Research
, Volume: 9( 1) DOI: 10.37532/J Food Sci Res.2024.09.95Effect of Sucrose on Quality Characterization of Different Types of Honey
- *Correspondence:
- Thameed Aijaz
Department of Food Technology, Islamic University of Science & Technology (IUST), Awantipora, Jammu and Kashmir, India
E-mail: tahmeed.shah@islamicuniversity.edu.in
Received: August 16, 2023, Manuscript No. TSFSR-23-110404; Editor assigned: August 21, 2023, PreQC No. TSFSR-23-110404 (PQ); Reviewed: October 05, 2023, QC No. TSFSR-23-110404; Revised: December 29, 2023, Manuscript No. TSFSR-23-110404 (R); Published: January 08, 2024, DOI: 10.37532/J Food Sci Res.2024.09.95
Citation:Aijaz T, Mohammad A, Maqbool N, et al. Effect of Sucrose on Quality Characterization of Different Types of Honey. J Food Sci Res. 9(1):95.
Abstract
Honey, a complex biological product produced by bees, is a nutrient-dense food with a substantial economic impact on the world. Honey is one of the foods that are prone to intentional or, unintentional adulteration. Sucrose is the most commonly utilised adulterant. In our present study, honey from different floral origin samples (wild shrub, acacia, mustard, brand 1 and brand 2) and their adulteration with sucrose syrup of 10%, 20%, 30%, 40% and 50% concentrations were analysed for physico-chemical properties, sugar profile and color analysis. Honey samples from different floral origins differ significantly (p 0.05), with moisture content ranging from 17.82 to 18.65%, protein content ranging from 0.37 to 0.77%, ash content ranging from 0.06 to 0.71%, HMF concentration ranging from 8.16 to 22.19 mg/kg, pH ranging from 3.19 to 3.88, and optical density ranging from 0.079 to 0.373. The sugar profile of honey samples showed significant variation with glucose content varied from 29.30 to 32.70%, fructose content for 32.24 to 38.61%, sucrose content for 1.04 to 1.69%, reducing sugars for 62.48 to 72.28% and total sugars varied from 69.30 to 78.67%. The L* value of color profile for different honey samples ranged from 19.78 to 45.23, a* value (6.72 to 16.80) and b*values from 24.59 to 38.31. The characteristics of honey samples from various floral origins met the standards for quality specified by international law and were suitable for consumption. The sucrose syrups of different concentration significantly (p ≤ 0.05) decrease the protein, ash, glucose, fructose, reducing sugars and a* value of honey samples. However significantly increases the moisture content, HMF, pH, optical density, sucrose content, total sugars, L* value and b* value of color.
Keywords
Honey; Physico-chemical; Sugar profile; HMF; Adulteration; Sucrose syrup
Introduction
Honey, a complex biological product produced by bees, is a nutrient-dense food with a substantial economic impact on the world. Although this product may be liquid, thick, or crystalline, its properties are mostly controlled by climatic and edaphic circumstances, agricultural practices, the type of flower that honey bees use as nectar, and postharvest honey management procedures [1]. Honey contains various components, which includes sugars such as fructose and glucose (60–85%) as monosaccharides, maltose and sucrose (10%) as disaccharides, enzymes like invertase, diastase, catalase, and glucose oxidase, Proline, gluconic acid, and acetic acid are the most abundant amino and organic acids, carotenoids, proteins, vitamins, hydroxymethylfurfural, numerous phytochemicals, and a number of minerals, including potassium, calcium, salt, magnesium, iron, and phosphorus, are also found [2]. Honey contains phenols, flavonoids, organic acids, minerals, amino acids, and other bioactive phenolic compounds that contribute to honey's antioxidant activity [3]. It also contains oligosaccharides, trace elements [4].
Honey is a good source of bioactive components with anti-inflammatory, anti-carcinogenic, wound healing, and anti-atherogenic characteristics, according to studies [5]. Bioactive components in honey vary widely depending on geographical origin, flora type, and honey handling and processing, all of which influence the Antioxidant Activity (AOA) of different honeys [6]. Honey moisture content is an important quality criterion that is controlled by a number of factors including maturity period, atmospheric factors harvesting time, and honey source [7]. The amount of Hydroxymethylfurfurals (HMF) is utilised as a quality control parameter with the hypothesis that they are indicative of honey heating, age, and adulteration by syrup-feeding bees during flowering. The HMF content for honey (tropical regions) must not exceed 80 mg/kg in order to ensure that the product has not undergone significant heating during processing and is suitable for use [8].
The pH is an effective predictor of the chance of microbial proliferation Silva, and it is significantly changed throughout extraction and storage, impacting the stability, texture, and shelf life of the honey. Honey's sugar content is impacted by processing, environmental, and storage circumstances, as well as composition, which is based on botanical and geographical origin [9]. Honey is a natural food product that is expected to be one of the top ten trends in the twenty-first century [10]. Promoting the consumption of honey, either directly or through value-added products such as honey beverages, honey wine, honey yoghurt, honey marmalade, and so on, is more beneficial.
The Government of India's Agricultural and Processed Food Products Export Development Authority (APEDA) reports that with 38177.08 metric tonnes exported for Rs. 705.87 crore (US $110.20 million) in the fiscal year 2015-16, India is the world's largest producer of honey. India's natural honey exports climbed by 32% in the fiscal year 2015-16. India's top honey-producing states include Punjab, Haryana, Rajasthan, Himachal Pradesh, Uttar Pradesh, Tamil Nadu, and West Bengal. Bangladesh, Morocco, Saudi Arabia, the United Arab Emirates, and the United States were the biggest exporters of honey in 2015-16.
