Original Article
, Volume: 15( 3)Benzohydrazide incorporated Imidazo [1,2-b] pyridazine: Synthesis, Characterization and in vitro Anti-tubercular Activity
- *Correspondence:
- Paidi KRS , Department of Chemistry, SK university, Anantapur, Andhra Pradesh, India, Tel: +91-9986244478; E-mail: ramanpk4u@gmail.com
Received: July 03, 2017; Accepted: August 07, 2017; Published: August 10, 2017
Citation: Paidi KR, Tatipamula VB, Kolli MK, et al. Benzohydrazide incorporated Imidazo [1,2-b] pyridazine: Synthesis, Characterization and in vitro Anti-tubercular Activity. Int J Chem Sci 2017;15(3):172
Abstract
The accumulating pharmacological importance of drug resistant pathogens has lent auxiliary urgency to new anti-tubercular compound development. In this regard, a novel series of imidazo [1,2-b] pyridazine comprising benzohydrazide derivatives have been synthesized, characterized by using spectral data and screened for anti-tuberculosis activity. The anti-tubercular activity of the synthesized compounds (6a-l) was determined by microplate alamar blue assay and the outcomes were screened in vitro against Mycobacterium tuberculosis H37Rv strain. Compounds 6a-l exhibited good to potent anti-tubercular activity when compared with the standard first line anti- tuberculosis drugs (ciprofloxacin, pyrazinamide and streptomycin). Some of the tested compounds exhibited highest inhibitory activity at 1.6 μg/mL minimal inhibition concentration.
Keywords
Benzohydrazide; Mycobacterium tuberculosis; Anti-tubercular activity; MABA; Minimal inhibition concentration
Introduction
Tuberculosis (TB) is an intercellular pathogen that infects, perseveres and reproduces in immune cells, which is endemic in several regions of the world and pursues to be a prime global health issue [1-3]. During the treatment of TB, the M. tuberculosis undergoes mutational changes and develops drug resistant pathogens which contributes to the elevated morbidity and mortality [4,5]. Therefore, the drug discovery of newer anti-TB agents with safe and potent biological action is required. This necessity had an impact on the synthesis for some novel hydrazides with the anti-tubercular activity [6].
Basically, hydrazide group plays a vital role in antimicrobial activity [6]. Moreover, hydrazones and benzohydrazides have been reviewed to possess anticonvulsant [7], antioxidant [8], antiviral [9], vasodilator activity [10], antitumor activity [9,11], antimicrobial [8,12,13], antitubercular [6,14,15], antidepressant [16], antimalarial [17], analgesic [18,19] and antiinflammatory [19] activities. Furthermore, some hydrazones have also been used as herbicides, insecticides, nematocides, rodenticides, and plant growth regulators [20] as well as plasticizers and stabilizers for polymers [21,22]. The metal complex of hydrazones have potential application as catalysts [23], luminescent probes [24], radioprotective properties [25], enzyme inhibitors [26] and molecular sensors [27]. With the target of attaining novel anti-tubercular compounds, we built a scaffold by introduction of the benzohydrazide linker to vinylbenzene and extension with imidazo [1,2-b] pyridazine. The introduction of heterocyclic moieties (like imidazo [1,2-b] pyridazine) impetus the molecule to possess more selectivity and potency [28]. The final synthesized compounds were investigated for in-vitro anti-TB activity against Mycobacterium tuberculosis H37Rv strain using microplate alamar blue assay (MABA) and ciprofloxacin, pyrazinamide and streptomycin as standard drugs.
