Research
, Volume: 17( 1) DOI: 10.37532/0974-7494.2023.17(1).190.IRON-CONTAINING MINERALS IN THE BIOX PROCESS
- *Correspondence:
- Kholmirzaeva Khilola Norboy KiziPh.D. Student, Nanochemistry, Nanophysics, and Nanotechnology, Department of Polymer Chemistry and Chemical Technology, Samarkand State University, Uzbekistan ; E-mail:khilolaxolmirzayeva@gmail.com
Received date: 12-March-2023, Manuscript No. tsnsnt-23-94059; Editor assigned: 16-March-2023, Pre-QC No. tsnsnt-23-94059 (PQ); Reviewed: 21-March-2023, QC No. tsnsnt-23-94059 (Q); Revised: 24-March-2023, Manuscript No. tsnsnt-23-94059 (R); Published: 31March-2023. DOI: 10.37532/0974-7494.2023.17(1).190.
Citation: Akhrorovich TI. Urol kizi SN, Norboy Kizi KK, et al. Iron-Containing Minerals in the Biox Process. Nano Tech Nano Sci IndJ.2023;17(1):190
Abstract
The rapid development of the industry leads to the use of various types of minerals in large quantities. In particular, the consumption of non-ferrous and rare metals is increasing, in addition, their reserves on an industrial scale are sharply decreasing. A quarter of the ore gold reserves are refractory gold-arsenic ores concentrated in large deposits. Due to the presence of finely disseminated submicroscopic gold in sulfides, the use of traditional cyanidation technology is ineffective, and the presence of elevated amounts of arsenic and antimony complicates the processing of concentrates at metallurgical plants. Deposits of refractory ores are not put into operation due to the lack of industrial technology for processing refractory concentrates. Therefore, the problem of developing and implementing in the gold mining industry an effective environmentally safe biohydrometallurgical technology for extracting gold from refractory gold-arsenic concentrates is of national importance and is very relevant. Processing or beneficiation of ores with a reduced content of metal, that is, unnecessary for use, requires their processing in large quantities. This significantly increases the cost of metal production. Given this, mining and metallurgical enterprises are recommended to find low-cost and efficient methods of processing low-metal ores and use them in order not to increase the cost. These include hydrometallurgical, and especially bacterial-chemical methods.
Introduction
Cyanidation is a royal road for the industrial extraction of gold (Au) from most ores in terms of extraction efficiency, time, and cost. However, due to its extreme toxicity, several greener alternatives have been investigated; thiosulfate and ammonia under alkaline conditions, and halides under acidic conditions [1-5].
The use of the bacterial oxidation process in the treatment of sulfide ores has been recognized as environmentally safe and economical. This process allows for the elimination of SO2 and As2O3 gases as present in the roasting process. Again due to the low operating temperatures of 35ºC - 45 ℃, the operating cost of bacterial oxidation is relatively lower.
However, Acid Mine Drainage (AMD) is a major problem all over the world, especially where coal and gold mine activities are common. AMD contamination is the release of toxic metals into the environment, which results from any mining procedure that causes the oxidation of iron sulfides. Once AMD is generated, it is difficult to control the process and the treatment also requires high cost [6, 7]. AMD causes severe environmental impacts, particularly on soil, water resources, and aquatic communities [8, 9]. The main source of AMD is the oxidation of sulfide mineral ores, which are initially exposed to the environment by intensive mining activities. In particular, among the metal sulfides, pyrite ore (FeS2, commonly known as fool's gold) is one of the main minerals responsible for the generation of AMD due to its ease of oxidation when exposed to oxygen, water, and microorganisms [10].
The oxidation reactions are:

The main end-product of the oxidation reaction is ferric sulfate. The hydrolysis reactions include:

The overall reaction is given by the combination of reactions:

Further reactions lead to the formation of jarosites.

The jarosite family of compounds, AFe3(SO4)2(OH)6, consists of compounds where A is: H3O+, Na+, K + [11].
In recent years, several types of research have been done to improve the bio-oxidation efficiency of refractory gold ores, which include using different nutritional types of microorganisms and different thermophilic types of microorganisms, adding nutrients that favor microbial growth, and proposing two-stage oxidation processes [12, 13]. The most promising process for industrial improvement is the two-stage oxidation process which includes a high-temperature ferric oxidation stage followed by a biological oxidation step [14, 15].