Food adulteration is the deliberate lowering of a food's quality through the addition or, substitution of unapproved alternative ingredients, the removal of valuable ingredients, or both according to A.H. Teen and G. A. Dykes 2014. Food adulteration is a very old and widespread issue that occasionally even affects certain industrialised nations. It is frequently encountered in low and middleincome countries. The severity of the issue is worse in low-income regions like Bangladesh, Indonesia, India, Vietnam, and African nations [11]. Honey is one of the foods that are prone to intentional or, unintentional adulteration.
Sucrose is the most commonly utilised adulterant in honey since it is a readily available and inexpensive source. Adulteration of honey with sugar has a negative impact on the nutritional and qualitative parameters of honey. If cheaper or inferior substances are used to replace honey in whole or in part, it is considered adulterated honey. The physico-chemical properties of honey samples from various floral origins were assessed in this study. Furthermore, adulterants such as sucrose were introduced to various floral honey samples and the quality characteristics were measured.
Materials and Methods
Raw material
The current study was conducted using five distinct floral honey samples such as wild shrub, acacia, mustard, brand 1 and brand 2 procured from local market of Awantipora, Kashmir.
Preparation of adultered honey samples
Sugar syrup for preparation adulteration of honey was prepared by mixing properly sugar (150 g) ingredient with distilled water (100 mL) straining and stored in refrigerator till further use. For the preparation of adultered honey samples, the prepared syrup at different concentrations of 0, 10, 20, 30, 40 and 50 mL per 100 mL of different honey samples. The samples of adulterated honey were agitated in a water-bath with a temperature control for 30 minutes at room temperature. To eliminate contaminants, the samples were centrifuged for three minutes at 2500 rpm using a LA3 centrifuge from Remi Instruments in Mumbai, India. All the honey samples were packed and sealed in glass bottles collected from local the market. Then the samples stored at 4? till further the analysis.
Physico-chemical properties of honey
Moisture content: An Abbe's refractometer (Nar 2T India) was used to measure the moisture content at 20°C. The appropriate value of the honey's refractive index was used to calculate the moisture content values using a common reference Table 1.
| Moisture content (g/100 g) |
Refractive index (20 |
Moisture content (g/100 g) |
Refractive index (20 |
| 13.0 | 1.5044 | 19.0 | 1.4890 |
| 13.2 | 1.5038 | 19.2 | 1.4885 |
| 13.4 | 1.5033 | 19.4 | 1.4880 |
| 13.6 | 1.5028 | 19.6 | 1.4875 |
| 13.8 | 1.5023 | 19.8 | 1.4870 |
| 14.0 | 1.5018 | 20.0 | 1.4865 |
| 14.2 | 1.5012 | 20.2 | 1.4860 |
| 14.4 | 1.5007 | 20.4 | 1.4855 |
| 14.6 | 1.5002 | 20.6 | 1.4850 |
| 14.8 | 1.4997 | 20.8 | 1.4845 |
| 15.0 | 1.4992 | 21.0 | 1.4840 |
| 15.2 | 1.4987 | 21.2 | 1.4835 |
| 15.4 | 1.4982 | 21.4 | 1.4830 |
| 15.6 | 1.4976 | 21.6 | 1.4825 |
| 15.8 | 1.4971 | 21.8 | 1.4820 |
| 16.0 | 1.4966 | 22.0 | 1.4815 |
| 16.2 | 1.4961 | 22.2 | 1.4810 |
| 16.4 | 1.4956 | 22.4 | 1.4805 |
| 16.6 | 1.4951 | 22.6 | 1.4800 |
| 16.8 | 1.4946 | 22.8 | 1.4795 |
| 17.0 | 1.4940 | 23.0 | 1.4790 |
| 17.2 | 1.4935 | 23.2 | 1.4785 |
| 17.4 | 1.4930 | 23.4 | 1.4780 |
| 17.6 | 1.4925 | 23.6 | 1.4775 |
| 17.8 | 1.4920 | 23.8 | 1.4770 |
| 18.0 | 1.4915 | 24.0 | 1.4765 |
| 18.2 | 1.4910 | 24.2 | 1.4760 |
| 18.4 | 1.4905 | 24.4 | 1.4755 |
| 18.6 | 1.4900 | 24.6 | 1.4750 |
| 18.8 | 1.4895 | 24.8 | 1.4745 |
TABLE 1. Relationship between honey's refractive index and moisture content.
pH: A digital pH metre was used to monitor pH (Orion, Star A215, Thermo Scientific). In a 250 ml beaker, 10 grammes of sample honey were dissolved in 75 mL of distilled water.
