Original Article
, Volume: 15( 3)HY Zeolite: An Efficient, Heterogeneous and Recyclable Catalyst for the One Pot Synthesis of ñ-Aminophosphonates
- *Correspondence:
- Nisar AD, Department of Chemistry, University of Kashmir, Srinagar-190006, India, Tel: +91-(194)227-2096; E-mail: nisarad@uok.edu.in
Received: June 12, 2017; Accepted: July 25, 2017; Published: August 03, 2017
Citation: Mira JA, Nisar AD, Khuroob MA, et al. HY Zeolite: An Efficient, Heterogeneous and Recyclable Catalyst for the One Pot Synthesis of α-Aminophosphonates. Int J Chem Sci. 2017;15(3):166
Abstract
An eco-friendly and efficient three-component protocol was developed for the one-pot synthesis of α-amino phosphonates from aldehydes, amines and trialkyl phosphates at room temperature using H-form zeolites as heterogeneous catalyst. The method is general with wide substrate scope giving products in excellent yields under mild reaction condition. The catalyst exhibits an excellent catalytic performance with good recyclability. The reusability and simple work up procedure make the protocol more attractive and eco-friendly.
Keywords
Zeolites; Heterogeneous catalysts; Recyclable; One pot synthesis; α-amino phosphonates
Introduction
The organic compounds of phosphorous and nitrogen like α-amino phosphonates are important class of pharmacologically active compounds and are reported to exhibit a diverse range of biological activities such as anticancer, syntheses inhibitors, HIV protease inhibitors, rennin inhibitors, enzyme inhibitors and serve as surrogates of α-amino acids [1-7]. Due to their diverse biological activities, various synthetic methods have been employed for the synthesis of α-aminophosphonates. The most commonly used method for the synthesis involves the reaction of imines with phosphorous nucleophiles in the presence of acidic catalysts [8]. One-pot three component procedure involving condensation of aldehydes, amines, and diethylphosphite or triethylphosphite has been reported in the presence of a variety of catalysts such as Lewis acids {Na2CaP2O7 Al(H2PO4)3, BiCl3 or Bronsted acids {oxalic acid, hypophosphorus acid, sulfamic acid}, base catalysts such as CaCl2 [9-15]. Recently, more efficient methodologies with some other catalytic systems, for instance ammonium metavanadate, FeCl3, Amberlyst-15, aluminum pillared clay, CeCl3-7H2O, CdI2, BF3-SiO2, NbCl5, TsCl, ZrOCl2-8H2O or ZrO (ClO4)2-6H2O [16-26], etc., have been developed. However, the reported methods are associated with some drawbacks as they involve the use of additional reagents, costly, non-recoverable, toxic catalysts, and some of the catalysts are difficult to synthesize. Thus, there is an unmet need for the development of simple, efficient, and environmentally benign methods for the synthesis of α aminophosphonates.
Due to economic and environmental concerns, development of heterogeneous catalysts is highly desirable [27]. These catalysts are generally inexpensive, can be handled and removed from the reaction mixture easily [28]. The acidic character of zeolites has been extensively reported [29] and much less explored for the synthesis of alpha amino phosphonates. Zeolites particularly hydrogen-exchanged zeolites whose framework-bound protons give rise to very high acidity [30] can prove efficient heterogeneous catalysts for the synthesis of α-aminophosphonates. In continuation of our interest in developing new and green synthetic methodologies [31-33], herein we report a green, mild and efficient synthesis for one pot procedure for the synthesis of α-aminophosphonates using zeolites as heterogeneous and recyclable catalysts.
Experimental
All the reagents and solvents for carrying out the present work were obtained from Sigma Aldrich and used as such. The chemical reactions were monitored by TLC on 0.25 mm silica gel 60 F254 plates (E. Merck) using KMnO4 or Dragendroff for developing sports on TLC. Purification of the compounds was carried out by column chromatography using Silica gel 60-120 mesh stationary phase or by recrystallization. 1H NMR and 13C NMR spectra were recorded on Bruker DPX 400 and DPX 500 instruments using CDCl3 or CD3OD as the solvents with TMS as internal standard. Mass spectra (LCMS) were recorded on Agilent Technologies 6540 instrument.