Materials and Methods
The research material was samples of sulfide ores from different stages of bacterial leaching, from ore preparation, and enrichment, up to thickening, neutralization, sorption cyanidation, and analysis of cyanidation tailings.
To prepare solid phase samples for spectral analysis, the following preparatory measures were performed. The solid residue after filtration and separation of the liquid phase was treated with concentrated nitric acid at a temperature of 600ºC and dried in an oven for 4 hours.
Next, the dry filtrate was subjected to alkaline treatment in a saturated solution of KOH at a temperature of 60℃ and dried in an oven for 4 hours. The obtained solid residue was subjected to firing in a muffle furnace at a temperature of 450℃. The analysis of the liquid phase of the samples was carried out by liquid chromatography, and the solid phase by X-ray diffraction spectral analysis on an ICP-MS-7500 instrument.
Result and Discussion
very significant number of currently known minerals are of great practical importance as mineral raw materials (provided, of course, that their accumulations in certain areas, called mineral deposits, have an industrial content and reserves sufficient to provide an enterprise for the development of the deposit). Some minerals, especially ore minerals, contain certain metals valuable for industry (iron, manganese, copper, lead, zinc, tin, tungsten, molybdenum, etc.), which are extracted during the metallurgical processing of ores. Other types of minerals may contain noble metals. Most often, there is a combination of 12-15 chemical elements that are part of all known minerals (TABLE 1).
TABLE 1. Mineral composition of sulfide ores
| Rock-forming minerals | Content,% | Accessory minerals | Content,% | Ore minerals | Content,% |
|---|---|---|---|---|---|
| Quartz and feldspars | 22-57 | Rutile Ilmenite |
0,2-0,7 0,02 |
Pyrite Arsenopyrite | 3,0-9,0 0,1-1,5 |
| Hydromicas, kaolinite, dickite, biotite | 25-60 | Magnetite | -«- | Antimonite | 3 |
| Ankerite, oligonite, siderite, calcite | 2-17 | Zircon Tourmaline |
-«- -«- |
Sphalerite Fahlore |
0,01 -«- |
| Carbonaceous matter | 0,02-0,2 | moissonite | -«- | Pyrrhotite | -«- |
| Barite | 0,05-0,2 | grenades | -«- | Boulangerite | -«- |
| Apatite | -«- | Chalcopyrite | Unit of grain | ||
| Olivine | -«- | Galena | -«- | ||
| Freisbergite | -«- | ||||
| Gold | -«- |
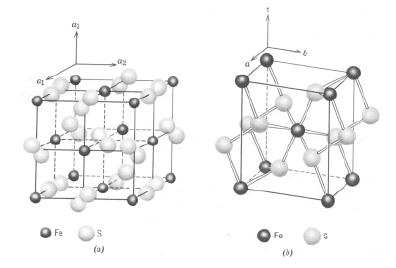
Iron ions are mainly in the composition of ore minerals - pyrite - FeS2 and arsenopyrite - AsFeS, as well as in the composition of fayalite - Fe2[SiO4] (FIG. 1.). Chemical composition: Fe - 46.6%, S - 53.4%. It often contains impurities in very small amounts: Co (cobalt pyrite), Ni, As, Sb, and sometimes Cu, Au, Ag, etc. In these cases, we are essentially dealing with solid pseudo-solutions—crystal sols.
Iron atoms in the composition of sulfide minerals are in the divalent form. During bacterial leaching in oxidation reactors in a sulfuric acid medium, they pass into solution in the form of ferrous and ferric sulfates (FIG. 1). In the process of bacterial leaching, ferrous iron, forming hydroxides, can also precipitate. Sulfide sulfur of pyrite is oxidized to sulfate, and ferrous iron to oxide, and two products are formed: sulfate ferrous oxide and sulfuric acid.
Sulfate ferrous oxide chemically oxidizes pyrite with the formation of ferrous sulfate and elemental sulfur. The ferrous iron is again oxidized by bacteria to the oxide iron, which can be used as an oxidizing agent for pyrite.
The leading role in the implementation of the considered transformations belongs to thionic bacteria - A. ferrooxidans, oxidizing sulfides and ferrous iron, and A. thiooxidans - oxidizing elemental sulfur.