Ash: A five-gram sample of honey was charred in a pre-weighed crucible dish and over a hot plate. The muffle furnace (STXMF117, Naberthem, Germany) was then used to overnight char the samples in the crucible dish at a temperature of 600°C. The procedure was repeated till a steady weight was obtained. After cooling, the samples were weighed. The ash content of samples was determined as: (1)
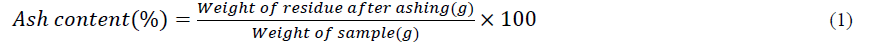
Protein content: The AOAC's conversion of the organic nitrogen in honey to (NH4)2SO4 in 2000 allowed for the estimate of the honey's overall crude protein content. In a digestion chamber at 370°C for 3–4 hours, a honey sample (300 mg) was put through digestion with the use of a copper catalyst, H2SO4, and 3 mL of deionized water. The resulting solution was distilled after the addition of NaOH, and the distillate was then collected in a flask with H3BO3 (4%) and mixed indicator using distillation apparatus. The mixture was then titrated with 0.1 M HCl till the end point (purple colour).
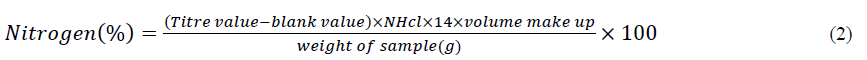
HMF: Using a Spectrophotometer UV-1700 (Japan), the White spectrophotometric method was used to assess the HMF level in honey. In this method, a honey solution's absorbance at 284 and 336 nm is compared with the same solution after bisulfate has been added (Germany). In mg/kg, the outcomes were presented [12].
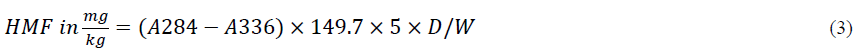
Where: A284 and A336 = absorbance at 284 nm and 336 nm, respectively

126=molecular weight of HMF
16830=molar absorptivity ε of HMF at λ= 284 nm
1000=conversion g into mg
10=conversion 5 into 50 ml
1000=conversion g of honey into kg
5=theoretical nominal sample weight
D=dilution factor, in case dilution is necessary
W=Weight in g of the honey sample
Optical density: A gram of honey was centrifuged for ten minutes at 3000 rpm after being diluted with nine mL of distilled water. The absorbance of the filtrate supernatant at 530 nm was measured using a spectrophotometer with distilled water used as a blank.
Estimation of Sugar
Reducing sugars
Preparation of test sample: Weigh 2 g of a homogenous honey sample (M), dissolve in distilled water, and afterwards dilution to 100 mL in a calibration flask (honey solution). Dilute 50 mL of the honey solution to 100 ml of distilled water, following the guidelines established by the International Honey Commission.
Standardization of the modified Fehling’s solution: Take 5 mL of Fehling's solutions A and B, which will effectively react with 0.050 g of invert sugar to produce a 25 mL diluted invert sugar solution (2 g/L) when combined.
Preliminary titration: Pipette 5 mL of Fehling's solution A into a 250 mL Erlenmeyer flask, followed by adding roughly 5 mL of Fehling's solution B. 15 mL of the burette's diluted honey solution should be added after the 7 mL of distilled water. On a hot plate, warm the cooled mixture to a boil for two minutes. To complete the titration within a total boiling time of 3 minutes, 3-5 drops of 0.2% aqueous methylene blue solution were added by adding miniscule amounts of diluted honey solution continuously while the mixture was still boiling. (Titration was continued until the blue colour was completely decolored to a brick-red end point.) The value of the titre was recorded.
Titration: Pipette roughly 5 mL of Fehling's solution B into a 250 mL Erlenmeyer flask following the addition of 5 mL of Fehling's solution A. The diluted honey solution volume from the preliminary titration should be added in 1.5 mL. Heat the cold mixture to boiling for 2 minutes on a hot plate. As the mixture is still boiling, add 1 mL of a 0.2% methylene blue solution, and finish the titration within three minutes of total boiling time by adding small amounts of diluted honey solution repeatedly until the indicator is decolored. Note the total volume (YmL).

M=Mass (g) of honey sample
Y=Volume (mL) of diluted honey solution consumed in the titration.
Sucrose content
Sample preparation: Dilute 250 mL of distilled water with 10 mL of the honey solution.
Hydrolysis: The test sample was heated to 65°C over a boiling water bath using the honey solution (50 mL) and 25 mL of distilled water in a graduated flask. 10 mL of hydrochloric acid was added when the flask was taken out of the water bath. The solution was heated to 20°C and neutralised with sodium hydroxide, using litmus paper as an indicator. It was then allowed to cool naturally for 15 minutes before the volume was adjusted to 100 mL (Diluted honey solution).
Preliminary titration: Pipette 5 mL of Fehling's solution A and add around 5 mL of Fehling's solution B into a 250 mL Erlenmeyer flask. 15 mL of the diluted honey solution from the burette should be added after the 7 mL of distilled water. On a hot plate, boil the cold mixture for 2 minutes. By adding small amounts of the diluted honey solution repeatedly while it was still boiling, 3-5 drops of the 0.2% aqueous methylene blue solution were added, and the titration was completed within the allotted three minutes of boiling time. (Titration was continued until the blue colour was completely decolored to brick-red end point.) The value of the titre was recorded.
Titration: Pipette 5 mL Fehling’s solution A into 250 mL Erlenmeyer flask and add approximately 5 mL Fehling’s solution B. Add 1.5 mL of the diluted honey solution volume determined in the preliminary titration. Heat the cold mixture to boiling for 2 minutes on a hot plate. Add 1 mL 0.2 % methylene blue solution whilst still boiling and complete the titration within a total boiling time of 3 minutes by repeated small additions of diluted honey solution until the indicator is decolorized. Note the total volume (YmL).