Material and Methods
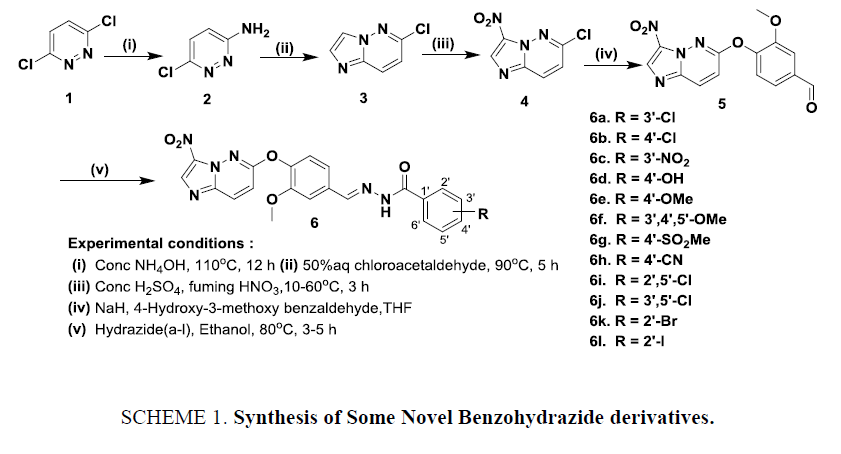
The solvents were purified according to standard procedures prior to use, and all commercial chemicals were used as received. For thin-layer chromatography (TLC) analysis, Merck pre-coated Plates (silica gel 60 F254) were used and eluting solvents are indicated in the procedures. Merck silica gel 60 (230-400 mesh) was used for flash column chromatography. Melting point (mp) determinations were performed by using Mel-temp apparatus and are uncorrected. 1H NMR spectra were recorded on a Varian Unity instrument at room temperature at 400 MHz. Chemical shifts are reported in ‘δ’ parts per million (ppm) downfield from tetramethylsilane (TMS) with reference to internal solvent and coupling constants ‘J’ in Hz. The mass spectra were recorded on Agilent ion trap MS (Scheme 1). All the acid chlorides used for the preparation of 6a-l were purchased from commercial sources.
Synthesis of 6-chloropyridazin-3-amine (2)
To the starting material 3,6-dichloro pyridazine (1) (10 g, 75.52 mmol), aqueous conc. ammonium hydroxide (100 mL) was added and warmed at 110°C for 12 h in a SS bomb vessel. After the completion of the reaction (confirmed by TLC), the reaction mixture was cooled to 0°C, the contents were filtered, and the resulting solid washed with water (2 × 15 mL) to obtain 2 as pale-yellow solid.
Yield: 80.8%; mp: 111-112°C; 1H NMR (400 MHz, DMSO-d6): δ 7.32 (d, J=12.4 Hz, 1H), 6.80 (d, J=12.4 Hz, 1H), 6.60 (br.s, 2H); 13C NMR (CDCl3): 160.7, 145.4, 129.4, 118; ESI-MS: m/z, 130.0 (M+1).
Synthesis of 6-chloroimidazo [1,2-b] pyridazine (3)
A mixture of 2 (30 g, 232.0 mmol) and chloroacetaldehyde (45 mL, 50% aqueous solution) was heated to 90°C for 5 h. Water was distilled off to obtain brown yellow solid. The crude compound was stirred with 100 mL of ethanol and filtered at the pump to afford 3 as brown solid.
Yield: 20 g (70.0%); mp: 114-116°C; 1H NMR (400 MHz, DMSO-d6): δ 8.61 (d, J=4.0 Hz, 1H), 8.46 (d, J=12.0 Hz, 1H), 8.22 (d, J=4.0 Hz, 1H), 7.76 (d, J=12.0 Hz, 1H); .13C NMR (100 MHz, DMSO-d6): 149.3, 136.1, 128.7, 126.1, 124.0, 118.8; ESI-MS: m/z, 154.2 (M+1).
Synthesis of 6-chloro-3-nitroimidazo [1,2-b] pyridazine (4)
To a stirred solution of 3 (5g, 33.55 mmol) in conc. sulphuric acid (7.1 mL, 130.7 mmol), fuming nitric acid (4.1 mL, 98.0 mmol) was added over a period of 15 min at 10°C and the reaction was continued for 3 h at room temperature. Then the reaction mixture was heated at 60°C for 1 h. The reaction mixture was poured into ice, filtered and the precipitated solids were washed with water and dried to obtain solid. The residual mother liquor was further basified with NaOH solution to pH 14 and the obtained 4 as off white solid.