In the absence of thionic bacteria, oxygen dissolved in water and sulfate oxide iron (III) can in principle serve as oxidizers of minerals. However, at normal pressure and low temperatures, the oxidation of sulfides with oxygen proceeds at very low rates, and sulfate oxide iron in an acidic environment without iron-oxidizing bacteria is practically not formed (iron remains in the ferrous form (II)).
Thus, using pyrite as an example, a twofold role of thionic bacteria in the leaching of non-ferrous metals was found: direct participation in the oxidation of sulfides and indirect participation due to the formation of leaching reagents (ferric sulfate oxide (III) and sulfuric acid).
Bacteria A. ferrooxidans are able to accelerate the oxidation of Fe2+ and sulfide minerals by several times, tens, and sometimes even hundreds and thousands of times compared to purely chemical oxidation under the same conditions - arsenopyrite can be oxidized 4-8 times faster, chalcopyrite - in 6 times-12 times, covellite and bornite - 18 times, pyrite - 1000 times, ferrous iron in an acidic environment - 200,000 times (compared to oxidation with atmospheric oxygen). Elemental sulfur is oxidized with the participation of bacteria to sulfuric acid. The oxidation reaction of pyrite with oxygen is sometimes written as two successive stages: first, the oxidation of sulfide sulfur with the formation of ferrous sulfate, and then the bacterial oxidation of ferrous iron to oxide. Gold and silver also often accompany sulfide minerals - they occur as fine inclusions in minerals such as pyrite, arsenopyrite, and stibnite. After the preliminary destruction of the crystal lattice of sulfide minerals by thionic bacteria, the exposed precious metals can be extracted with any suitable solvent.
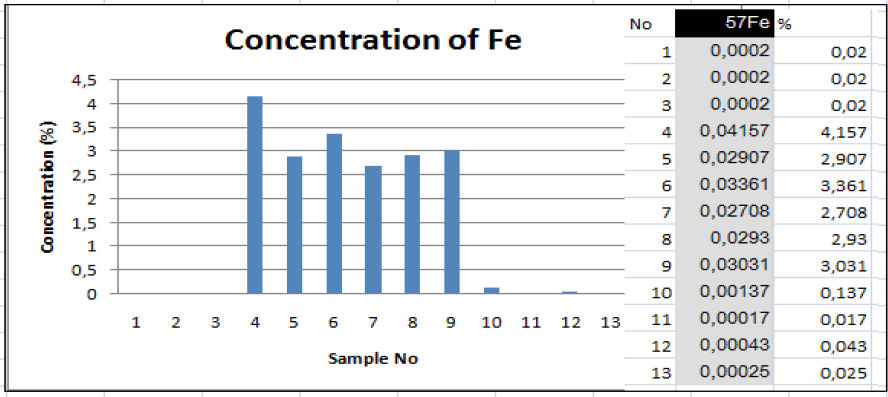
Quantitative analysis of the presence of iron ions showed a wide variation in its amount in the liquid phase as a percentage, especially in oxidation reactors - from 0.02 to 0.04% (FIG. 2).
Iron hydroxide residues at the alkalization stage in an amount of 7 g/l to 9 g/l. participating in reactions with sodium cyanate binds a certain amount of it and forming ferrocyanide, reduces the activity of cyanide. The demonstrated approaches to the study of bacterial leaching of sulfide minerals give some idea of the nature of these interactions; At the same time, they show that the bacterial leaching of metals is a very complex and theoretically still insufficiently developed process.
In the general case, it is necessary to consider the system ore (mineral) - water (and gas) medium - microorganism simultaneously from the standpoint of biochemistry and physical chemistry, taking into account the determining factors of the process.
The most intensive processes of destruction of rocks of useful deposits occur with a high activity of microorganisms, while later changes are mainly associated with non-biological oxidation-reduction processes.
