Original Article
, Volume: 9( 5)Acridine Orange Staining For Identifying Viral Infection of Cells In-Vitro and Cellular DNA
Ronald B*
University of Nebraska, College of Arts & Sciences, Durham Science Center, 6001 Dodge Street, Omaha Nebraska 68182, USA
- *Correspondence:
- Ronald Bartzatt, University of Nebraska, College of Arts & Sciences, Durham Science Center, 6001 Dodge Street, Omaha Nebraska 68182, USA, Tel: 4025542651, E-mail: rbartzatt@unomaha.edu
Received: May 19, 2016; Accepted: June 10, 2016; Published: June 27, 2016
Citation: Bartzatt R. Acridine Orange Staining For Identifying Viral Infection of Cells In-Vitro and Cellular DNA. ChemXpress. 2016;9(5): 102.
Abstract
There has also been an increasing usage of dyes in the medicine sector. In clinical diagnoses, dyes act as a vital ingredient in many of the medical tests conducted on human patients. Most of the abnormalities can be seen in photographs or by automated instrumentation. Acridine orange is also known as Acridine Orange Base, Acridine Orange NO, and Basic Orange 14. Evidence of the efficacy of acridine orange staining is presented here for determination of the presence of single-strand RNA virus HIV within white blood cells and RAJI cells. Prior to the staining protocol the cells are mounted on glass slides and fixed using Carnoy solution. The cells mounted are stained. When examined by dark field ultraviolet microscopy the fluorescence (if any) indicates the presence or lack there-of virus particles. The relative number of cells infected and non-infected with HIV can be estimated. The fluorescence emission is stark and easily observed. Fluorescence emission is sufficiently strong for photographic recording.
Keywords
Acridine orange; HIV; Staining; HTLVIII; White blood cells
Abbreviations
HIV: Human Immunodeficiency Virus; AO: Acridine Orange
Introduction
The dye acridine orange (AO) interacts with DNA and RNA by intercalation and electrostatic attraction, respectively [1]. This dye is cell permeable and interacts with double-strand DNA by intercalation and fluoresces green, while, electrostatic single-strand RNA and DNA fluoresces red [1]. Acridine orange staining has been shown to useful for measuring apoptosis (a process of programmed cell death that occurs in multicellular organisms) [1]. During illumination by ultraviolet light and under dark field microscopy, viral particles present, whether single strand or double strand, will show emission and be observable.Acridine orange is the most common of fluorochromes that are utilized for studies of whole blood [2-4], reticulocyte counting [5], and for observation with identification of nucleic acids [6]. AO has been applied and found to be effective for studies of vaginal and cervical cancer tissue [7], the investigation of blood parasites [8], and various instrumental automated techniques [9]. Acridine orange staining is used to examine wet mounts for diagnosis of T. vaginalis infection [10]. Clinicians have used AO to correctly diagnose for T. vaginalis for a multitude of patients because of the advantage for early detection of T. vaginalis [10]. AO staining does not allow the distinction of acidic vesicles having different internal pH, however, it is phototoxic which allows the bursting of dye loaded vesicles [11]. This staining methodology has been applied to the rapid screening of urine for bacterial infection and by utilizing automated systems [12]. The staining method has been used parallel gram staining to successfully describe and characterize infectious keratitis [13]. This staining has been used and is effective for the early detection of positive blood cultures [14]. Some investigators, using AO has shown it to be superior to gram stain for detection of microorganisms in cerebrospinal fluid and various clinical specimens [15].
Acridine orange staining has been successful in the clinical detection and diagnoses of Plasmodium falciparum [16]. This staining methodology is repeatedly utilized successfully for blood smears examination for presence of malaria parasites [17]. Veterinary applications exist, AO staining has been used for detection of the presence of bovine respiratory syncytial virus [18]. Although reverse transcriptase assay has been utilized to detect human immunodeficiency virus (HIV) [19] this study demonstrates the detection of HIV infected lymphocytes can be accomplished by AO dye staining. Previous studies have shown the efficacy of examining laboratory cell lines [20,21]. Utilizing the dye acridine orange will continue to be useful for clinical diagnosis and laboratory research.
Experimental
Instrument and reagents
All reagents used were obtained from SIGMA (Sigma-Aldrich, PO Box 14508, St. Louis, MO 63178 United States). A Zeiss ultraviolet microscope was utilized for dark field microscopic examination. A NIKON 35 mm camera with suitable microscope interface was utilized to obtain images. All images were gathered at 400X magnification with EKTA chrome film at 200 ASA.