Sucrose content= (invert sugar (after inversion-before inversion) × 0.95 (6)
Glucose
Iodimetric measurements in a weak alkaline solution were used to calculate the percentage of glucose. In an essence, 40 mL of 0.05 N iodine solution and 25 mL of 0.1 N NaOH solutions were added to a 50 mL honey solution (0.5%) in a flask. The solution was acidified with 5 mL of concentrated H2SO4 and immediately titrated against 0.05 N sodium thiosulphate solutions after holding the stoppered flask in the dark for 20 minutes. Honey solution was substituted with 50 ml of water as the blank (BIS 1994). The glucose was calculated using the following form:

B-ml of thiosulphate solution used in blank
S-ml of thiosulphate solution used in sample
Fructose
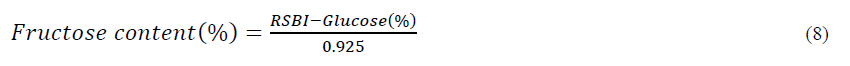
RSBI = Reducing sugar content before inversion
Total sugars
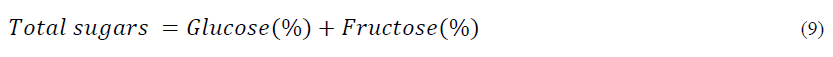
Color analysis
Using a Hunter Color Flex colorimeter, visual colour was assessed in terms of L (lightness), a* (redness and greenness), and b* (brightness) (yellowness and blueness). A conventional black-and-white tile was used to calibrate the instrument (45/0 geometry, 10 observers), which was then followed by sample measurements.
Statistical analysis
The results were expressed as mean of three replicates, standard deviation and Analysis of Variance (ANOVA) followed by Least Significant Difference (LSD) test (p ≤ 0.05) to separate means (p ≤ 0.05) using the Statistical Package for Social Science software 18 (SPSS Inc., Chicago, IL, USA).
Results and Discussion
Physico-chemical properties of honey
Moisture content: The amount of moisture in honey affects its ability to resist granulation and fermentation while being stored, making it an essential quality aspect. In our study, mean values of moisture content for five varieties of honey samples varied from 17.82 to 18.99%. The highest moisture content of 18.99% was observed for mustard sample followed by brand 2, brand 1, acacia and lowest for wild shrub honey sample (Table 2). The variations in the floral sources of the samples, the temperature and humidity levels in the environment at the time of honey production, and the beekeepers' methods could all be responsible for the considerable fluctuation in moisture content between different honey samples [13]. The effect of sugar syrup as adulterant showed significant increase in moisture content of different honey samples with increase in percentage of sugar syrup. Analysis of all the samples demonstrated moisture content lower than the limit (20%) for all honey samples permitted by the European directive, Codex and special grade of BIS. However, adultered honey samples showed moisture content above the limit of 20 % after 30 % sugar syrup. Similar results were reported in previous studies, honey is categorised into three groups depending on moisture content: "Special grade," or 20%; "grade A," or 22%; and "standard grade," or 25%.
| Honey samples | Moisture (%) | Protein (%) | Ash (%) | HMF (mg/Kg) | pH | Optical density |
|---|---|---|---|---|---|---|
| Wild Shrub | 17.82a ± 0.30 | 0.37a ± 0.03 | 0.17b ± 0.4 | 21.65d ± 0.91 | 3.19a ± 0.16 | 0.373e ± 0.1 |
| Acacia | 18.39b ± 0.20 | 0.77d ± 0.09 | 0.06a ± 0.02 | 8.16a ± 0.65 | 3.53b ± 0.02 | 0.182d ± 0.1 |
| Mustard | 18.99d ± 0.11 | 0.64b ± 0.09 | 0.07a ± 0.01 | 22.19d ± 0.46 | 3.63c ± 0.13 | 0.101b ± 1.9 |
| Brand 1 | 18.51c ± 0.09 | 0.91e ± 0.06 | 0.71c ± 0.07 | 9.85b ± 0.16 | 3.80d ± 0.02 | 0.079a ± 0.1 |
| Brand 2 | 18.65cd ± 0.02 | 0.68bc ± 0.1 | 0.28d ± 0.03 | 12.48c ± 0.48 | 3.88d ± 0.05 | 0.108c ± 0.1 |
| C.D (p = 0.05) | 0.17 | 0.03 | 0.01 | 2.04 | 0.15 | 0.08 |
| Note: Values were expressed as the average of triplicates ± standard deviation; Different superscript letters (a-e) in the same column indicate a significant difference between honey samples | ||||||
TABLE 2. Physico chemical properties of different types of honey.
PH: All of the honey samples had pH values that ranged from 3.19 to 3.88 as shown in Table 2 and differed significantly (P ≤ 0.05). As the concentration of sugar syrup increases, pH of adultered honey increases significantly. The presence of sugar content has positive correlation with pH content of honey. In our study, highest pH content of adultered samples was observed in Brand 2 (4.18 to 6.62) followed by Brand 1(4.10 to 6.62), mustard (3.97 to 6.51), Acacia (3.84 to 6.32) and lowest in wild shrub (3.45 to 5.45). The increased pH of adultered honey might be due to decrease in acidity of honey samples Gebremariam and Brhane. The pH readings of the honey samples agreed with those noted by other researchers Nayik et al., Azeredo et al., Ouchemoukh et al., Ozcan and Olmez., Kumar et al, low pH generally hinders microbes from growing and multiplying, hence honey's pH level affects its texture, stability, and shelf life. Pyruvic acid, gluconic acid, citric acid, maleic acid, lactones or inorganic ions (phosphate, chloride, and sulphate), as well as esters, all contribute to maintaining the pH of honey at a steady level. This makes pH a helpful indicator for the proliferation of the bacteria in honey [14].