In the biological cycle, along with living organisms, the most important component of all bioinert systems is involved - water, which is the main carrier of energy. Water also determines the classes of water migration (acid, sulphate, gley, etc.), the acid-base zoning of bio-inert systems, the replacement of acid horizons by alkaline ones, which determines their unity. Thus, the acidic environment in the biosphere is created by carbon (carbonic and organic acids), sulfur (H2S, H2SO4), locally chlorine (HCl) and Fluorine (HF). The most striking manifestation in this cycle of elements is played by aerobic acidophilic thionic microorganisms, which have taken their rightful place in this geological niche and play a leading role in the concentration and dispersion of elements. TABLE 2 shows that the composition of the ore and concentrate contains mainly the same type of rock-forming and ore minerals. In a sulfuric acid environment, in the presence of microorganisms with an active supply of oxygen, new minerals are formed due to the dispersion of elements and their assembly in a new quality.
TABLE 2. X-ray diffraction analysis of samples of sulfide ores in the process of bacterial leaching.
| № | Name of samples | Name of minerals |
|---|---|---|
| 1. | neutral, pH - 7.8 |
Quartz, albite, calcite, muscovite, ankerite, pyrite, chalcopyrite (arsenopyrite) |
| 2. | Concentrate | The same as in ore. |
| 3. | Sulfate pH - 1.5, 96 hours |
Albite Na[AlSi3O8], anorthite Ca[Al2Si2O8], pyrite, phengite, yansite (CaMnMn), todorokite, vashigite, quartz, orthoclase K[AlSi3O8]. |
| 4. | decantation, pH - 4.5 |
The same as in the sulfuric acid environment. |
| 5. | alkalization, pH - 11.5 |
The same as in the sulfuric acid environment. |
| 6. | cyanide tailings, pH - 9.5 |
The same as in the sulfuric acid environment. |
| 7. | cinder of cyanide tailings | Quartz, hematite Fe2O3, berlinite, calcite, analbite, silicon aluminum phosphate, muscovite. |
The formed new minerals remain unchanged when the pH of the medium changes and cyanides are added. Only when exposed to a strong high-temperature factor, the appearance of minerals characteristic of volcanic rocks was noted.
Bases and strong bases - alkalis (Na, K, Ca, Mg), the Clarke of which is much higher than the acidic components, neutralize acids when acidic waters interact with rocks. The acidic environment of the upper horizons (or less alkaline) is replaced by the alkaline (or less acidic) of the lower horizons, causing zoning.
Thus, in bioinert systems, redox and acid-base zoning is observed with predominant dominance of acid. The results obtained showed that, apparently, in the natural environment, the geological evolution of minerals occurred precisely with the acidic dominance of bio-inert systems in mineral deposits, where mineral formation can occur to the maximum extent. Alkaline, even if long-term, dominance in bio-inert systems does not give such a high degree of variability and to a lesser extent affects the migration and mineral-forming properties of elements.
TABLE 2 shows that if sulfide ores and concentrates contain the same rock-forming minerals - quartz, albite, calcite, and ore minerals - muscovite, ankerite, pyrite, chalcopyrite, and arsnopyrite, then in a sulfuric acid medium with the participation of thionic microorganisms, new minerals are added - anorthite, phengite, yansite, todorokite, and vashigite. The characteristics of these new minerals and their chemical structure, when they were searched on the Internet, did not give positive results. The roasting of sulfide ores also gave rise to new minerals - hematite, berlinite, analbite, and silicon-aluminum phosphate. Thus, the artificial appearance of sharp extreme factors, such as bio-inert sulfuric acid environment, as well as roasting, led to the mineral formation.
Minerals containing valuable metals are represented by the third group of rock-forming minerals - ore minerals. These include sulfide minerals - pyrite, arsenopyrite, antimonite, sphalerite, pyrrhotite, fahlore, chalcopyrite, boulangerite, galena, freisbergite, etc. Their content in the composition of the ore ranges from 0.1 to 3 and 9%. If the ore contains native gold, then it can be represented by single grains.
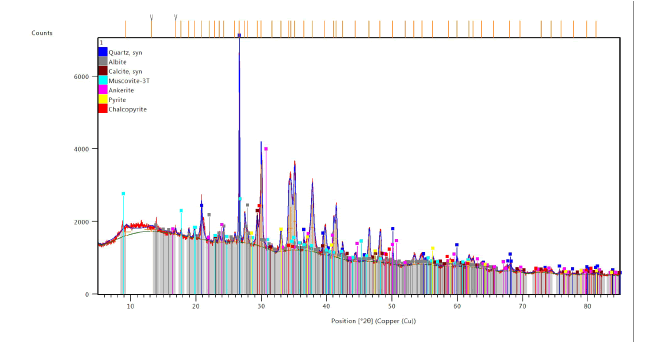
X-ray diffraction analysis of the solid phase of the ore revealed the following minerals in descending quantitative order- quartz, albite, calcite, muscovite, ankerite, pyrite, and chalcopyrite (FIG. 3).