Tissue culture
For growth of human lymphocytes, culture media utilized was 90% RPMI 1640 having a 10% fetal bovine serum presence. Antibiotics present were penicillin (100 units/mL) and streptomycin (100 μg/mL).
Solutions for cell handling and staining
Phosphate buffered saline (PBS): For 1 L of solution: Sodium chloride 8 g, Potassium chloride 0.2 g, Sodium phosphate, dibasic anhydrous 1.44 g, Potassium phosphate, monobasic 0.24 g, then filter sterilize.
Carnoy Solution: Ethanol 6 mL, Chloroform 3 mL, Glacial acetic acid 1 mL.
Calcium chloride (0.1M): Dissolve 11.09 g CaCl2 into 1 L of distilled water.
Magnesium sulfate (0.2M): Dissolve 4.93 g MgSO4
Phosphate buffer: 0.067 molar Na2HPO4 and KH2PO4 in distilled water (pH 6.0).
L-Lysine treatment of slides prior to staining: It is necessary to treat class slides with 1% L-Lysine in distilled water prior to staining to assure that target cells are attached to the slide itself through the staining procedure. 1) Take a fresh clean glass slide and immerse in a solution of 1% L-Lysine for at least five minutes; 2) Remove slide with forceps or gloved hand to maintain cleanliness and allow to air dry.
Acridine orange solution: 0.01% acridine orange in phosphate buffer (pH 6.0).
Staining protocol
If cells used in tissue culture are attached, then the cells should be detached by treatment with 0.05% trypsin in normal saline. The specific staining procedure method is as follows: 1) Wash the infected cells in PBS and pellet softly at low speed; 2) Resuspend cells in minimal PBS and drop cells onto glass slides pre-coated with L-Lysine to attach cells; 3) Let air dry; 4) Fix slides in Carnoy fluid, for 4 minutes; 5) Treat rapidly by immersion in successive 95%, 80%, 70%, and 50% ethanol, respectively for 1 minute each; 6) Rinse for one minute into 0.002 molar MgSO4 solution; 7) Rinse in 1% acetic acid for one minute; 8) Rinse in 0.002 molar MgSO4 solution for one minute; 9) Rinse in phosphate buffer pH 6.0 for 2 minutes; 10) Stain in 0.01% acridine orange solution in same phosphate buffer for 3 minutes; 11) Rinse in same phosphate buffer (pH 6.0) for 3 minutes minimum; 12) Differentiate in 0.1 molar calcium chloride solution containing 0.004 molar MgSO4 for 10 minutes; 13) Rinse in phosphate buffer pH 6.0 for 10 minutes; 14) Drain off excess fluid and allow to air dry; 15) Examine under dark field UV microscopy.
Results and Discussion
There has also been an increasing usage of dyes in the medicine sector. Here, dyes act as an important ingredient in many of the medical tests conducted on humans. These dyes in coordination with latest diagnosis equipment allow flawless handling and high accuracy in tests that are carried out on patients. Most of the microbial or cellular abnormalities can be seen in photographs or by automated instrumentation. Accurate tissue evaluations facilitate correct diagnosis and allow for more immediate implementation of treatment plans.
