Original Article
, Volume: 15( 4)Kinetics and Mechanism of Oxidation of Indigo Carmine with Potassium Bromate: Effect of CTAB and SDS Micelles
- *Correspondence:
- Ramakrishna K Department of Chemistry, College of Science, Gitam University, Visakhapatnam, India, Tel: +91-9866234551; E-mail: karipeddirk@gmail.com
Received: March 23, 2017; Accepted: October 30, 2017; Published: November 06, 2017
Citation: Kalyana Chakravarthy M, Ramakrishna K, Subba Rao PV. Kinetics and Mechanism of Oxidation of Indigo Carmine with Potassium Bromate: Effect of CTAB and SDS Micelles. Int J Chem Sci. 2017;15(4):220.
Abstract
The oxidation of indigo carmine with potassium bromate both in absence and presence of SDS, CTAB micelles is investigated. The kinetic runs were carried out in mercuric acetate which scavenges the bromate-bromide reaction. The pseudo first order rate constants are under the condition [IC]˂[BrO3-1]. The reaction shows first order kinetics with indigo carmine, H+ ion and Bromate ion. The reaction rate is increased with increase in the [SDS] and [CTAB]. The effect of micelles on reaction rate is explained by Berezin treatment and the binding constant of reacting substances with micelles is determined.
Keywords
Potassium bromate; Indigo carmine (IC); Sodium dodecyl sulphate (SDS); Cetyl trimethyl ammonium bromide (CTAB)
Introduction
Textile industries are one of the chief sources for release of waste water containing unused dyes. On the basis of functional group and structure, dyes were classified as anionic, neutral and cationic [1]. Indigo carmine with chemical name 5, 51 indigo disulphonic acid sodium salt is an oldest anionic dye which belongs to the class of indigoids which are extensively used in dyeing cotton clothes (blue jeans). The primary component of the dye is indigotin synthesized from the extract of indigofera tinctoria [2]. In aqueous solutions, the dye possess intense blue colour which is because of cross conjugation or H-chromophore attached at double bond [3]. In the structure of this dye, there are two NH donor and two CO acceptor groups attached to the double bonded carbon atoms [4]. The dye shows its role as an indicator in quantitative analysis [5,6] and in drug synthesis [7]. The dye has appreciable toxicity and show adverse effects when comes in contact with eyes, skin and also effects reproductive, developmental and neuronal systems [8]. Certain methods were in force in decolorization of this dye like adsorption [9], Nano degradation using TiO2 as catalyst [10], photo catalytic methods using visible and UV light [11], advanced oxidation processes [12-16] and chemical oxidation [17,18]. Jonnalgadda et al. [19] investigated the oxidation of indigo carmine with bromate in aqueous sulphuric acid at high concentration and reported a competitive mechanism. The present authors carried out the kinetic study in low [acid] in presence of CTAB and SDS micelles.
Experimental
Preparation of solutions
The solutions of indigo carmine, potassium bromate, sulphuric acid and sodium sulphate were prepared from the samples procured from E-Merck-India Pvt. Ltd., Mumbai, of AR grade quality. SDS (AG, Fluka) and CTAB (Sigma Aldrich) is prepared as per the literature and the cmc is determined [20,21]. The solutions are standardized as per the procedure available in the literature.
Stoichiometry
The stoichiometry of the reaction is determined by mole ratio method. The reaction between IC and Bromate is carried out by fixing the [IC] and varying the [BrO31-] in the range of (1.0 to 9.0) × 10-5 moldm-3 in aqueous sulphuric acid and in presence of mercuric acetate. The reaction stoichiometry is determined from the plot of absorbance vs. mole ratio which is found to be 3:2 (i.e., 3 moles of Indigo carmine reacts with 2 moles of Bromate ion). The stoichiometric equation is presented as:
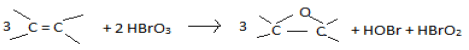
Product analysis
Indigo carmine solution (1.0 × 10-5 moldm-3) was mixed with an equivalent amount of bromate (1.0 × 10-5 moldm-3) in 0.1 moldm-3 sulphuric acid and in presence of mercuric acetate. After the discharge of the blue colour, a yellow coloured solid was obtained as product. The product is identified as Isatin-5-monosulphonic acid by carrying out the spot test prescribed by Feigl [21]. The test consists in heating the product in a micro test tube with a drop of alcoholic solution of 4-NitroPhenyl Hydrazine for 5 minutes. After cooling the solution a drop of 10% sodium hydroxide and a drop of 1% magnesium nitrate were added. The formation of blue colour precipitate confirmed the product as Isatin-5-monosulphonic acid.