During gravity and flotation enrichment, not only the element planned for extraction goes into the concentrate, but also a large number of rock-forming minerals, some of which can be completely dissolved in the sulfuric acid medium, the rest tend to dissolve partially, there are also minerals that are insoluble in any media for a long time.
Academician A.E. Fersman describes the environment around us, as consisting of ninety-nine percent of only twelve to twenty chemical elements, and by combining these elements in various ways with each other, we get the whole variety of minerals. The most important are, in descending order, iron, oxygen, magnesium, nickel, sulfur, calcium, aluminum, sodium, manganese, potassium, carbon, hydrogen, phosphorus, and cobalt. The same chemical elements form their basis, 12-20 elements seem to be the main ones, and among them, the first place is occupied by iron, silicon, magnesium, and gases- hydrogen and oxygen.
When analyzing samples of sulfide ores taken at various stages of processing, the maximum quantitative change in 26 chemical elements was found, which were most susceptible to dissolution and were noted in the liquid phase of the studied samples (TABLE 3). Academician A.E. Fersman was right when he spoke about the basic elements that make up all minerals. In our case, we are talking about the inclusion of an additional factor that increases the rate of transformation of minerals by several tens of times - this is a technological process with an acidic environment and microorganisms. Indeed, microorganisms are an important evolutionary process that catalyzes the emergence of new mineral compounds and accelerates morphogenesis. TABLE 3 shows 6 main chemical elements (from 1 to 6), which have the highest content (expressed in%) in the composition of sulfide minerals - muscovite, ankerite, pyrite, and chalcopyrite. Of the rock-forming minerals, the presence of quartz, albite, and calcite was noted in descending order (FIG. 2).
TABLE 3. The degree of solubility of chemical elements in the liquid phase of sulfide samples (µg/l).
Content |
Dissolved elements in the composition of sulfide minerals | ||||||||
|---|---|---|---|---|---|---|---|---|---|
Na, % |
Si, % |
P, % |
K, % |
Ca,% |
Fe,% |
Li | Mg | Al | |
| Neutral, pH – 7,8 |
0,095 | 0,37 | 0,019 | 0,061 | 0,16 | 0,02 | 1397,3 | 73143,8 | 13973,2 |
| Concentrate | 0,092 | 0,374 | 0,02 | 0,057 | 0,22 | 0,02 | 1395,2 | 76411,2 | 14607,4 |
| Sulfuric pH- 1,5, 96 hours |
0,0009 | 0,406 | 0,026 | 0,052 | 0,309 | 4,157 | 2114,7 | 1603438,5 | 667836,1 |
| Decantation, pH – 4,5 |
0,0005 | 0,322 | 0,019 | 0,046 | 0,267 | 0,137 | 1636,7 | 852911,23 | 20165,2 |
| alkalized., pH - 11,5 |
0,0091 | 0,388 | 0,019 | 0,053 | 0,273 | 0,043 | 1392,3 | 22897,0 | 16939,7 |
| Tails cyanide., pH – 9,5 |
0,0021 | 0,383 | 0,043 | 0,104 | 0,309 | 0,025 | 1457,6 | 41118,1 | 24699,5 |
These chemical elements, presented in descending order-Si, Ca, Na, K, Fe and P, are part of all known types of ores-rock-forming, accessory and ore minerals. Sulfur S and carbon C should be added to these elements, which are amenable to the action of microorganisms and the amount of which in the composition of sulfide ores is quite high, especially after the creation of optimal processes for them that support their vital activity. The content of silicon in the classifier is 0.37%. During bacterial leaching into the content of Si slightly increases and varies from 0.388% to 0.406%.
The decomposition of aluminosilicates is accompanied by the transition into solution of a number of elements, including silicon, aluminum, uranium, beryllium, etc. The above elements, which are capable of maximum dissolution under the influence of microorganisms, are apparently the main sources of shaping and migration processes in sulfide ore deposits.