General procedure for the preparation of alpha aminophosphonates
In a typical procedure aldehyde (1.0 mmol), amine (1.0 mmol), triethylphosphite (1.1 mmol) and catalyst (20 mol%) were taken in oven dried round bottom flask containing THF (10 ml). The resulting heterogeneous mixture was stirred on magnetic stirrer at room temperature till the reaction was complete which was monitored by TLC. After completion, the catalyst was removed by filtration and the reaction mixture (filtrate) was dried on rota-evaporator. The crude extract was then purified by recrystallizing with methanol or by column chromatography using 60-120 mesh silica gel with dichloromethane (DCM) and methanol as eluting solvents to afford the pure compounds. The purified compounds were characterized by spectroscopic techniques i.e., 1H NMR, 13C NMR and MS (supporting information).
Results and Discussion
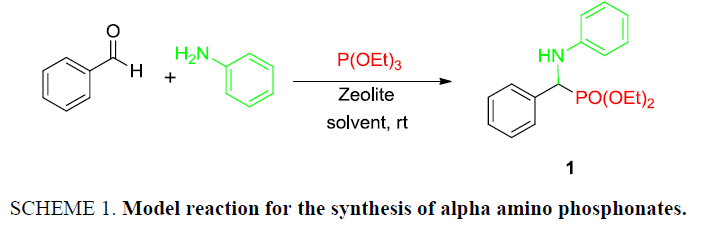
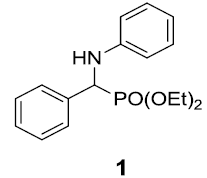
At the onset of this work, the reaction of benzaldehyde (1.0 mmol), aniline (1.0 mmol) and triethylphosphite (1.1 mmol) using 15 mol% zeolite-y as a catalyst in DCM was chosen as a template reaction (Scheme 1). The desired aminophosphonate (1) was obtained with 75% yield at room temperature in 12 h. The conditions for the model reaction were then optimized by using different zeolites, varying their concentration, using different reaction mediums. Initially, screening of different zeolites proved that H-form of zeolite give better results in terms of yield and reaction time (Table 1).
Scheme 1: Model reaction for the synthesis of alpha amino phosphonates.
| Entry | Catalyst | Time (h) | Yield (%) |
|---|---|---|---|
| 1. | No catalyst | 20 | traces |
| 2. | Mordenite (10 mol%) | 15 | 68 |
| 3. | Mordenite (20 mol%) | 15 | 73 |
| 4. | HY zeolite (5 mol%) | 15 | 63 |
| 5. | HY zeolite (10 mol%) | 12 | 71 |
| 6. | HY zeolite (15 mol%) | 12 | 77 |
| 7. | HY zeolite (20 mol%) | 10.5 | 89 |
| 8. | HY zeolite (30 mol%) | 10.5 | 89 |
Table 1: Screening of various zeolites and their concentration on the synthesis of α-aminophosphonates.
The conversion rate and yields were found to increase with increasing amounts of the catalyst, (Table 1). With 5 mol% of H-form of zeolite, 63% yield of the desired product was formed (Table 1, entry 4). The yield increased to 71% when 10 mol% of the catalyst was used (Table 1, entry 5) and finally 20 mol% was found to be the optimum concentration of the catalyst in terms of yield and time (Table 1, entry 7). Increasing the catalyst above this amount could not bring any significant increase in the product yield (Table 1, entry 8).