Ash content: Ash refers to the inorganic component in honey and is a measure for determining the floral source of honey and identifying different types of honey. The Codex Alimentarius have given acceptable limit for ash content in honey ranged from 0.02 to 1.03%. The results in our study showed that the different honey samples analysed were significantly different (p ≤ 0.05) with each other. The honey samples with ash content ranged from 0.06 to 0.71% (Table 2). The highest ash content was observed for Brand 1and lowest for acacia honey sample. Increase in addition of sugar syrup from 0 to 50% showed significant decrease in ash content of all honey samples. Among the adultered honey samples, highest ash content was observed in Brand 1 (0.68 to 0.54%), followed by Brand 2 (0.27 to 0.18%), wild shrub (0.16 to 0.10), mustard (0.06 to 0.02%). and lowest was observed in acacia (0.05 to 0.01%) with increase in sugar syrup (10 to 50%) respectively.
Similar results were obtained by Oroian and Sorina; Chuttong et al. Getachew et al; Ouchemoukh et al. The floral source, geographic region, and honey maturity level may indeed contribute to the observed variation in ash content.
Protein content: Trace amounts of the proteins in honey come from pollen and enzymes produced by honeybees Habib et al., 2014. In our study, mean value of protein content of five varieties of honey samples varied from 0.37 to 0.91%. The highest protein of 0.91 % was observed for Brand 1 followed by acacia, brand 2, mustard and lowest for wild shrub honey sample. The protein content in all the varieties of honey samples were significantly (p ≤ 0.05) different. The effect of sugar syrup as adulterant showed significant decrease in protein content of different honey samples with increase in percentage of sugar syrup.
The highest mean value of protein content was observed in brand 1 (0.89 to 0.80 %), followed by Acacia (0.72 to 0.62 %), mustard (0.63 to 0.52%), brand 2 (0.66 to 0.57 %) and lowest was observed in wild shrub (0.36 to 0.21%). Similar results were reported in previous studies Buba et al., Kumar et al., Oyeyemi et al. The presence of honeybee enzymes, various floral sources, and various pollen types could be the cause of the variation in the protein composition of various types of honey samples Azeredo et al., Saxena et al., Escuredo et al., El Sohaimy et al., Khan et al. The usual level of nitrogen in honey is low, at around 0.04%, though it can go as high as 0.1%. In general, the outcomes fell within the parameters set by India's official methods [15].
HMF: Hydroxymethylfurfural (HMF) is a main indicator of honey for determination of freshness and quality. The HMF content of all honey samples showed lower value ranged from 8.16 to 22.19 mg/kg as recommended by the Codex Alimentarius Commission (40 mg/kg). The maximum content of HMF was observed for mustard (22.19 mg/kg) followed by wild shrub (21.65 mg/kg), brand 2 (12.48 mg/kg), brand 1 (9.85 mg/kg) and lowest for acacia (8.16 mg/kg).
The results of HMF of all the honey samples were differed significantly at 5 % level of significance presented in Table 2. The HMF content of all honey samples increases significantly with increase in concentration of sugar syrup from 10 to 50%. Highest HMF content was observed in Mustard (33.52 to 53.68 mg/kg) followed by wild shrub (31.37 to 51.33 mg/kg), Brand 2 (24.74 to 54.28 mg/kg), acacia (24.53 to 52.16 mg/kg) and Brand 1 (20.86 to 52.16 mg/kg) with increase in sugar syrup from 10 to 50% respectively. Elevated HMF content in honey samples may be sign of adulteration by adding invert syrup, while heating of sugar with an acid for inversion of sucrose can produce HMF Capuano and Fogliano, 2011. Our results were in agreement with the values reported by Ozcan and Olmez, 2014; Nayik et al., Duman et al., reported HMF content in honey samples at the average of 4.52 mg/kg. In other studies, HMF was reported at the level of 3.3% also observed high HMF content (12.0–32.6 mg/kg) in Egyptian honeys.
Optical density: Optical Density (OD) of honey samples is an important parameter for assessing its color and freshness. The optical density of honey showed laevorotatory because of presence of higher content laevulose sugars. Fresher and lighter honey samples have lower ODs than darker and stored honey samples, which have greater ODs. Generally, lighter honeys have more consumer demand than darker honey. Optical density of honey samples revealed higher OD values for wild shrub (0.373), acacia (0.182), brand 2 (0.108), mustard (0.101) lowest for brand 1 (0.079) as shown in Table 2.
The optical density of honey samples increased significantly with the increase in concentration of adulterant (sugar syrup) from 10 to 50 %. The optical density of adultered samples ranged for wild shrub (0.892 to 1.986), acacia (0.611 to 1.587), Brand 1 (0.123 to 1.256), mustard (0.103 to 1.256), and brand 2 (0.152 to 1.035) with increase in sugar syrup (10 to 50%) respectively. The variation in OD may be due to difference in chemical compositions and color pigments associated with different honey samples. The increased OD of honey sample with addition of sucrose syrup might be increase in total soluble solids of adultered honey. The results obtained in the present study were lower than the results found by Manzoor.
