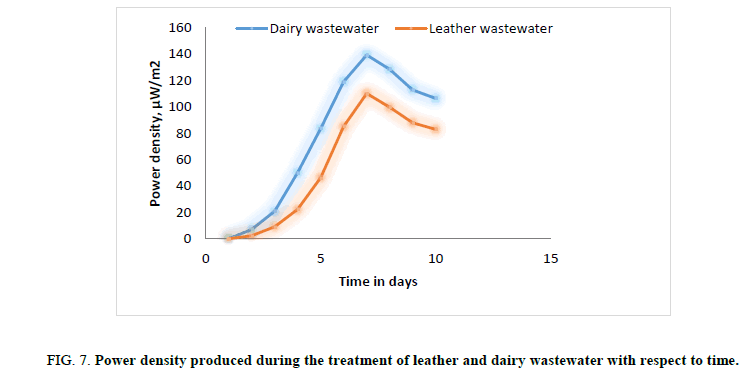
In first phase of experimental run using dairy and leather wastewater as substrate for the microorganism, the experiment is conducted with water as the catholyte and Sacchromyces cerevesiae is used as microorganism to degrade the organic matter. No mediator is being used. The current and voltage generated by the MFC were noted down on daily basis for about ten days with the help of multimeter. The parameters such as power, power density, current densities were calculated and graph is being plotted for detailed analysis (Figures. 3 and 7). Figures. 3 and 7 illustrates the growth curve of microorganism as the voltage increases during the exponential phase but enters a standing voltage phase and decreases as the setup enters the decline stage due to death of microbes attributing to the exhaustion of nutrients present in the anodic chamber [16]. The maximum current and voltage obtained during the treatment of dairy wastewater was found to be 0.67 mA and 0.57 V, and 0.58 mA and 0.52 V for leather effluent. Maximum current density and power density obtained during the treatment of dairy and leather wastewater was found to be 242.15 mA/m2 and 139.23 μW/m2; 209.68 mA/m2 and 110.04 μW/m2 respectively.
Figure 3: Voltage produced during the treatment of domestic, leather and dairy wastewater with respect to time.
Figure 4: Current produced during the treatment of domestic, leather and dairy wastewater with respect to time.
Figure 5: Power generated during the treatment of domestic, leather and dairy wastewater with respect to time.
Figure 6: Current density produced during the treatment of leather and dairy wastewater with respect to time.
Figure 7: Power density produced during the treatment of leather and dairy wastewater with respect to time.
Physiochemical analysis: The dairy and leather wastewater was initially characterized for its physiochemical parameters and it was then treated with fabricated microbial fuel cell (MFC) using Saccromyces cereviseae. Table 3 shows the physiochemical characteristics estimated for leather and dairy wastewater before and after treatment using MFC. The pH of untreated leather and dairy wastewater was found to be 5.8 and 6.1 and after treatment the pH had increased to 6.7 and 6.5 which show that treatment of wastewater using MFC had changed acidic nature of effluent to nearer neutral. The total dissolved solids (TDS) and total suspended solids (TSS) in the effluent were measured and percentage of removal was found to be 50%, 66% for leather effluent and 58%, 73% for dairy effluent respectively. Other parameters like COD and BOD were also estimated and percentage of removal was found to be 80.4%, 59% for leather effluent and 83.4%, 64% for dairy effluent respectively.
| Parameters | Leather wastewater | Dairy wastewater | ||
|---|---|---|---|---|
| Untreated | Treated | Untreated | Treated | |
| Colour | Greyish black | Greyish black | Milky white | Milky white |
| Odour | Pungent | Pungent | Bad | Bad |
| pH | 5.8 | 6.7 | 6.1 | 6.5 |
| BOD (mg/L) | 920 | 340 | 562 | 226 |
| COD (mg/L) | 2754 | 882 | 1711 | 388 |
| TDS (mg/L) | 1846 | 923 | 2134 | 896 |
| TSS (mg/L) | 3010 | 1023 | 2640 | 711 |
Table 3. Physiochemical characteristics of leather and dairy wastewater.