Yield: (5 g, 77.3%); mp: 143-146oC; 1H NMR (400 MHz, DMSO-d6): δ 8.84 (s, 1H), 8.52 (d, J=9.6 Hz, 1H), 7.82 (d, J=9.6 Hz, 1H); 13C NMR (100 MHz, DMSO-d6): 149.6, 140.5, 137.4, 134.6, 129.6, 125.0; ESI-MS: m/z, 199.1 (M+1).
Synthesis of 4-(3-nitroimidazo [1,2-b] pyridazin-6-yloxy)-3-methoxybenzaldehyde (5)
To a stirred solution of 4-hydroxy-3-methoxy benzaldehyde (5.75 g, 1.5 eq) in tetrahydrofuran, added a screw cap vial containing a suspension of sodium hydride (1.5 g, 37.87 mmol, 1.5 eq) in dry anhydrous THF. The reaction mixture was stirred at room temperature for 30 min before addition of 4 (5 g, 25.25 mmol, 1 eq). The stirring was continued at room temperature for overnight, the reaction mixture was taken up in dichloromethane, brine and extracted with dichloromethane. The combined organic layer was dried with sodium sulphate and evaporated to crude. The crude product was triturated with diethyl ether obtained the compound 5.
Yield: 76.9%; mp: 150-165°C; 1H NMR (400 MHz, DMSO-d6): δ 10.9 (s, 1H), 8.69 (s, 1H) 8.49 (d, J=13.04 Hz, 1H) 7.70-7.58 (m, 4H), 3.82 (s, 3H); 13C NMR (100 MHz, DMSO-d6):192.5, 160.5, 151.8, 146.2, 140.1, 136.5, 135.4, 134.6, 130.4, 124.3, 123.4, 116.7, 113.0, 56.6; MS (EI+): m/z=315.0 (exact mass=314.2).
General procedure for the preparation of benzylidene hydrazide derivatives (6a-6l)
To the mixture of compound 6 (300 mg, 0.955 mmol, 1.0 eq) in ethanol corresponding hydrazides (1.0 eq, a-l) were added and heated to 80°C for 3-5 h. After completion of the reaction confirmed by TLC, the reaction mixture was evaporated to crude product. The crude product was triturated with diethyl ether and filtered to obtain the pure final compound 6a-l.
(22E)-N'-((4-(3-nitroimidazo [1,2-b] pyridazin-6-yloxy)-3-methoxyphenyl) methylene)-3-chlorobenzohydrazide (6a)
Off white solid; Yield: 68%; mp: 120-122°C; 1H NMR (400 MHz, DMSO-d6): δ 12.02 (s, 1H), 8.69 (s, 1H), 8.49-8.45 (m, 2H), 7.97-7.88 (m, 2H), 7.69-7.67 (m, 1H), 7.60-7.57 (m, 3H), 7.45-7.38 (m, 2H), 3.81 (s, 3H); 13C NMR (100 MHz, DMSO) 161.4, 152.1, 144.1, 143.1, 140.7, 137.0, 136.2, 135.2, 134.6, 133.5, 132.5, 131.3, 130.7, 129.5, 125.2, 123.7, 122.3, 117.2, 116.0, 111.4, 56.2; MS (EI+): m/z=467.0 (exact Mass=466.08).
(22E)-N'-((4-(3-nitroimidazo [1,2-b] pyridazin-6-yloxy)-3-methoxyphenyl) methylene)-4-chlorobenzohydrazide (6b)
Off white solid; Yield: 70%; mp: 110-113°C; 1H-NMR (400 MHz, DMSO-d6): δ 12.00 (s, 1H), 8.68 (s, 1H), 8.49-8.45 (m, 2H), 7.96-7.94 (m, 2H), 7.63-7.57 (m, 4H), 7.45-7.39 (m, 2H), 3.81 (s, 3H); 13C NMR (100 MHz, DMSO) 162.6, 161.0, 151.6, 149.0, 148.0, 143.0, 140.1, 137.0, 136.5, 134.6, 133.7, 132.6, 130.2, 130.0, 129.0, 123.2, 121.3, 116.7, 111.0, 56.5, 49.0; MS (EI+) m/z=466.9 (exact Mass=466.08).