With 20 mol% of H-form of zeolite as optimum concentration, we proceeded to examine the role of various solvents on the yield and rate for the model reaction. Solvents like THF, dimethyl formamide (DMF), acetone, acetonitrile, dichloromethane (DCM), methanol, water and various combinations of miscible aqueous-organic solvents (1:1) like DMF–H2O, THF–H2O, CH3CN–H2O, MeOH–H2O, acetone–H2O (Table 2, entries 1-12) were screened and in general, the reaction occurred in every solvent. Among organic solvents (Table 2, entries 1-6), best results were obtained in THF (91% yield) in a reaction time of 10.5 h (Table 2, entry 1). Increase in the reaction time could not improve the yield of the desired product. Water as a medium resulted in poor yield even after employing a long reaction time due to very poor solubility of organic substrates (Table 2, entry 12). However, water miscible organic solvent combinations improved the yield (Table 2, entries 7-11) but to a lesser extent than organic solvents alone.
| Entry | Solvent | Time (h) | Yield (%) |
|---|---|---|---|
| 1. | Tetrahydrofuran (THF) | 10.5 | 91 |
| 2. | Dimethylformamide (DMF) | 10.5 | 89 |
| 3. | Acetone | 10.5 | 79 |
| 4. | Acetonitrile | 10.5 | 87 |
| 5. | Dichloromethane (DCM) | 10.5 | 67 |
| 6. | Methanol | 10.5 | 90 |
| 7 | DMF-water | 10.5 | 53 |
| 8 | THF-water | 10.5 | 69 |
| 9 | Acetonitrile-water | 10.5 | 67 |
| 10 | Methanol-water | 10.5 | 65 |
| 11 | Acetone-water | 10.5 | 63 |
| 12 | Water | 15.0 | 35 |
Table 2: Screening of various solvents for the synthesis of a-aminophosphonate.
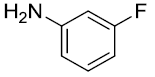
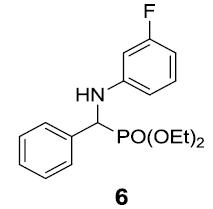
After optimization, scope of one-pot protocol for the synthesis of alpha amino phosphonates was explored for various substituted benzaldehydes and anilines (Scheme 2-3), the representative results are summarized in (Table 3). It was found that all the reactions were neat and clean affording the desired products in excellent yields. Benzaldehydes bearing electron withdrawing substituents (Table 3, entries 3,4,5,7,8,9) reacted faster than those bearing electron donating groups (Table 3, entries 2,3,4,5,7,10). Reverse was observed in case of anilines, anilines with electron withdrawing groups were found to react slower than anilines bearing electron releasing groups (Table 3, entries 2,3,4,5,7). Also, ortho bearing benzaldehydes were found to react slowly and produced comparatively lower yields.