Mediator MFC (Phase II)
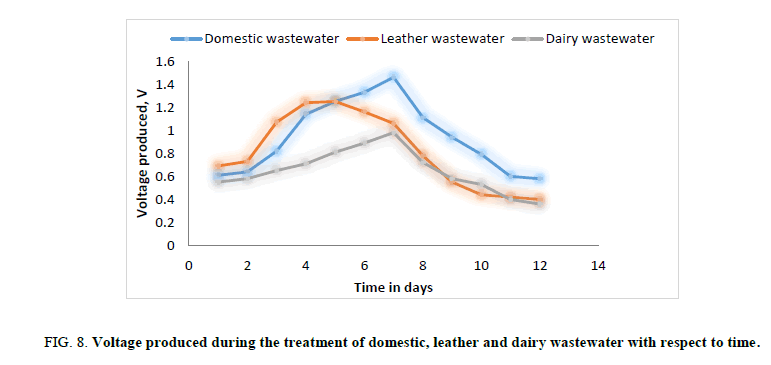
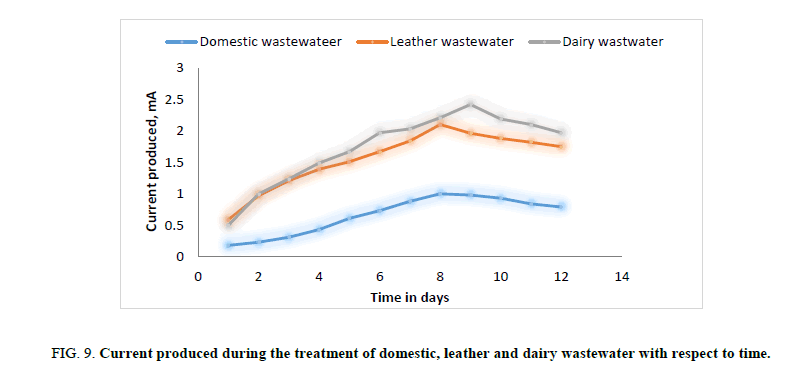
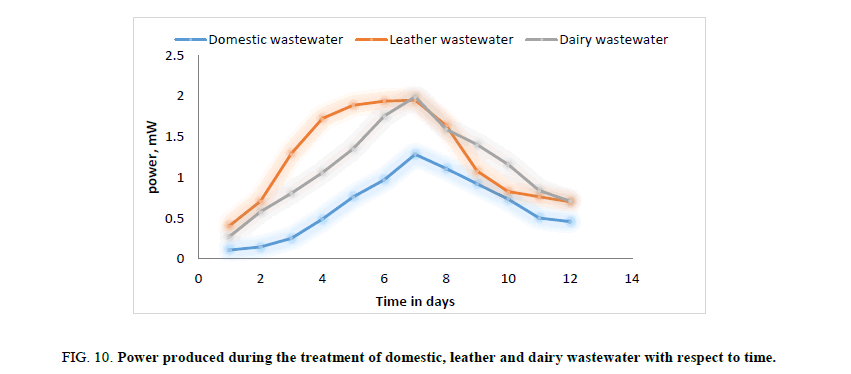
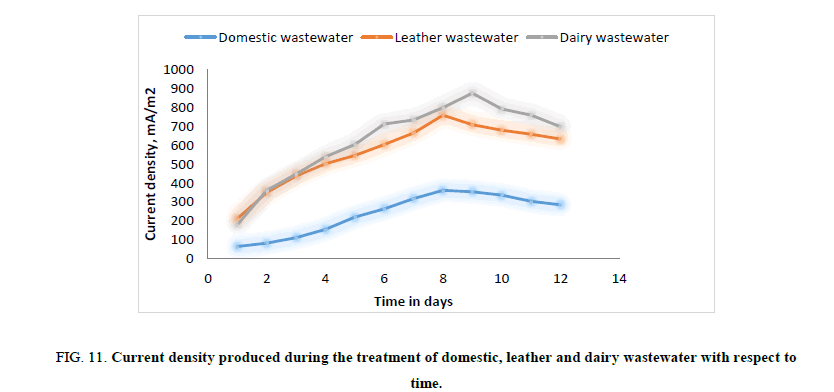
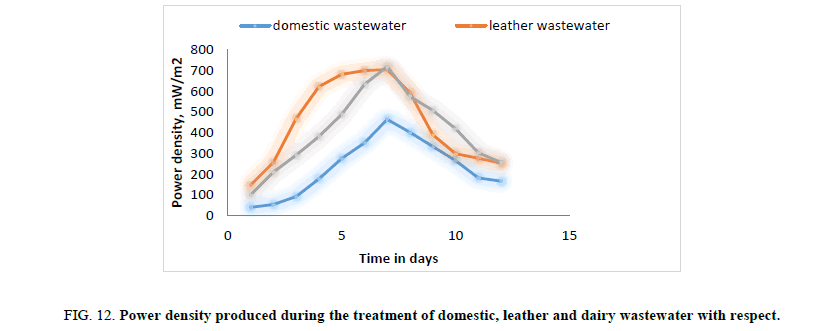
In the second phase of experimental run, domestic wastewater, dairy wastewater, and leather wastewater is used as substrate for the microorganism Sacchromyces cerevesiae with potassium permanganate as catholyte and ferroin indicator as artificial mediator to increase the efficiency of the fabricated model in terms of current generation. Potassium permanganate (KMnO4), a strong oxidizing agent is used as catholyte which can increase the flow of electrons between anode and cathode. The current and voltage produced by the fabricated MFC model were noted down for every 24 hours for about twelve days. Figures. 8 and 12 illustrates the growth curve of microorganism as the voltage increases during the exponential phase but enters a standing voltage phase and decreases as the setup enters the decline stage due to death of microbes attributing to the exhaustion of nutrients present in the anodic chamber. Maximum current and voltage obtained during the treatment of dairy, leather and domestic wastewater were found to be 2.42 mA and 0.98 V; 2.1 mA and 1.25 V; 1 mA and 1.46 V respectively. Maximum current density and power density obtained during the treatment of dairy, leather and domestic wastewater was found to be 874.90 mA/m2 and 719.23 mW/m2; 759.21 mA/m2 and 705.13 mW/m2; 361.53 mA/m2 and 464.49 mW/m2 respectively.
Figure 8: Voltage produced during the treatment of domestic, leather and dairy wastewater with respect to time.
Figure 9: Current produced during the treatment of domestic, leather and dairy wastewater with respect to time.
Figure 10: Power produced during the treatment of domestic, leather and dairy wastewater with respect to time.