(22E)-N'-((4-(3-nitroimidazo [1,2-b] pyridazin-6-yloxy)-3-methoxyphenyl) methylene)-3-nitrobenzohydrazide (6c)
Yellow solid; Yield: 24%; mp: 98-100°C; 1H-NMR (400 MHz, DMSO-d6): δ 12.25 (s, 1H), 8.77-8.68 (m, 2H), 8.53-8.37 (m, 4H), 7.85 (t, J=7.20 Hz, 1H), 7.59-7.58 (m, 2H), 7.47-7.40 (m, 2H), 3.82 (s, 3H); MS (EI+): m/z=477.9 (exact Mass=477.1).
(22E)-N'-((4-(3-nitroimidazo [1,2-b] pyridazin-6-yloxy)-3-methoxyphenyl) methylene)-4-hydroxybenzohydrazide (6d)
Off white solid; Yield: 75%; mp: 130-133°C; 1H-NMR (400 MHz, DMSO-d6): δ 11.71 (s, 1H), 10.13 (s, 1H), 8.68 (s, 1H), 8.47-8.45 (m, 2H), 7.82-7.80 (m, 2H), 7.59-7.34 (m, 4H), 6.87-6.85 (m, 2H), 3.80 (s, 3H); 13C NMR (100 MHz, DMSO) 162.8, 161.4, 152.2, 148.7, 143.4, 140.7, 137.0, 136.4, 135.4, 134.2, 132.6, 131.6, 130.8, 128.3, 127.5, 123.6, 123.8, 121.9, 117.2, 111.5, 57.0; MS (EI+): m/z=448.9 (exact Mass=448.11).
(22E)-N'-((4-(3-nitroimidazo [1,2-b] pyridazin-6-yloxy)-3-methoxyphenyl) methylene)-4-methoxybenzohydrazide (6e)
Off white solid; Yield: 65%; mp: 123-128°C; 1H-NMR (400 MHz, DMSO-d6): δ 11.80 (s, 1H), 8.68 (s, 1H), 8.49-8.45 (m, 2H), 7.93-7.91 (m, 2H), 7.59-7.55 (m, 2H), 7.44-7.37 (m, 2H), 7.08-7.06 (m, 2H), 3.83 (s, 3H), 3.80 (s, 3H); 13C NMR (100 MHz, DMSO) 207.0, 160.2, 155.5, 143.2, 145.0, 142.3, 138.8, 137.0, 134.0, 130.2, 129.0 (2C), 127.6, 120.2 (2C), 117.3, 116.3, 113.7 (2), 36.2, 22.7; MS (EI+): m/z=463.0 (exact Mass=462.41).
(22E)-N'-((4-(3-nitroimidazo [1,2-b] pyridazin-6-yloxy)-3-methoxyphenyl) methylene)-3,4,5-trimethoxybenzohydrazide (6f)
Brown solid; Yield: 61%; mp: 115-118°C; 1H-NMR (400 MHz, DMSO-d6): δ 11.79 (s, 1H), 8.69 (s, 1H), 8.52-8.46 (m, 2H), 7.60-7.57 (m, 2H), 7.45-7.37 (m, 2H), 7.24 (s, 2H), 3.87 (s, 6H), 3.81 (s, 3H), 3.73 (s, 3H); 13C NMR (100 MHz, DMSO) 163.0, 162.8, 162,5, 160.9, 151.6, 147.0, 152.6, 142.3, 140.1, 136.5, 134.6, 134.0, 130.2, 130.0, 125.9, 123.2, 121.2, 116.6, 114.2, 111.0, 56.4 (2C), 55.9 (2C); MS (EI+): m/z = 523.0 (exact Mass=522.47).
(22E)-N'-((4-(3-nitroimidazo [1,2-b]pyridazin-6-yloxy)-3-methoxyphenyl)methylene)-4-methylsulfonyl)benzohydrazide (6g)
Off white solid; Yield:72%; mp: 135-149°C; 1H-NMR (400 MHz, DMSO-d6): δ 12.15 (s, 1H), 8.69 (s, 1H), 8.51-8.46 (m, 2H), 8.16-8.03 (m, 4H), 7.60-7.58 (m, 2H), 7.46-7.40 (m, 2H), 3.81 (s, 3H), 3.29 (s, 3H), 162.5, 160.9, 151.7, 148.6, 143.8, 142.9, 140.2, 138.4, 136.5, 134.6, 133.6, 130.2, 129.2 (2C), 127.7 (2C), 123.3, 121.4, 116.7, 111.0, 56.5, 43.7; MS (EI+): m/z=510.9 (exact Mass=510.1).