| Entry | Benzaldehyde | Anilines | Product | Yield (%) |
|---|---|---|---|---|
| 1. |  |
 |
 |
91 |
| 2. | 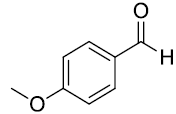 |
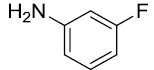 |
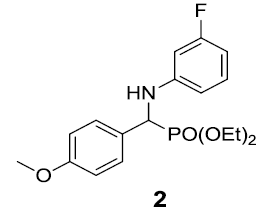 |
89 |
| 3. | 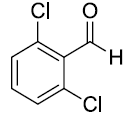 |
 |
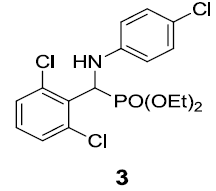 |
83 |
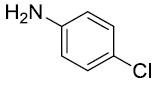
| 4. | 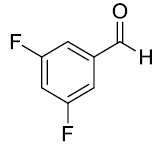 |
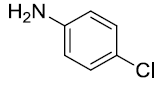 |
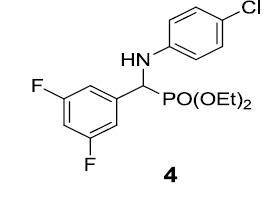 |
92 |
| 5. | 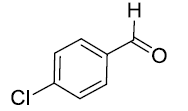 |
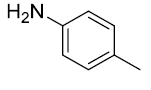 |
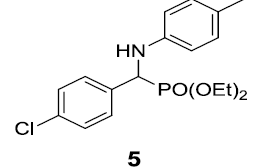 |
90 |
| 6. | 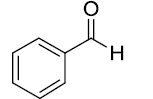 |
 |
 |
87 |
| 7. | 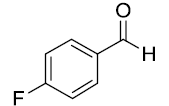 |
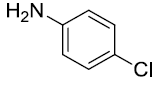 |
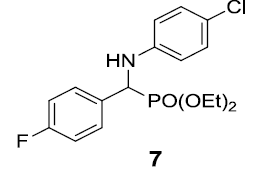 |
90 |
| 8 | 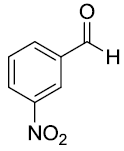 |
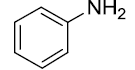 |
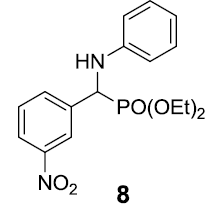 |
93 |
| 9 | 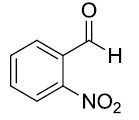 |
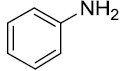 |
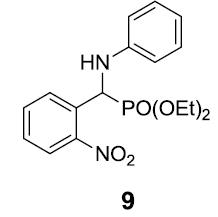 |
91 |
| 10 | 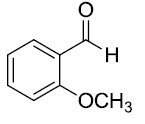 |
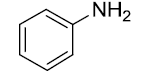 |
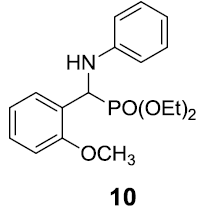 |
87 |
Table 3: Substrate scope for one pot synthesis of a-amino phosphonates.
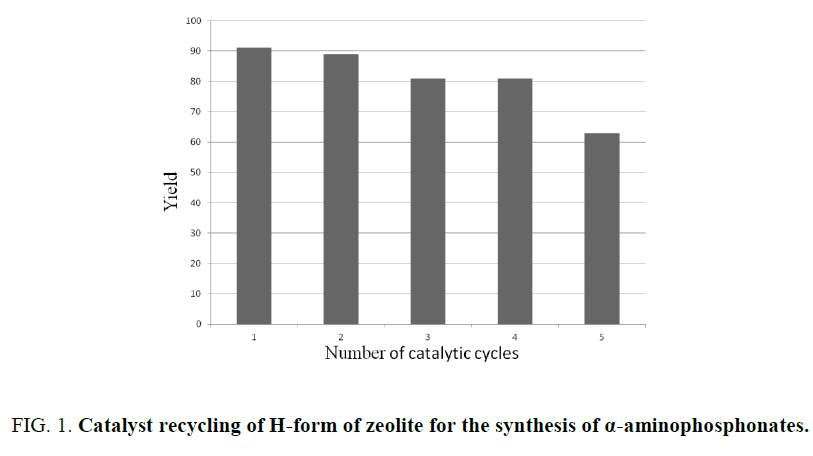
Recycling of HY zeolite catalyst
The reusability of the catalyst was examined for the model reaction. The catalyst was removed from the reaction mixture by simple filtration, washed with acetone and dried in an own at 150°C and finally calcinated at 550°C. The recovered catalyst was reused for at least four times without any significant loss in catalytic activity (Figure 1).
Regarding the mechanism, the reaction is believed to involve formation of an activated imine by the transfer of acidic proton from zeolite so, that addition of the phosphite is facilitated to give a phosphonium intermediate to give the α- aminophosphonate [31-33].