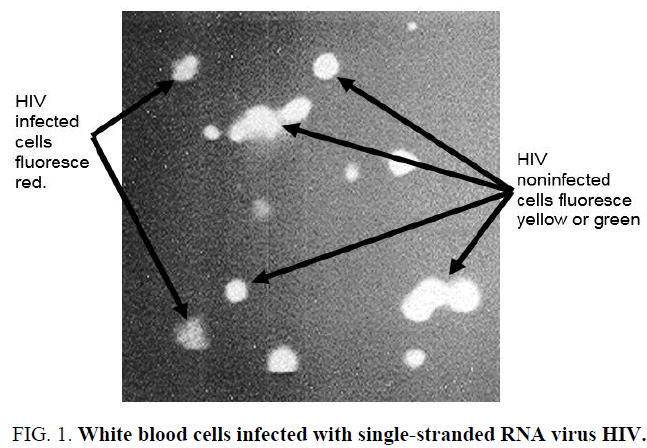
Acridine orange compound (N,N,N',N'-Tetramethylacridine-3,6-diamine) is also referred to as: Acridine Orange Base, Acridine Orange NO, Basic Orange 14, Euchrysine Rhoduline Orange, and Rhoduline Orange N [1,11,19]. It has the chemical formula C17H19N3, formula weight of 265.36, and is an orange powder. It has a center pyridine ring flanked on two sides by aromatic rings bearing a tertiary amine substituent, this structure enabling it to act as a fluorescent cationic dye [22,23]. The recognition/diagnosis of microbial infection is a major component of clinical care, and the use of dyes for preparation of specimens enables a fast and accurate recognition of disease. This is particularly the case for HIV incidence and patients. The proper collection of whole blood (not coagulated) from incoming patients and the methodical preparation of mounted and fixed blood cells, to be followed by acridine orange staining (per Materials and Methods) can reveal the actual infected white blood cells (see Figure 1). Figure 1 shows results of staining process for human white blood cells and examination under dark field microscopy. The red fluorescence observed is of HIV infected white blood cells. These will be vividly and starkly set apart from non-infected white blood cells that will emit yellow or green color. This is easy to see and is comparatively distinctive.
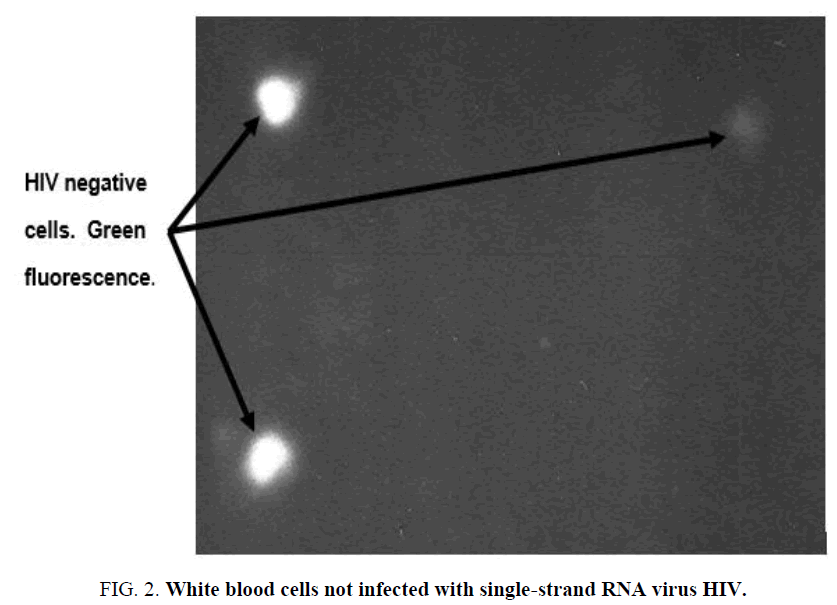
The white blood cells in Figure 2, all emit a green fluorescence which is descriptive of double-strand DNA and not single-strand RNA virus HIV. The green fluorescence is stark against the dark background characteristic of dark-field microscopy. The HIV negative cells are clearly identified and can be estimated for comparison of the extent of infection/non-infection of a patient blood sample.