(22E)-N'-((4-(3-nitroimidazo [1,2-b] pyridazin-6-yloxy)-3-methoxyphenyl) methylene)-4-cyanobenzohydrazide (6h)
Light yellow solid; Yield: 59%; mp: 140-145°C; 1H-NMR (400 MHz, DMSO-d6): δ 12.08 (s, 1H), 8.54 (s, 1H), 8.54-8.46 (m, 2H), 8.29-8.21 (m, 1H), 8.12-8.05 (m, 3H), 7.60-7.58 (m, 2H), 7.47-7.40 (m, 2H), 3.82 (s, 3H); 13C NMR (100 MHz, DMSO) 163.0, 161.0, 154.7, 151.7, 148.1, 142.1, 140.1, 136.5, 134.6, 133.6, 132.9, 131.8, 130.1, 129.3, 128.3, 123.2, 121.3, 119.0, 116.7, 112.5, 111.0, 56.5; MS (EI+): m/z=457.8 (exact Mass=457.11).
(22E)-N'-((4-(3-nitroimidazo [1,2-b] pyridazin-6-yloxy)-3-methoxyphenyl) methylene)-2,5-difluorobenzohydrazide (6i)
Pale yellow solid; Yield: 69%; mp: 118- 122°C; 1H NMR (400 MHz, DMSO-d6): δ 12.20-12.01 (m, 1H), 8.69-8.67 (m, 1H), 8.48-8.42 (m, 1H), 8.36 (s, 1H), 7.59-7.55 (m, 2H), 7.45-7.32 (m, 4H), 7.25-7.11 (m, 1H), 3.80 (s, 3H); 13C NMR (100 MHz, DMSO) 160.9, 159.7, 159.4, 157.0, 151.6, 148.3, 143.7, 142.9, 140.1, 136.5, 134.6, 133.4, 130.2, 123.2, 121.4, 120.0, 118.5,117.0, 116.7, 111.4, 56.5; MS (EI+): m/z=468.9 (exact Mass=468.1).
(22E)-N'-((4-(3-nitroimidazo [1,2-b] pyridazin-6-yloxy)-3-methoxyphenyl) methylene)-3,5-dichlorobenzohydrazide (6j)
Off white solid; Yield: 74%; mp: 126-130°C; 1H-NMR (400 MHz, DMSO-d6): δ 12.08 (s, 1H), 8.69 (s, 1H), 8.47-8.46 (m, 2H), 7.94-7.89 (m, 3H), 7.60-7.57 (m, 2H), 7.46-7.39 (m, 2H), 3.81 (s, 3H); 13C NMR (100 MHz, DMSO) 161.4, 152.2, 149.1, 143.5, 140.7, 137.6, 137.0, 136.0, 135.4, 135.1, 134.7, 134.0, 132.1, 131.0, 130.8, 127.4, 123.8, 122.0, 117.2, 111.5, 57.0; MS (EI+): m/z=500.9 (exact Mass=500.04).
(22E)-N'-((4-(3-nitroimidazo [1,2-b] pyridazin-6-yloxy)-3-methoxyphenyl) methylene)-2-bromobenzohydrazide (6k)
Light brown solid; Yield:65%; mp: 130-134°C; 1H NMR (400 MHz, DMSO-d6): δ 12.1-11.9 (m, 1H), 8.69-8.67 (m, 1H), 8.48-8.41 (m, 2H), 8.31 (s, 1H), 7.74-7.67 (m, 2H), 7.59-7.49 (m, 2H), 7.46-7.29 (m, 2H), 7.20-7.05 (m, 1H), 3.81-3.61 (m, 3H); 13C NMR (100 MHz, DMSO) 171.4, 165.4, 161.0, 151.6, 147.6, 142.8, 140.1, 138.6, 136.4, 134.6, 133.6, 131.6, 130.7, 130.2, 129.0, 123.2, 121.3, 119.8, 116.6, 111.6, 56.5; MS (EI+): m/z=510.9 (exact Mass=510.08).