Kinetic procedure
The reaction was followed spectrophotometrically at known intervals of time using “Systronics 106 visible spectrophotometer”. The course of the reaction was followed at a wavelength of 610 nm in presence of SDS micelles and at 570 nm in presence of CTAB micelles till the completion of 90% reaction [22]. All the kinetic runs were carried out in a temperature controlled water bath (± 0.1°C). The kinetic runs were carried out under pseudo first order conditions [BrO31-] >> [IC] in presence of mercuric acetate (1.0 × 10-3 moldm-3) which scavenge bromide in the reaction. The pseudo first order rate constants are determined from the slopes of the linear plots drawn between log (absorbance) vs. time. The kinetic runs were carried out in duplicate and rate constants found to agree within ± 5%.
Results and Discussion
The kinetic results are summarized as follows:
1) The reaction obeys first order kinetics with indigo carmine in presence of SDS and CTAB micelles. The rate data is presented in TABLE 1.
| In SDS | In CTAB | ||
|---|---|---|---|
| 105[IC] moldm-3 | 103 k1 sec-1 | 105[IC] moldm-3 | 103 k1 sec-1 |
| 0.5 | 1.15 | 1.65 | 1.34 |
| 1.0 | 1.15 | 2.48 | 1.35 |
| 1.5 | 1.15 | 3.30 | 1.27 |
| 2.0 | 1.15 | 4.13 | 1.26 |
| 3.0 | 1.15 | 4.96 | 1.22 |
TABLE 1. Effect of varying concentration of indigo carmine.
[BrO3-]=1.0 × 10-3 moldm-3, [H+]=0.1 moldm-3, [SDS]=7.0 × 10-3 moldm-3, [Hg(OAc)2]=1.0 × 10-3 moldm-3, [BrO3-]=1.0 × 10-3 moldm-3,[H+]=0.05 moldm3, [CTAB]=5.0 × 10-4 moldm-3,[Hg(OAc)2]=1.0 × 10-3 moldm-3.
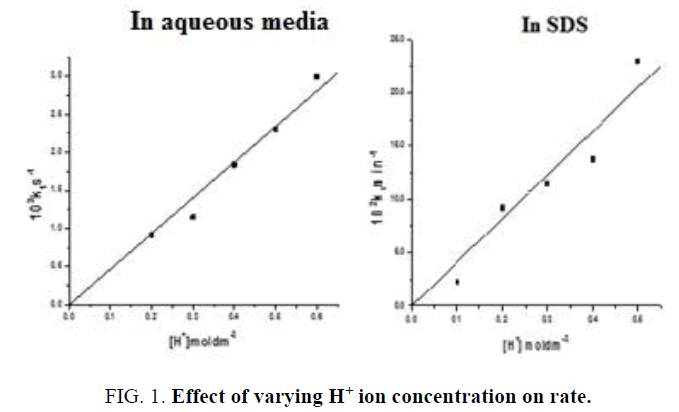
2) The reaction obeys first order kinetics with respect to [H+] ion in absence and presence of SDS micelles. This is evident from the linear plots of k1 and [H+] ion passing through origin. (FIG. 1 ). In presence of CTAB micelles, the reaction rate is inhibited by [H+]. (TABLE 2).
| [H+] moldm-3 |
103 k1 sec-1 No micelle |
[H+] moldm-3 |
102 k1 sec-1 In CTAB |
[H+] moldm-3 |
102 k1 min-1 In SDS |
|---|---|---|---|---|---|
| 0.2 | 0.92 | 0.3 | 4.03 | 0.1 | 2.30 |
| 0.3 | 1.15 | 0.4 | 3.45 | 0.2 | 9.21 |
| 0.4 | 1.84 | 0.5 | 2.76 | 0.3 | 11.5 |
| 0.5 | 2.30 | 0.7 | 2.30 | 0.4 | 13.8 |
| 0.6 | 3.0 | 0.8 | 1.84 | 0.5 | 23.0 |
TABLE 2. Effect of varying H+ ion concentration.