Sugar analysis: Sugars, which make up the majority of honey, are primarily impacted by floral and geographic sources and less so by seasonal, processing, and storage factors Dobre. On the basis of floral as well as geographic origin, honey samples have been differentiated based on sugar composition. Glucose content of honey depends upon the source of nectar. Five types of honey samples were evaluated and the glucose level ranged from 29.30 to 32.70% (Table 3). All the analysed samples of honey were significantly (p ≤ 0.05) different with each other. This glucose content of adultered honey samples decrease significantly with the increase in concentration of adulterant (sugar syrup).
The highest glucose content was found in wild shrub ranges from 30.59 to 24.44 % followed by Brand 2 (30.26 to 21.65%), mustard (30.17 to 23.61%) and acacia (29.52 to 22.78%) and lowest was observed in brand 1 (28.38 to 22.71%). These adultered samples were significantly (p ≤ 0.05) different from each other. The similar results of glucose content were reported by Nayik. The data presented in our study for glucose content fully agreed with the range 22.0–40.7 % given by White et al. These values are lower than the range of 29.4–42.0% reported by Ouchemoukh, but higher than the ranges 13.5–36.3% and 24.0–39.7% observed by Lazaridou [16].
| Honey samples | Glucose (%) | Fructose (%) | Sucrose (%) | Reducing sugars (%) | Total sugars (%) |
|---|---|---|---|---|---|
| Wild shrub | 32.07c ± 1.74 | 38.45d ± 2.09 | 1.28b ± 0.18 | 72.28d ± 0.56 | 74.72c ± 0.77 |
| Acacia | 32.70e ± 1.17 | 32.24a ± 0.45 | 1.47c ± 0.19 | 66.11c ± 0.12 | 69.30a ± 1.68 |
| Mustard | 32.37d ± 1.21 | 38.61d ± 0.84 | 1.26b ± 0.04 | 62.48a ± 0.88 | 78.67d ± 1.2 |
| Brand 1 | 29.30a ± 1.05 | 37.57c ± 0.65 | 1.04a ± 0.01 | 62.88a ± 0.32 | 77.00d ± 1.00 |
| Brand 2 | 31.89b ± 0.64 | 35.92b ± 0.48 | 1.69d ± 0.02 | 64.78b ± 0.48 | 70.55b ± 1.67 |
| C.D (p = 0.05) | 0.26 | 1.75 | 0.05 | 1.34 | 2.11 |
| Note: Values were expressed as the average of triplicates ± standard deviation; Different superscript letters (a-e) in the same column indicate a significant difference between honey samples. | |||||
TABLE 3. Sugar profile estimation of different types of honey.
Fructose content of honey acts as sweetener. In our study fructose content of all honey samples ranged from (32.24 to 38.61%) and were significantly different from each other at 5 % level of significance Table 3. The fructose content observed from adultered samples with addition of sugar syrup as adulterant showed highest fructose content in wild shrub (35.58 to 29.59%) followed by Brand 1 (32.89 to 24.96%), mustard (32.78 to 22.74%), Brand 2 (31.23 to 22.27%) and lowest fructose content was found in acacia (30.69 to 21.27%). Thus, it was found that as the concentrations of adulterant increases (sugar syrup) from 10 to 50%, the fructose content of all honey samples showed significant decrease. The result of fructose content observed in our study were same as reported by de La Fuente et al., Nayik et al. These values fall within the range of 32.0 – 48.4 % obtained by Finola et al. and Lazaridou et al. The sucrose content of honey samples is shown in Table 3 ranged from 1.69 to 1.04. The sucrose all the honey samples were significantly different at 5 % level of significance. The maximum sucrose content was found in Brand 2 (1.69%) followed by Acacia (1.47%), mustard (1.26 %), wild shrub (1.28 %) and lowest level of sucrose in Brand 1 (1.04%). Maximum sucrose content of 5 % is recommended by the food regulations of many countries and proposed Codex Alimentarius (1969). The addition of sugar syrup showed significant increase in sucrose level of all the adulterated honey samples. The highest level of sucrose was found in brand 2 (7.11 to 22.27%) followed by Acacia (6.65 to 20.26 %), mustard (6.46 to 20.89 %), wild shrub (6.24 to 18.23%) and lowest level of sucrose brand 1 (5.88 to 19.79%). The sucrose content in honey samples were found to be within the range of 0.2–7.6 % given by White et al., 1962. 0.08–5.31 % reported by Ouchemoukh et al., 0.14-11.49 % by Serrano et al. Also, the same results were obtained by Kumar et al., Nayik et al., Buba et al. The various honey types' differences in sucrose concentration may be caused by the transglucosylation reaction, which is started by the transfer of α -D-glucopyranosyl unit from sucrose to an acceptor molecule. The amount of sucrose in honey varied according to the different floral source and invertase enzyme activity [17].
Reducing sugars of honey samples present in Table 3 varied from 62.48 to 72.28 %. The highest reducing sugar was observed in wild shrub honey sample and lowest reducing sugar in mustard honey sample. The results of reducing sugar in all honey samples showed significant (p ≤ 0.05) differences. The reducing sugars of all the honey samples showed significant decrease with increase in sugar syrup from 10 to 50%. The highest value of reducing sugars was found in wild shrub ranged from 69.84 to 52.16% followed by Acacia (63.46 to 48.48%), brand 2 (61.54 to 47.57%), brand 1 (60.89 to 44.26 %) and lowest in Mustard (60.22 to 46.27 %). The results are compatible with the findings of Nayik and Nanda, who reported a range of 60.6 to 72.81%, which is in agreement with the standards proposed by EU Directive 2001/110.Similar results were reported by Khalil et al., Getachew et al., Gobessa et al., Gebremedhin et al.