Figure 11: Current density produced during the treatment of domestic, leather and dairy wastewater with respect to time.
Figure 12: Power density produced during the treatment of domestic, leather and dairy wastewater with respect.
Physiochemical analysis
Table 4 shows the physiochemical characteristics estimated for leather, dairy and domestic wastewater before and after treatment using MFC. The pH of untreated leather, dairy and domestic wastewater was found to be 5.6, 5.1 and 6.2 and after treatment the pH had increased to 6.8, 6.7 and 6.5 which show that treatment of wastewater using MFC had changed acidic nature of effluent to neutral. The total dissolved solids (TDS) and total suspended solids (TSS) in the wastewater were measured and percentage of removal was found to be 65%, 76% for leather effluent 71%, 76% for dairy effluent 53%, 57% for domestic wastewater respectively. Other parameters like COD and BOD were also estimated and removal percentage was found to be 80%, 64% for leather wastewater, 85.4%, 79% for dairy wastewater and 65%, 47% for domestic wastewater respectively.
| Parameters | Leather wastewater | Dairy wastewater | Domestic wastewater | |||
|---|---|---|---|---|---|---|
| Before | After | Before | After | Before | After | |
| Colour | Greyish black | Greyish black | Milky white | Milky white | Light green | Black |
| Odour | Pungent | Pungent | Bad | Bad | Bad | Bad |
| pH | 5.6 | 6.8 | 5.1 | 6.7 | 6.3 | 6.5 |
| BOD (mg/L) | 920 | 190 | 522 | 184 | 280 | 146 |
| COD (mg/L) | 2754 | 540 | 1686 | 273 | 547 | 188 |
| TDS (mg/L) | 1846 | 642 | 2050 | 582 | 475 | 220 |
| TSS (mg/L) | 3010 | 720 | 2720 | 639 | 262 | 112 |
| Sulphate (mg/L) | 340 | 230 | 210 | 100 | 70 | 30 |
| Chloride (mg/L) | 929 | 1516 | 538 | 1468 | 538 | 2446 |
| Copper (mg/L) | - | - | - | - | <0.05 | 13.7 |
| Chromium (mg/L) | 29.3 | 0.88 | - | - | - | - |
Table 4. Physiochemical characteristic of leather, dairy and domestic wastewater.