In aqueous media: [IC]=2.0 × 10-5 moldm-3, [BrO31-]=1.0 × 10-2 moldm-3, µ=0.8 moldm-3, [Hg (OAc) 2]=1.0 × 10-3 moldm-3,
In CTAB: [IC]=1.0 × 10-5 moldm-3, [BrO31-]=1.0 × 10-2 moldm-3, [Hg+2]=1.0 × 10-3 moldm-3, [CTAB]=1.0 × 10-3 moldm-3, µ=0.8 moldm-3,
In SDS: [IC]=3.0 × 10-5 moldm-3, [Bromate]=1.0 × 10-2 moldm-3, [SDS]=7.0 × 10-3 moldm-3, µ=0.8 moldm-3, [Hg+2]=1.0 × 10-3 moldm-3.
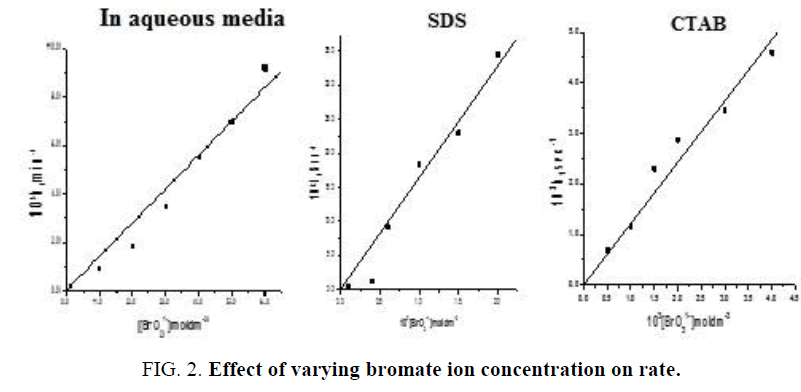
3) The reaction shows first order kinetics with respect to [BrO31-] ion both in absence and presence of SDS, CTAB micelles. Plots drawn between [BrO31-] and k1 are linear passing through origin. (FIG. 2). The kinetic data is presented in TABLE 3.
| [BrO31-]102 moldm-3 |
103 k1 min-1 In aqueous media |
[BrO31-]103 moldm-3 |
103 k1 sec-1 In CTAB |
[BrO31-]102 moldm-3 |
102 k1 sec-1 In SDS |
|---|---|---|---|---|---|
| 1.0 | 0.92 | 0.5 | 0.69 | 0.1 | 0.46 |
| 2.0 | 1.84 | 1.0 | 1.15 | 0.4 | 1.15 |
| 3.0 | 3.45 | 1.5 | 2.30 | 0.6 | 9.21 |
| 4.0 | 5.5 | 2.0 | 2.87 | 1.0 | 18.4 |
| 5.0 | 7.0 | 3.0 | 3.45 | 1.5 | 23.03 |
| 6.0 | 9.2 | 4.0 | 4.60 | 2.0 | 34.5 |
TABLE 3. Effect of varying bromate ion concentration on rate.
In aqueous media: [IC]=2.0 × 10-5 moldm-3, [H+]=0.2 moldm-3, µ=0.8 moldm-3, [Hg (OAc)2]=1.0 × 10-3 moldm-3
In CTAB: [IC]=2.0 × 10-5 moldm-3, [acid]=0.2 moldm-3, [CTAB]=1.0 × 10-3 moldm-3, [Hg+2]=1.0 × 10-3 moldm-3
In SDS: [IC]=1.0 × 10-5 moldm-3, [H+]=0.1 moldm-3, [SDS]=5.0 × 10-3 moldm-3, [Hg+2]=1.0 × 10-3 moldm-3.
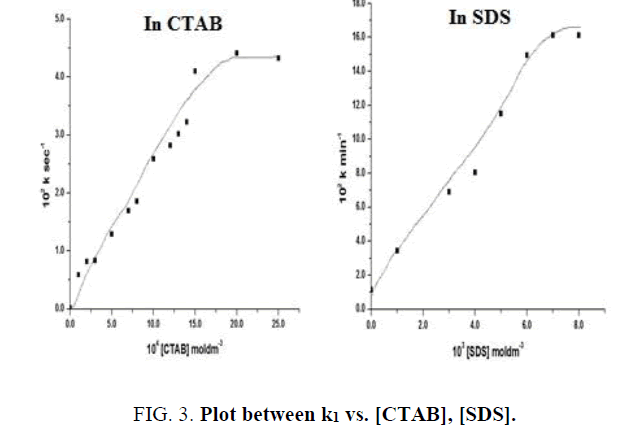
4) The reaction rate is increased by 232 times with increase in the [CTAB] and by 15 times with increase in the [SDS] and limiting value is reached (FIG. 3). TABLE 4.