(22E)-N'-((4-(3-nitroimidazo [1,2-b] pyridazin-6-yloxy)-3-methoxyphenyl) methylene)-2-iodobenzohydrazide (6l)
Pale yellow solid; Yield: 85%; mp: 118- 122°C; 1H-NMR (400 MHz, DMSO-d6): δ 12.07-11.93 (m, 1H), 8.69-8.67 (m, 1H), 8.48-8.41 (m, 1H), 8.31 (s, 1H), 7.59-7.06 (m, 8H), 3.81-3.62 (m, 3H); 13C NMR (100 MHz, DMSO) 192.5, 170.0, 160.8, 151.6, 151.3, 147.7, 143.1, 142.9, 142.1, 140.1, 138.6, 137.9, 136.5, 135.4, 127.7, 124.3, 123.2, 121.3, 119.8, 116.6, 111.1; MS (EI+): m/z=558.9 (exact Mass=558.01).
Anti-tubercular activity
The in vitro anti-tubercular assessment of 6a-l have been tested against Mycobacterium tuberculosis H37Rv strain ATCC 27294 using the microplate alamar blue assay (MABA) and ciprofloxacin, pyrazinamide, streptomycin as standard drugs [29]. All the compounds 6a-l are screened for anti-tubercular activity in three sets (n=3). Initially, to lessen the evaporation of the medium in the aseptic 96 well plates during incubation, 200 μL of sterile deionized water was placed on all outer circumference wells of plate. Then to each well, 100 μL of the Middlebrook 7H9 broth was added and serial dilutions of the compounds 6a-l were made directly on the plate (i.e., 100-0.8 μg/mL). Then the 96 well plates was accurately occluded with parafilm and incubated at 37°C for five days. Finally, 25 μL of freshly prepared combination of almar blue (Accumed International, Westlake Ohio) reagent and 10% tween 80 (1:1) were added to the well plate and incubated for one day. A blue and pink colour was elucidated as no bacterial growth and growth respectively. The Minimal Inhibition Concentration (MIC) is the lowest drug concentration, which averted a colour change from blue to pink.
Results and Discussion
All the synthesized compounds were evaluated for in vitro anti-tubercular against M. tuberculosis and the results were tabulated in Table 1. Compounds 6c, 6d, 6f, 6g, 6i, 6j and 6k were very potent against M. tuberculosis H37Rv strain at MIC 1.6 μg/mL. Compounds 6b, 6e, 6h and 6l showed equivalent activity as that of standard drugs (pyrazinamide, ciprofloxacin) with a MIC 3.125 μg/mL. The compound 6a was found to be active against M. tuberculosis strain at MIC of 6.25 μg/mL similar to MIC of standard drug streptomycin.
| Compound | Substiutetns (R)/ Structure | MIC* values (μg/mL) |
|---|---|---|
| 6a | 3’-Cl | 6.25 |
| 6b | 4’-Cl | 3.125 |
| 6c | 3’-NO2 | 1.6 |
| 6d | 4’-OH | 1.6 |
| 6e | 4’-OMe | 3.125 |
| 6f | 3’,4’,5’ -OMe | 1.6 |
| 6g | 4’-SO2 Me | 1.6 |
| 6h | 4’-CN | 3.125 |
| 6i | 2’,5’-Cl | 1.6 |
| 6j | 3’,5’ -Cl | 1.6 |
| 6k | 2’ -Br | 1.6 |
| 6l | 2’ -I | 3.125 |
| Pyrazinamide | 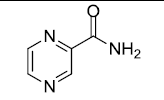 |
3.125 |
| Ciprofloxacin | 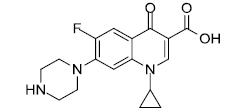 |
3.125 |
| Streptomycin | 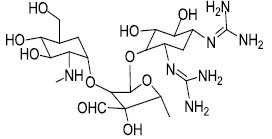 |
6.25 |
Table 1: In vitro anti-tubercular activity of the 6a-l against M. tuberculosis H37RV strain.