Conclusion
An eco-friendly and efficient method has been developed for the one pot synthesis of alpha amino phosphonates from carbonyl compounds, amines and triethyl phosphate at room temperature by using H-form zeolites. Operational simplicity, metal free, and use of heterogeneous and reusable catalyst are the features of the developed protocol. This reaction has a wide substrates scope with excellent yields under mild conditions. The reusability and simple work up procedure make the protocol more attractive and eco-friendly.
References
- Agawane SM, Nagarkar JM. Nano ceria catalyzed synthesis of α-aminophosphonates under ultrasonication. Tetrahedron Letters. 2011;52(27):3499-504.
- Stowasser B, Budt KH, Jian-Qi L, et al. New hybrid transition state analog inhibitors of HIV protease with peripheric C 2-symmetry. Tetrahedron Letters. 1992;33(44):6625-8.
- Patel DV, Rielly-Gauvin K, Ryono DE. Preparation of peptidic α-hydroxy phosphonates a new class of transition state analog renin inhibitors. Tetrahedron Letters. 1990;31(39):5587-90.
- Burke TR, Barchi JJ, George C, et al. Conformationally constrained phosphotyrosyl mimetics designed as monomeric Src homology 2 domain inhibitors. J Med Chem. 1995;38(8):1386-96.
- Pan W, Ansiaux C, Vincent SP. Synthesis of acyclic galactitol-and lyxitol-aminophosphonates as inhibitors of UDP-galactopyranose mutase. Tetrahedron Letters. 2007;48(25):4353-6.
- Jin L, Song B, Zhang G, et al. Synthesis, X-ray crystallographic analysis, and antitumor activity of N-(benzothiazole-2-yl)-1-(fluorophenyl)-O, O-dialkyl-α-aminophosphonates. Bioorganic and Med Chem letters. 2006;16(6):1537-43.
- Kafarski P, Lejczak B. Aminophosphonic acids of potential medical importance. Curr Med Chem-Anti-Cancer Agents. 2001;1(3):301-2.
- Azizi N, Rajabi F, Saidi MR. A mild and highly efficient protocol for the one-pot synthesis of primary α-amino phosphonates under solvent-free conditions. Tetrahedron Letters. 2004;45(50):9233-6.
- Akbari J, Heydari A. A sulfonic acid functionalized ionic liquid as a homogeneous and recyclable catalyst for the one-pot synthesis of α-aminophosphonates. Tetrahedron Letters. 2009;50(29):4236-8.
- Elmakssoudi A, Zahouily M, Mezdar A, et al. Na2CaP2O7 a new catalyst for the synthesis of α-amino phosphonates under solvent-free conditions at room temperature. Comptes Rendus Chimie. 2005;8(11):1954-9.
- Maghsoodlou MT, Habibi?Khorassani SM, Heydari R, et al. Al(H2PO4)+3 as an efficient and reusable catalyst for one?pot three?component synthesis of α?amino phosphonates under solvent?free conditions. Chinese Journal of Chemistry. 2010;28(2):285-8.
- Zhan ZP, Li JP. Bismuth (III) chloride-catalysed three?component coupling: Synthesis of α?amino phosphonates. Synthetic Communications. 2005;35(19):2501-8.
- Bhattacharya AK, Kaur T. An efficient one-pot synthesis of α-amino phosphonates catalyzed by bismuth nitrate pentahydrate. Synlett. 2007;pp:0745-8.
- Heydari A, Arefi A. One-pot three-component synthesis of α-amino phosphonate derivatives. Catalysis Communications. 2007;8(7):1023-6.
- Kudrimoti S, Bommena VR. (Bromodimethyl) Sulfonium bromide: An inexpensive reagent for the solvent-free, one-pot synthesis of α-aminophosphonates. Tetrahedron Letters. 2005;46(7):1209-10.
- Vahdat SM, Baharfar R, Tajbakhsh M, et al. Organocatalytic synthesis of α-hydroxy and α-aminophosphonates. Tetrahedron Letters. 2008;49(46):6501-4.