| 104[CTAB] moldm-3 |
102 k sec-1 |
|---|---|
| 0.0 | 0.019 |
| 1.0 | 0.59 |
| 2.0 | 0.82 |
| 3.0 | 0.83 |
| 5.0 | 1.29 |
| 7.0 | 1.69 |
| 8.0 | 1.86 |
| 10.0 | 2.59 |
| 12.0 | 2.82 |
| 15.0 | 4.10 |
| 20.0 | 4.41 |
| 103[SDS] moldm-3 | 102 k min-1 |
|---|---|
| 0.0 | 1.15 |
| 1.0 | 3.45 |
| 3.0 | 6.90 |
| 4.0 | 8.06 |
| 5.0 | 11.51 |
| 6.0 | 14.96 |
| 7.0 | 16.12 |
| 8.0 | 16.12 |
TABLE 4. Micellar effects.
CTAB effect: [IC]=4.0 × 10-5 moldm-3, [KBrO3]=1.0 × 10-2 moldm-3, [H+]=0.2 moldm-3, [Hg (OAc)2]=1.0 × 10-3 moldm-3
SDS effect, [IC]=3.0 × 10-5 moldm-3, [BrO3-]=1.0 × 10-2 moldm-3, [H+]=0.2 moldm-3, [Hg+2]=1.0 × 10-3 moldm-3.
Jonnalagadda et al. [19] reported the oxidation of indigo carmine with bromate in aqueous sulphuric acid at high concentration in the range of (1.5 to 3.0) moldm-3. They reported consecutive – competitive mechanism and reported the rate expression assuming the active oxidizing species as HOBr.
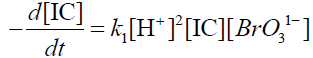
The present authors employed a different approach in the kinetic study of this reaction. It is known that when the reaction is carried out in presence of mercuric acetate under the condition [Hg (CH3COO)2] >>[indigo carmine] the mercuric ion Hg+2 forms a complex with Br1- thus scavenging it and now only BrO31- oxidise IC. Since [BrO31-] >>[IC], the Br1- concentration found in the reaction is equivalent to Indigo carmine and almost all the bromide will be bound by Hg+2 as a complex.
The present author found that in presence of no auto catalysis is present, presumably due to elimination of BrO31- Br1- reaction forming Br2. Plot of log (absorbance) vs. time is a good straight line even up to 95% of the reaction with no sign of auto catalysis or auto inhibition showing clear first order kinetics with respect to Indigo carmine. The reaction obeys first order kinetics with respect to BrO31- and H+ ion. The second order path in H+ ion is absent because the author employed much lower H+ ion concentration than Jonnalagadda et al. [19]. The [H+] ion catalysis can be explained as due to the formation of more reactive species HBrO3 by interaction with BrO31- in a pre-equilibrium which interacts with indigo carmine in the subsequent rate determining step.
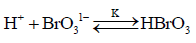 (1)
(1)
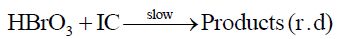 (2)
(2)
The protonated BrO31- i.e, HBrO3 is more effective oxidizing species than the negatively charged BrO31-ion. The rate equation for the rate determining step (2) will be,
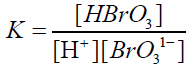 (3)
(3)
Rate=k [HBrO3] [IC] (4)
Hence Rate=kK[H+][IC][BrO31-] (5)
The rate law explains the first order kinetics with respect to [H+], IC and BrO31-.
Effect of CTAB
The author investigated the micellar effect of CTAB on oxidation of Indigo carmine by BrO31- in aqueous sulphuric acid medium and in presence of excess mercuric acetate which scavenges Br1- and eliminates the auto catalysis – complication. The author found that CTAB micelle exerts more catalytic effect than SDS. The reaction exhibits first order kinetics in BrO31- and Indigo carmine and H+ ion inhibited the reaction. The H+ ion inhibition is due to BrO31- is preferred by CTAB to HBrO3, the former being the active oxidizing species for the reaction in the CTAB micelles.