From the received samples, the liquid and solid phases were separated and they were analyzed for quality and quantity for the main chemical elements. Density and pH were preliminarily determined, the results of which are attached in TABLE 4. The Table shows that the pH of the medium compared to the pH of the operating reactors is quite high and is more than 2. This circumstance is due to the stagnation of the pulp and the inhibition of the activity of microorganisms from a lack of oxygen and mixing of the pulp. Trivalent iron dissolved in the liquid phase tends to become divalent and precipitate.
TABLE 4. Characteristics of samples by density, ratio solid phase and medium pH
| № | Sample name | Density, g/cm3 | Solids ratio | medium pH | |
|---|---|---|---|---|---|
| g | % | ||||
| 1 | Top drain classifier | 1,146 | 135 | 11,78 | 8,07 |
| 2 | Flotoconc. | 1,239 | 252 | 20,33 | 8,45 |
| 3 | Reactor 2-1 | 1,210 | 216 | 17,85 | 2,05 |
| 4 | Reactor 2-2 | 1,195 | 226 | 18,91 | 2,15 |
| 5 | Reactor 2-3 | 1,185 | 114 | 9,62 | 2,10 |
| 6 | Reactor 2-4 | 1,180 | 125 | 10,59 | 2,08 |
| 7 | Reactor 2-5 | 1,175 | 148 | 12,59 | 2,10 |
| 8 | Reactor 2-6 | 1,173 | 139 | 11,84 | 2,10 |
| 9 | ATD-1 | 1,068 | 4,26 | ||
| 10 | ATD-3 | 1,145 | 328 | 28,64 | 6,15 |
| 11 | Nutrition CHEMIX | 1,308 | 486 | 37,15 | 9,82 |
| 12 | Cyanidation tailings | 1,180 | 253 | 21,44 | 11,95 |
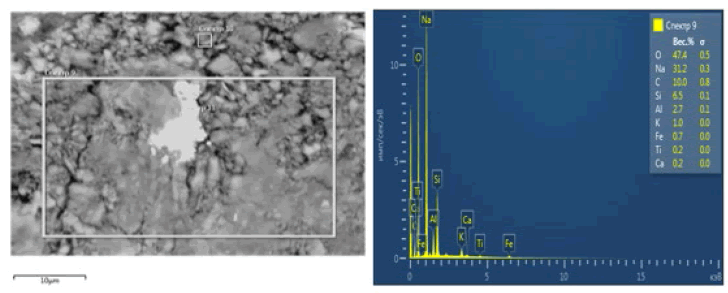
The standard enthalpy of formation ΔН0298, entropy ΔS0298 and Gibbs energy ΔG0298 of some substances at 298 K are given in TABLE 5. The results of the spectral analysis of the solid residue after acid and alkaline treatment are given, where alkali and alkaline earth elements prevail to the maximum- Na-31.2%, C-10.0%. Other elements-silicon -6.5%, aluminum -2.7%, potassium-1.0%, iron - 0.7%, titanium-0.2% and calcium 0.2% have a lower content. The appearance of cerium in the amount of 13.4% was noted (FIG. 4).
TABLE 5. Thermodynamic characteristics of some substances involved in bacterial oxidation processes.
| Substance | Physical state | Enthalpy, Kj/mole |
Entropy, J/mole*K |
Free energy (Gibbs), KJ/mol |
|---|---|---|---|---|
| FeS2 | Crystal | -177,40 (-163,2) |
52,99 | -266,05 |
| FeSO4 | Crystal | -927,59 (-3016) |
107,53 | -819,77 |
Thionic microorganisms in natural conditions, for a long geological time, with their organic secretions - metabolites, expressed by various types of organic compounds, contribute to the emergence of bio-inert matter and create optimal habitat for themselves. This evolutionarily fixed biochemical property, under artificially created production conditions, can be activated on an extremely large scale and, apparently, copy the evolution of sulfide minerals in an accelerated form, while under natural conditions these processes would proceed for millions of years.