It was found that compounds substituted with m-nitro, p-hydroxyl, m,p,m-trimethoxy, p-sulfonylmethyl, o,m/m,m-dichloro and o-bromo at R exhibited potent anti-tubercular activity, while p-chloro, p-methoxy, p-cyano and o-iodo derivatives showed good anti-mycobacterial activity. Based on structural similarities with the first line anti-tuberculosis drugs (i.e., rifampicin), the synthesized compounds may act by inhibiting bacterial RNA polymerase (enzyme essential for DNA transcription) [30].
Conclusion
In this study, novel benzohydrazide derivatives were designed, synthesized and screened for anti-tubercular activity against M. tuberculosis H37Rv strain. The structures of the synthesized compounds were characterized by 1H NMR, 13C NMR and Mass spectroscopic results. All compounds screened for anti-TB activity using MABA, the compounds 6a-l showed anti-tubercular activity within MIC range of 1.6 to 6.25 μg/mL, more or equivalent to those for standard anti-Tb drugs (pyrazinamide, ciprofloxacin with MICs 3.125 μg/mL; streptomycin MIC 6.25 μg/mL). From the above results, due to the presence of novel structure and potent anti-tubercular activity it can be concluded that the tested compounds could be a best initiation to find new lead to class of anti-tubercular agents in the future.
Acknowledgement
The authors acknowledge Maratha Mandal Dental College, Belgaum for biological screening support.
References
- Jabado N, Gros P. Tuberculosis: The genetics of vulnerability. Nature. 2005;434:709-11.
- Behar SM, Baehrecke EH. Tuberculosis: Autophagy is not the answer. Nature. 2015;528:482-3.
- Kaufmann SHE. Tuberculosis: Deadly combination. Nature. 2008;453:295-6.
- Mao J, Yuan H, Wang Y, et al. Synthesis and anti-tuberculosis activity of novel mefloquine-isoxazole carboxylic esters as prodrugs. Bioorganic & Medicinal Chemistry Letters. 2010;20:1263-8.
- Umaa K, Kannan K, Krishnakumar K. Synthesis and comparative antimycobacterial evaluation of certain substituted benzimidazoles. Int J Chem Sci. 2009;7:80-6.
- Bedia K, Elcin O, Seda U, et al. Synthesis and characterization of novel hydrazide-hydrazones and the study of their structure-antituberculosis activity. European J of Med Chem. 2006;41:1253-61.
- Dimmock JR, Vashishtha SC, Stables JP. Anti-convulsant properties of various acetylhydrazones, oxamoylhydrazones and semicarbazones derived from aromatic and unsaturated carbonyl compounds. Eur J Med. Chem. 2000;35:241-8.
- Randhawa H, Kamboj A, Saluja AK. Synthesis, pharmacological evaluation and computational studies of some novel hydrazine derivatives of thiophene chalcone as antimicrobial and antioxidant agents. World Journal of Pharmaceutical Research. 2014;3:3146-59.
- El-Sabbagh OI, Rady HM. Synthesis of new acridines and hydrazones derived from cyclic β-diketone for cytotoxic and antiviral evaluation. Eur J Med Chem. 2009;44(9):3680-6.
- Kümmerle AE, Raimundo JM, Leal CM, et al. Studies towards the identification of putative bioactive conformation of potent vasodilator arylidene N-acylhydrazone derivatives. Eur J Med Chem. 2009;44(10):4004-9.
- Nasr T, Bondock S, Youns M. Anticancer activity of new coumarin substituted hydrazide-hydrazone derivatives. Eur. J. Med. Chem. 2014;76:539-48.
- Kandile NG, Mohamed MI, Zaky H, et al. Novel pyridazine derivatives: Synthesis and antimicrobial activity evaluation. Eur J Med Chem. 2009;44:1989-96.
- Wu J, Wang J, Hu D, et al. Synthesis and antifungal activity of novel pyrazolecarboxamide derivatives containing a hydrazone moiety. Chem Central J. 2012;6:51.