The percentage of the total sugar content in the five honey samples shown in Table 3 ranged from 69.30 to 78.67%. The highest total sugar was observed in mustard honey sample and lowest total sugar in Acacia honey sample. Significant differences (p ≤ 0.05) were observed in total sugar content of all honey samples. The total sugar content of all adultered honey samples are present in Table 3. The total sugar of adultered honey samples showed significant increase with increase in sugar syrup from 10 to 50%. The maximum total sugar content was observed in Mustard honey ranged from 80.22 to 89.21% followed by brand 1 (79.37 to 88.73%) wild shrub (77.35 to 87.21%), brand 2 (74.39 to 86.45%) and minimum total sugar content in acacia (72.48 to 83.23 %) [18]. The values obtained in the present study were same as reported by Ouchemoukh et al., Khalil et al., Nayik et al., Manzanares et al. The percentage of total sugar content forms the major fraction of honey. The values obtained in our present study were located within the range of 60.6 –79.4% and same was reported by Ibrahim et al., and were lower than the values obtained by Serrano et al., who reported in range of 80.0-83.5% [19].
Color profile
The color of honey is an important quality parameter related to its commercialization and attractive attribute toward end use consumers. The chemical makeup, amount of ash, temperature of the hive, and length of storage period all have a direct impact on the colour of honey samples. The honey during thermal process significantly effects color parametes due to caramelization and maillard reactions. The color values are expressed as in terms of L*(darkness/lightness), a* (greenness to redness) and b* (blueness to yellowness) are present in Table 4. The color profile of all varieties of honey samples with L* values of 45.23, 31.27, 26.38, 23.91 and 19.78 was observed for observed for wild shrub, acacia, mustard, brand 1 and brand 2, respectively. a* and b* color values for wild shrub, Acacia, Mustard, Brand 1 and Brand 2 was observed as 16.80 and 37.43, 11.84 and 31.23, 8.26 and 24.59, 6.72 and 38.31 and 7.75 and 32.96, respectively. The L* color value of all adultered honey samples showed significant increase with the increase in concentration of sugar syrup from 10 to 50 %. The highest L* value was observed in wild shrub varied from 46.78 to 58.46, acacia (33.15 to 49.11), mustard (28.26 to 40.46), brand 1 (27.34 to 41.32) and lowest in brand 2 (22.75 to 37.86). The a* value of adultered honey samples decrease significantly as the concentration of adulterant (sugar syrup) increases. The a* color value for wild shrub ranged from 15.27 to 9.23, acacia (11.22 to 5.78), mustard (7.93 to 4.12), brand 1 (6.34 to 3.54) and brand 2 (7.14 to 3.17). The b* value of adultered honey samples showed significant increase with increase in concentration of adulterant (sugar syrup) from 10 to 50%. The values b* observed in adultered samples observed for wild shrub ranged from 39.37 to 50.25, acacia (33.48 to 59.59), mustard (26.42 to 42.58), brand 1 (39.74 to 54.68) and brand 2 (34.15 to 45.17). Similar results were reported by Ahmed et al., and Can [20].
| Honey samples | L* | a* | b* |
|---|---|---|---|
| Wild shrub | 45.23e ± 0.01 | 16.80e ± 0.12 | 37.43c ± 0.01 |
| Acacia | 31.27d ± 0.03 | 11.84d ± 0.01 | 31.23b ± 0.11 |
| Mustard | 26.38c ± 0.04 | 8.26c ± 0.10 | 24.59a ± 0.03 |
| Brand 1 | 23.91b ± 0.01 | 6.72a ± 0.06 | 38.31c ± 0.03 |
| Brand 2 | 19.78a ± 0.01 | 7.75b ± 0.04 | 32.96b ± 0.05 |
| C.D (p = 0.05) | 1.27 | 0.05 | 2.24 |
| Note: Values were expressed as the average of triplicates ± standard deviation; Different superscript letters (a-e) in the same column indicate a significant difference between honey samples | |||
TABLE 4. Color profile of different honey.