Atomic Absorption Spectroscopy (AAS) was used to analyze the amount of the heavy metals such as chromium and copper present in the wastewater and percentage of removal of chromium was found to be 96%. The amount of copper was found to be high in treated water when compared to untreated wastewater due to oxidation of the electrode copper rod by losing electrons. The reduced cu+ ions are released into anodic chamber resulting in increase in the amount of copper. Other chemicals such as sulphate and chloride were also estimated and percentage removal of sulphate was found to be 32% for leather effluent, 52% for dairy effluent and 57% for domestic wastewater respectively. The amount of chloride is high in treated wastewater than in untreated wastewater due to the transfer of chloride ions along with protons from anode to cathode through salt bridge.
Conclusion
Power generation during the treatment of dairy, leather, domestic wastewater using microbial fuel cell (MFC) was successfully demonstrated in this study. The experimental runs were carried out without and with mediator as first and second phase respectively. From the results, it was evident that second phase of experimental run in MFC had produced much higher power due to the addition of mediator. The mediator molecule had increase the power generation by shuttling the electrons produced inside the microbes and transferred it to the anode with the help of redox reaction. The reduction in COD and BOD after the treatment process showed the successful treatment of dairy, leather and domestic wastewater in both phases of experimental runs. The results also showed that level of heavy metals such as chromium and sulphate were also reduced after the treatment of wastewater. In both phases of experimental run, power generated during the treatment of dairy wastewater was more. This showed that the organism Saccharomyces cerevesiae has effectively degraded the organic matter present in the dairy wastewater and produced more electrons when compared to leather and domestic wastewater.
References
- Coyle ED, Simmons RA. Understanding the global energy crisis, Purdue University Pres. 2014.
- Vernona C, Thompson E, Cornella S. Carbon dioxide emission scenarios: Limitations of the fossil fuel resource. Procedia Environ Sci. 2011;6:206-15.
- Potte MC. Electrical effects accompanying the decomposition of organic compounds. Royal Society (Formerly Proceedings of the Royal Society). 1911;84:260-76.
- Allen RM, Bennetto HP. Microbial fuel-cells: Electricity production from carbohydrates. Appl Biochem Biotechnol. 1993;39:27-40.
- Davis F, Higson SPJ. Biofuel Cells: Recent advances and applications. Biosens Bioelectron. 2007;22:1224-35.
- Rahimnejad M, Adhami A, Darvari S, et al. Microbial fuel cell as new technology for bioelectricity generation: A review. Alexandria Eng J. 2015;54:745-56.
- Das S, Mangwani N. Recent developments in Microbial fuel cells: A review. J Sci Indus Res. 2010;69:727-31.
- Shrivastava S, Bundela H. Power generation through double chamber MFC operation by slurry mixed with different substrates. Int J Eng Trends Technol. 2013;4:4201-5.
- Alterman P, Rabaey K, Clauwaert P, et al. Microbial fuel cell from wastewater treatment. Water Sci Technol. 2006;54:9-15.
- Reddy LV, Kumar SP, Wee YJ. Microbial fuel cells (MFCs): A novel source of energy for new millennium. Curr res technol edu topic appl microbiol microbial biotechnol. 2010;3:956-64.
- Logan BE, Hamelers B, Rozendal R, et al. Microbial fuel cells: Methodology and technology. Environ Sci Technol. 2006;40:5181-92.
- Chandra I. Waste to energy: Microbial fuel cell a novel approach to generate bio-electricity. Int J adv Biotechnol Bioinf. 2012;1:33-40.
- American Public Health Association. Standard Methods for the Examination of Water and Wastewater. 19th edition. APHA, Washington, DC, USA. 2005.
- Rahimnejad M, Mokhtarian N, Najafpour G, et al. Low voltage power generation in a biofuel cell using anaerobic cultures. World Appl Sci J. 2009;6:1585-8.
- Gopala Rao G, Viswanath SG. Simple conditions for the use of ferroin indicator in cerimetric titrations of antimony(III) alone and in mixtures with arsenic(III) Analytica Chimica Acta. 1977;92:163-9.
- Nair k, Renganathan K. Barathi S, et al. Performance of salt-bridge microbial fuel cell at various agarose concentrations using hostel sewage waste as substrate. Int J Adv Res Technol. 2013;2:326-30.