- Mamolo GM, Falagiani V, Zampieri D, et al. Synthesis and antimycobacterial activity of [5-(pyridin-2-yl)-1,3,4-thiadiazol-2-ylthio]acetic acid arylidene-hydrazide derivatives. II Farmaco 2001;56(8):587-92.
- Turan-Zitouni G, Ozdemir A, Kaplancikli ZA, et al. Synthesis and antituberculosis activity of new thiazolylhydrazone derivatives. Eur J Med Chem. 2008;43:981-5.
- Pandeya SN, Manjula H, Singh PN. Anti-depressant activity of some phenylaceticacid hydrozones and 2-chlorophenyl semicarbazones. Indian J Physiol Pharmacol 2000;44(4):509-10.
- Gemma S, Kukreja G, Fattorusso C, et al. Synthesis of N1-arylidene-N2-quinolyl- and N2-acrydinylhydrazones as potent antimalarial agents active against CQ-resistant P. falciparum strains. Bioorg Med Chem Lett. 2006;16(20):5384-8.
- Almasirad A, Tajik M, Bakhtiari D, et al. Synthesis and analgesic activity of N-arylhydrazone derivatives of mefenamic acid. J of Pharmand Pharmaceut Sci. 2005;8:419-25.
- Júnior WB, Alexandre-Moreira MS, Alves MA, et al. Analgesic and anti-inflammatory activities of salicylaldehyde 2-chlorobenzoyl hydrazone (H2LASSBio-466), salicylaldehyde 4-chlorobenzoyl hydrazone (H2LASSBio-1064) and their Zinc(II) complexes. Molecules. 2011;16:6902-15.
- Al-Hazmi GA, El-Asmy AA. Synthesis, spectroscopy and thermal analysis of copper (II) hydrazone complexes. J Coord Chem. 2009;62:337-45.
- Ibrahim KM, Gabr IM, Zaky RR. Synthesis and magnetic, spectral and thermal eukaryotic DNA studies of some 2-acetylpyridine- [N-(3-hydroxy-2-naphthoyl)] hydrazone complexes. J Coord Chem. 2009;62:1100-11.
- El-Behery M, El-Twigry H. Synthesis, magnetic, spectral, and antimicrobial studies of Cu(II), Ni(II0 Co(II), Fe(III), and UO2(II) complexes of a new Schiff base hydrazone derived from 7-chloro-4-hydrazinoquinoline. Spectrochimica Acta Part A: Molecular and Biomolecular Spectroscopy. 2007;66:28-36.
- Pouralimardan O, Chamayou A, Janiak C, et al. Hydrazone Schiff base-manganese(II) complexes: Synthesis, crystal structure and catalytic reactivity. Inorg Chim Acta. 2007;360:599-1608.
- Basu C, Chowdhury S, Banerjee R, et al. A novel blue luminescent high-spin iron(III) complex with interlayer O-H···Cl bridging: Synthesis, structure and spectroscopic studies. Polyhedron. 2007;26:3617-24.
- Arapov OV, Alferova OF, Levocheskaia EI, et al. Radioprotective efficacy of acyl hydrazones. Radiobiologiia. 1987;27:843-6.
- Siemann S, Evanoff DP, Marrone L, et al. N-arylsulfonyl hydrazones as inhibitors of IMP-1 metallo-β-lactamase. Antimicrob Agents Chemother. 2002;46:2450-7.
- Bakir M, Green O, Mulder WH. Synthesis, characterization and molecular sensing behavior of [ZnCl2(η3-N,N,O-dpkbh)] (dpkbh=di-2-pyridyl ketone benzoyl hydrazone). J Mol Struct. 2008;873:17-28.
- Sridhar P, Alagumuthu M, Ram B, et al. Drugs against neurodegenerative diseases: Design and synthesis of 6-amino-substituted imidazo [1,2-b]pyridiazines as acetylcholinesterase inhibitors. Chemistry Select. 2017;2(2):842-7.
- Lourenco MCS, Souza MVN, Pinheiro AC, et al. Evaluation of anti-tubercular activity of nicotinic and isoniazid analogues. Arkivoc. 2007;14:181-91.
- Simons FE. Advances in H1-antihistaminesN Engl J Med. 2004;351:2203-17.