Conclusion
Honey is a natural product that honeybees have harvested and altered by mixing with particular ingredients from plant nectar or secretions from live sections of plants. Honey, a precise biological product made by bees, is a healthy food with economic significance all over the world. The characteristics of this product, which might be liquid, viscous, or crystalline, are primarily influenced by climatic and edaphic conditions, agricultural practises, the type of flower that provides the honey bees with nectar, and postharvest honey processing operations. The physicochemical parameters (moisture, protein, ash, HMF, OD, pH, color parameters, glucose, fructose, sucrose) were used to analyze the different floral honey samples. In this work, honey samples from different floral origins were assessed for their physico-chemical parameters. Moreover, sucrose as adulterants was added to different floral honey samples and were analysed for their change in quality parameters. The present study was done with aim of studying the physicochemical properties, identifying the purity and quality of different types of honey by comparing several characteristics and properties of the honey and also effect of different properties and characteristics of honey on addition of sugar syrup. The physico chemical, sugar profile and color analysis of the all five floral honey samples were within the limits recommended by European Commission and the codex Alimentarius. These characteristics are of great importance in honey trade as they influence its quality, granulation, texture as well as its nutritional efficiency. Mustard honey sample was observed to contain the highest level of moisture, Fructose and HMF compared to the other types of honey. The results also revealed that brand 1 honey was highest in Ash, protein, b value; brand 2 honey was highest in sucrose and pH. Wild shrub honey in total sugars, L value, a value and optical density and acacia was highest in glucose content. The five different floral honey samples were adultered with different levels of sugar syrup at a ratio of 0, 10, 20, 30, 40 and 50 mL per 100 mL of honey sample (v/v) showed that significant affect in physico chemical, sugar profile and color profile properties. Results showed that moisture content, HMF, pH, optical density, sucrose, total sugars, L value and b value increases in all honey samples with the increase in concentration of sugar syrup. Protein, ash, glucose, fructose, reducing sugars and a value decrease significantly with increase in sucrose syrup. Physical-chemical evaluations of honey are essential tools for determining its floral source and for implementing quality control measures that eventually influence consumer preferences for this product. As a result, these standards are fundamental for worldwide trade as well as being essential for quality control and certification.
References
- Ahmed J, Prabhu ST, Raghavan GSV, et al. Physicochemicalrheological, calorimetric and dielectric behavior of selected Indian honey. J Food Eng. 2007;79(4), 1207-1213.
- Aljadi AM, Kamaruddin MY. Evaluation of the phenolic contents and antioxidant capacities of two Malaysian floral honeys. Food Chem. 2004;85:513–518.
- Ayansola AA, Banjo AD. Physico-chemical evaluation of the authenticity of honey marketed in Southwestern Nigeria. J Basic Appl Sci Res. 2011;13339-3344.
- Azeredo LC, Azeredo MAA, Souza SR et al. Protein content and physicochemical properties in honey samples of Apismellifera of different floral origins," Food Chem. 2003;80(2):249-254.
- Belay A, Solomon WK, Bultossa G, et al. Physicochemicalproperties of the Harenna forest honey, Bale, Ethiopia. Food Chem. 2013;141(4):3386-3392.
[Crossref] [Google Scholar] [PubMed]
- Beretta G, Granata P, Ferrero M, et al. Standardization of antioxidant properties of honey by combination of spectrophotometric/fluorimetric assays and chemometrics. Anal Chim Acta. 2005;533:185–191.
- Bertoncelj J, Dobersek U, Jamnik M, et al. Evaluation of the phenolic content, antioxidant activity and colour of Slovenian honey. Food Chem. 2007;105: 822–828.
- Bertoncelj J, Golob T, Kropf U, et al. Characterisation of Slovenian honeys on the basis of sensory and physicochemical analysis with a chemometric approach. Int J Food Sci Technol. 2011;46:1661–1671.
- Buba F, Gidado A, Shugaba A. Analysis of biochemical composition of honey samples from North-East Nigeria. Biochem Anal Biochem. 2013;2(3):139.
- Can Z, Yildiz O, Sahin H, et al. An investigation of Turkish honeys: their physico-chemical properties, antioxidant capacities and phenolic profiles. Food Chem. 2015;180:133-141.
[Crossref] [Google Scholar] [PubMed]
- Cantarelli MA, Pellerano RG, Marchevsky EJ, Camiña JM. Quality of honey from Argentina: Study of hemical composittion and trace elements. J Argent Chem Soc. 2008;96(1-2):33-41.
- Capuano E, Fogliano V. Acrylamide and 5-hydroxymethylfurfural (HMF): A review on metabolism, toxicity, occurrence in food and mitigation strategies. LWT - Food Sci Technol. 2011;44(4):793-810.
- Chefrour C, Draiaia R, Tahar A, et al. Physicochemical characteristics and pollen spectrum of some north-east Algerian honeys. African J Food, Agriculture, Nutrition Develop. 2009;9(5).
- Chuttong B, Chanbang Y, Sringarm K, et al. Physicochemical profiles of stingless bee (Apidae: Meliponini) honey from South east Asia (Thailand). Food Chem. 2016;192:149-155.
[Crossref] [Google Scholar] [PubMed]
- Cimpoiu C, Hosu A, Miclaus V, et al. Determination of the floral origin of some Romanian honeys on the basis of physical and biochemical properties. Spectrochim Acta A Mol Biomol Spectrosc. 2013;100:149-154.
[Crossref] [Google Scholar] [PubMed]
- Cotte JF, Casabianca H, Chardon S, et al. Application of carbohydrate analysis to verify honey authenticity. J Chromatogr A. 2003;1021(1-2):145-155.
[Crossref] [Google Scholar] [PubMed]
- da Silva PM, Gauche C, Gonzaga LV, et al. Honey: Chemical composition, stability and authenticity. Food Chem. 2016;196:309-323.
[Crossref] [Google Scholar] [PubMed]
- De La Fuente E, Ruiz-Matute AI, Valencia-Barrera RM, et al. Carbohydrate composition of Spanish unifloral honeys. Food Chem. 2011;129(4):1483-1489.
- Dobre I, Georgescu LA, Alexe P, et al. Rheological behavior of different honey types from Romania. Int Food Res. 2012;49(1):126-132.
- El Sohaimy SA, Masry SHD, Shehata MG. Physicochemical characteristics of honey from different origins. Ann Agric Sci. 2015;60(2):279-287.