Conclusion
Thus, as can be seen from the above, microbiological leaching of elements occurs with the participation of microorganisms in chemical transformations of a redox nature. The redox reactions carried out by microorganisms are vital for them and most often have an energy value. Atoms in the composition of minerals (or the aqueous medium), which are in their lower valences, are potential substrates for bacteria - i.e. during their bacterial oxidation, a certain amount of energy is released, which is necessary for bacteria for various energy-consuming processes: transportation of nutrients, biosynthesis of organic compounds of protoplasm, etc. The main six elements that are able to dissolve as much as possible under the influence of microorganisms, apparently, are the main sources of shaping and migration processes in sulfide ore deposits.
An increase in the ore composition of the amount of colloidal types of clay or easily degraded minerals, which, when combined with metabolites of microorganisms, interact in the microorganism-environment-mineral system, contribute to the formation of new types of minerals. The presence of iron-bearing minerals that are easily soluble in sulfuric acid solutions, for example, limonite, which ensure the entry of iron ions into the leaching solution, is apparently also due to the action of microorganisms on minerals. In the natural environment, the geological evolution of minerals took place precisely under the acidic dominance of bio-inert systems in mineral deposits, where mineral formation can occur to the maximum extent. From an evolutionary point of view, an acidic environment leads to the transition of cationic elements into a dissolved state, which, when they enter an alkaline environment, precipitate, which contributes to the emergence of new types of minerals.
REFERENCES
- Sousa R, Regufe MJ, Fiuza A, et al. A systematic review of sustainable gold extraction from raw ores using alternative leaching reagents. Extr Ind Soc 2022; 9: 101018.
- Xie F, Chen JN, Wang J, et al. Review of gold leaching in thiosulfate-based solutions. Transactions of Non-ferrous Metals Society of China. 2021 1;31(11):3506-29.
- Gui Q, Khan MI, Wang S, Zhang L. The ultrasound leaching kinetics of gold in the thiosulfate leaching process catalysed by cobalt ammonia. Hydrometallurgy.20201;196:105426.
- Azizitorghabeh A, Wang J, Ramsay JA, et al. A review of thiocyanate gold leaching–Chemistry, thermodynamics, kinetics and processing. Minerals Engineering.20211;160:106689.
- Ahtiainen R, Lundstrom M, Liipo J. Preg-robbing verification and prevention in gold chloride-bromide leaching. Minerals Engineering. 2018 1;128:153-9.
- Aguiar AO, Andrade LH, Ricci BC, et al. Gold acid mine drainage treatment by membrane separation processes: An evaluation of the main operational conditions. Separation and Purification Technology. 2016 1;170:360-9.
- Qureshi A, Maurice C, Ohlander B. Potential of coal mine waste rock for generating acid mine drainage. Journal of Geochemical Exploration. 2016 1;160:44-54.
- Galhardi JA, Bonotto DM. Hydrogeochemical features of surface water and groundwater contaminated with acid mine drainage (AMD) in coal mining areas: a case study in southern Brazil. Environmental Science and Pollution Research. 2016;23:18911-27.
- Shim MJ, Choi BY, Lee G, et al. Water quality changes in acid mine drainage streams in Gangneung, Korea, 10 years after treatment with limestone. Journal of Geochemical Exploration. 2015 1;159:234-42.
- Blodau C. A review of acidity generation and consumption in acidic coal mine lakes and their watersheds. Science of the total environment. 2006 1;369(1-3):307-32.
- Ampofo NA. The impact of Jarosites in Biox® product on CIL processes-a case study of AngloGold Ashanti (Obuasi mine) (Doctoral dissertation).
- Mubarok MZ, Winarko R, Chaerun SK, et al. Improving gold recovery from refractory gold ores through bio oxidation using iron-sulfur-oxidizing/sulfur-oxidizing mixotrophic bacteria. Hydrometallurgy. 2017 1;168:69-75.
- Yang H, Li J, Tong L, et al. Role of hydrolyzed rice husk in pyrite bio-oxidation. Geochemistry. 20211;81(4):125775.
- Wang G, Xie S, Liu X, et al. Bio-oxidation of a high-sulfur and high-arsenic refractory gold concentrate using a two-stage process. Minerals Engineering. 2018 1;120:94-101.
- Muravyov M. Two-step processing of refractory gold-containing sulfidic concentrate via bio oxidation at two temperatures. Chemical Papers. 2019 23;73:173-83.