The positive CTAB micelles interact with both BrO31- and indigo carmine which are bound by these micelles. The micellar catalysis did not conform to the bimolecular pattern of Berezin’s where in the rate initially increases with increase in the [CTAB] for greater than critical micellar concentration (cmc) but shows limiting at higher value of [CTAB]. The author found that rate-[CTAB] profile has a plateau with no maximum. Therefore, the Berezin rate law will be:
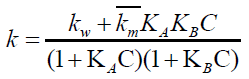 (6)
(6)
Where C=[CTAB]-critical micellar concentration of CTAB (9.2 × 10-4 moldm-3)
KA and KB represents the binding constants of bromate ion and Indigo carmine respectively
Since HBrO3 is hydrophilic, it may not be strongly bound CTAB and KAC ≅ 1 (where A represents bromate ion) and can be neglected. The equation now reduces to,
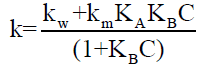 (7)
(7)
This equation 7 explains the binding pattern of plot of k vs. C.
As the value of kw in the above equation is found to very small in comparison with k, it can be neglected. The equation further reduces to,
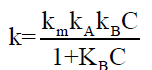 (8)
(8)
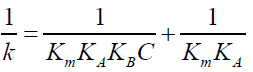 (9)
(9)
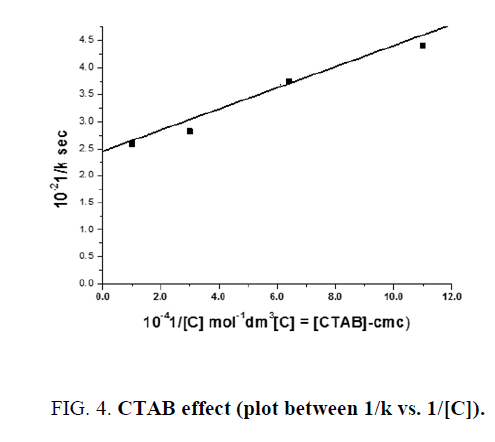
The author found that the plot of 1/k vs. 1/C, where C=[CTAB]-cmc, is linear with positive intercept (FIG. 4). From the value of slope and intercept obtained the value of KB (binding constant of Indigo carmine with CTAB micelles) and 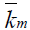 have been determined and found to be 2307.0 ± 115.35 dm3 mol1- and 6.66 × 10-2 sec-1.
have been determined and found to be 2307.0 ± 115.35 dm3 mol1- and 6.66 × 10-2 sec-1.
Effect of SDS
In the presence of SDS micelles the reaction obeys first order kinetics with respect to bromate ion, H+ ion and Indigo carmine and the hydrogen ion catalysis can be explained by assuming that HBrO3 but not BrO31- as the active oxidizing species in the presence of SDS involving the following rate-determining steps:
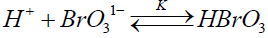 (10)
(10)
HBrO3 + I. C → products (11)
The Berezin equation can be written:
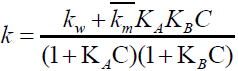 (12)
(12)
Where KA and KB are binding constants of Bromate ion and Indigo carmine respectively,
The rate- [Surfactant] profile shows a limiting behaviour at higher SDS concentration. It is easy to explain this behaviour because HBrO3 being hydrophilic will have a low value of binding constant (KA) and (1+ KAC) ≅ 1.
Then the Berezin equation undergoes modification to,
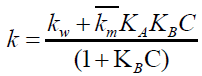 (13)
(13)
Since the uncatalyzed component is small compared to micellar component, kw can be neglected. Then the equation 16 reduces to,
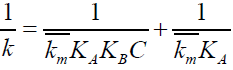 (14)
(14)
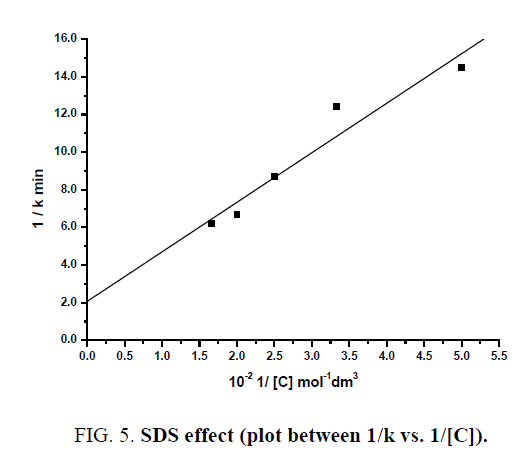
The plot of 1/k vs. 1/C is linear with a positive intercept (FIG. 5) and from the slope and intercept of the plot, KB (Binding constant of Indigo carmine with SDS) and 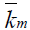 KA has been found to be 78.2 ± 3.91 dm3 mol-1 and 4.86 × 10-3 min-1.