- Kaboudin B, Jafari E. One-pot synthesis of 1-aminophosphinic acids using 50% hypophosphorus acid under microwave irradiation. J Iran Chem Soc. 2008;5:S97-S102.
- Mitragotri SD, Pore DM, Desai UV, et al. Sulfamic acid: An efficient and cost-effective solid acid catalyst for the synthesis of α-aminophosphonates at ambient temperature. Catalysis Communications. 2008;9(9):1822-6.
- Dar BA, Chakraborty A, Sharma PR, et al. Grinding-induced rapid, convenient and solvent free approach for the one pot synthesis of α-aminophosphonates using aluminium pillared interlayered clay catalyst. J of Ind Eng Chem. 2013;19(3):732-8.
- Rezaei Z, Firouzabadi H, Iranpoor N, et al. Design and one-pot synthesis of α-aminophosphonates and bis (α-aminophosphonates) by iron (III) chloride and cytotoxic activity. Eur J of Med Chem. 2009;44(11):4266-75.
- Tajbakhsh M, Heydari A, Alinezhad H, et al. Coupling of aldehydes, amines, and trimethyl phosphite promoted by amberlyst-15: Highly efficient synthesis of α-aminophosphonates. Synthesis. 2008;pp:352-4.
- Jafari AA, Nazarpour M, Abdollahi?Alibeik M. CeCl3· 7H2O?catalyzed one?pot Kabachnik-Fields reaction: A green protocol for three?component synthesis of α?aminophosphonates. Heteroatom Chemistry. 2010;21(6):397-403.
- Kabachnik MM, Minaeva LI, Beletskaya IP. Synthesis of novel α-aminophosphonates containing adamantyl fragment. Synthesis. 2009;14:2357-60.
- Reddy MV, Dindulkar SD, Jeong YT. BF3· SiO 2-catalyzed one-pot synthesis of α-aminophosphonates in ionic liquid and neat conditions. Tetrahedron Letters. 2011;52(37):4764-7.
- Jun?Tao H, Gao JW, Zhang ZH. NbCl5: An efficient catalyst for one?pot synthesis of α?aminophosphonates under solvent?free conditions. App Organomet Chem. 2011;25(1):47-53.
- Trost BM. Atom economy-a challenge for organic synthesis: Homogeneous catalysis leads the way. Angewandte Chemie International Edition. 1995;34(3):259-81.
- Corma A, García H. Lewis acids: From conventional homogeneous to green homogeneous and heterogeneous catalysis. Chemical Reviews. 2003;103(11):4307-66.
- Hölderich W, Hesse M, Näumann F. Zeolites: Catalysts for organic syntheses. Angewandte Chemie International Edn. 1988;27(2):226-46.
- Deng C, Zhang J, Dong L, et al. The effect of positioning cations on acidity and stability of the framework structure of Y zeolite. Scientific Reports.2016;6:23382. 30.
- Dangroo NA, Dar AA, Dar BA. An efficient protocol for domino one pot synthesis of 1, 2, 3-triazoles from natural organic acids and phenols. Tetrahedron Letters. 2014;55(49):6729-33.
- Dar BA, Dangroo NA, Gupta A, et al. Iodine catalyzed solvent-free cross-dehydrogenative coupling of arylamines and H-phosphonates for the synthesis of N-arylphosphoramidates under atmospheric conditions. Tetrahedron Letters. 2014;55(9):1544-8.
- Dangroo NA, Dar AA, Shankar R, et al. An efficient synthesis of phosphoramidates from halides in aqueous ethanol. Tetrahedron Letters. 2016;57(25):2717-22.
- Pettersen D, Marcolini M, Bernardi L, et al. Direct access to enantiomerically enriched α-amino phosphonic acid derivatives by organocatalytic asymmetric hydrophosphonylation of imines. The J of Org Chem. 2006;71(16):6269-72.