KA has been found to be 78.2 ± 3.91 dm3 mol-1 and 4.86 × 10-3 min-1.
References
- Mishra G, Tripathy M. A critical review of the treatment for decolorisation of textile effluent. Colourage. 1993;40:35-8.
- Andreotti A, Bonaduce I, Colombini MP, et al. Characterisation of natural indigo and shellfish purple by mass spectrometric techniques. Rapid Communications in Mass Spectrometry. 2004;18(11):1213-20.
- Saggioro EM, Oliveira AS, Pavesi T, et al. Solar CPC pilot plant photocatalytic degradation of indigo carmine dye in waters and wastewaters using supported-TiO2: Influence of photodegradation parameters. Int J Photo Energy. 2015;2015:12.
- Vautier M, Guillard C, Herrmann JM. Photocatalytic degradation of dyes in water: Case study of indigo and of indigo carmine. J Catalysis. 2001;201(1):46-59.
- Curr E. Encyclopedia of microscopic stain (Leonard Hall, London, 1960). pp:235.
- Scholz F. Electro analytical methods, guide to experiments and applications, Springer-Verlag, Berlin. pp:51.
- El Hamd MA, Derayea SM, Abdelmageed OH, et al. Spectrophotometric method for determination of five 1,4-dihydropyridine drugs using N-bromosuccinimide and indigo carmine dye. Int J Spectroscopy. 2013;2013:7.
- Sanroman MA, Pazos M, Ricart MT, et al. Electrochemical decolourisation of structurally different dyes. Chemosphere. 2004;57(3):233-9.
- Rehman R, Zafar J, Nisar H. Adsorption studies of removal of indigo caramine dye from water by formaldehyde and urea treated cellulosic waste of citrus reticulata peels. Asian J Chem. 2014;26(1):43.
- Yogesh MV, Shrivastava VS. Degradation of indigo caramine dye by sol gel deposited nanocrystaline cadmium sulphide thin film. Adv Sci Lett. 2012;5(1):173-7.
- Vinod SS. Removal of indigo caramine dye by using nanosized semiconducting photocatalyst in aqueous media. Advances in Applied Science Research. 2011;2(3):280-286.
- Sano T, Puzenat E, Guillard C, et al. Improvement of photocatalytic degradation activity of visible-light-responsive TiO2 by aid of ultraviolet-light pretreatment. J Phys Chem. 2009;113(14):5535-40.
- Khataee AR, Vatanpour V, Ghadim AA. Decolorization of CI acid blue 9 solutions by UV/Nano-TiO2, Fenton, Fenton-like, electro-Fenton and electrocoagulation processes: A comparative study. J Hazar Mat. 2009;161(2):1225-33.
- Liu G, Wu T, Zhao J, et al. Photoassisted degradation of dye pollutants. 8. Irreversible degradation of alizarin red under visible light radiation in air-equilibrated aqueous TiO2 dispersions. Environ Sci Technol. 1999;33(12):2081-7.
- Konstantinou IK, Albanis TA. TiO2-assisted photocatalytic degradation of azo dyes in aqueous solution: Kinetic and mechanistic investigations: A review. Applied Catalysis B: Environmental. 2004;49(1):1-4.
- Fujishima A, Rao TN, Tryk DA. Titanium dioxide photocatalysis. Journal of Photochemistry and Photobiology C: Photochemistry Reviews. 2000;1(1):1-21.
- Murty PS, Subbaiah KV, Rao PS. Kinetics of oxidation of indigo carmine by potassium peroxydisulfate. Reaction Kinetics and Catalysis Letters. 1979;11(1):79-83.
- Edokpayi JN, Iyun JF, Idris SO. Kinetics and mechanism of the electron transfer reaction between tetraoxoiodate (VII) ion and indigo carmine in aqueous hydrochloric acid medium. Adv App Sci Res. 2011;2(3):1-9.
- Jonnalagadda BS, Simoyi RH. A kinetic study of the oxidation of indigo carmine with acidic bromate. J Chem Soc. Perkin Trans. 1988.
- Goheen CS, Matson SR. Determining the critical micelle concentration of surfactants using a binary mixing system. Journal of the American Oil Chemists' Society. 1989;66(7):994-7.
- Feigl F. Spot Tests in Organic Analysis” (Elsevier Publishing Company, London), 1966, p:540.
- Martinek K, Yatsimirskii AK. Physicochemical foundations of micellar catalysis. Russian Chemical Reviews. 1973;42(10):787-802.