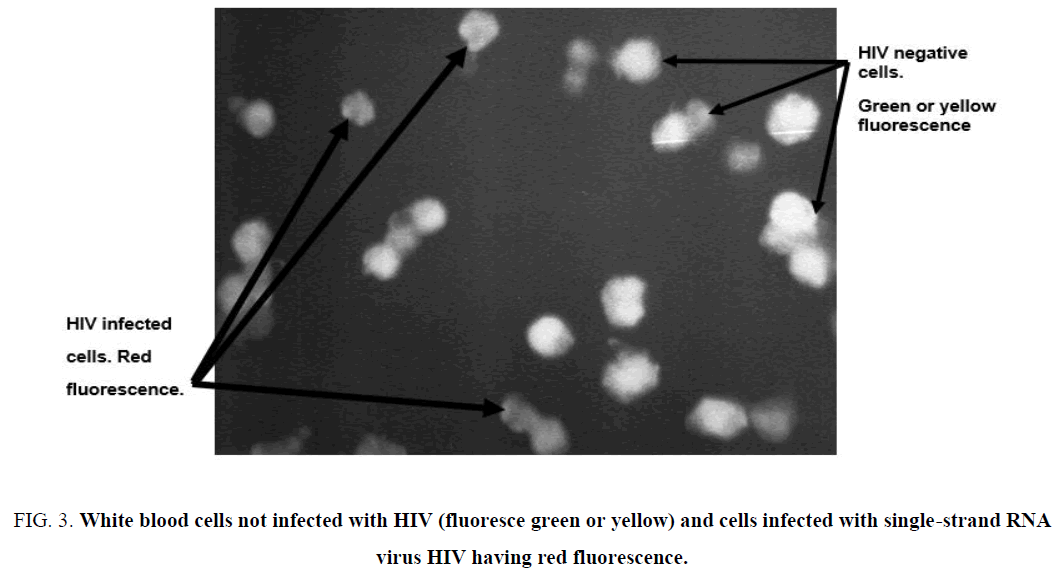
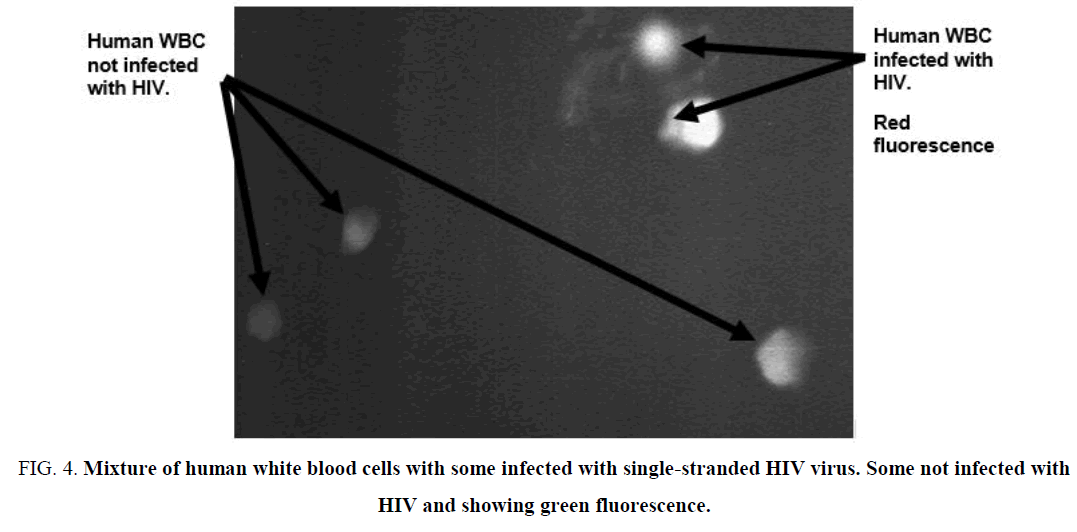
Further evidence of the efficacy of the application of acridine orange staining methodology for examination of potential presence of single-stranded RNA virus presence. In Figure 3, is additional plate of fixed cells attached to glass slide (see Experimental) having a high density of present human white blood cells. This is in addition to results presented in Figure 4, in which, a higher focus onto the fixed cells is accomplished. There is still stark and clear definition of non-infected and HIV infected white blood cells. Here, the human white blood cells must be fixed appropriately to the glass slides, which is accomplished by use of Carnoy solution (see Experimental).
The use of L-Lysine for pre-preparation of glass slides is very important. The coating of glass slides with L-Lysine helps tremendously to assure that the desired cells are attached to glass slides strongly, prior to the staining steps.
For attachment of human white blood cells to glass slides, the concentration of L-Lysine at 1% in solution is the minimal concentration for appropriate preparation of human blood samples (see Experimental). The firm attachment of blood cell samples to glass slides is important preparation prior to the fixing of the cells to the slides.
The Carnoy fixative solution method is shown to be suitable and efficient for human white blood cell fixation (see Experimental). The cells are strongly attached to the glass slides. The strong attachment of cells is highly important prior to the staining steps utilizing acridine orange. The attached cells must survive several steps with various mixtures during the acridine orange dye injection into fixed cells. The staining procedure should be carried out by use of protective gloves that allow the grasping and handling of fragile glass slides. The handling of the slides must be done in such a manner as not to contact or rub off the fixed cells from the glass slides. In addition, the transfer of glass slides from solution to solution should be done carefully and not subjecting prepared glass slides to trauma or undesired contact from gloves, forceps, etc.
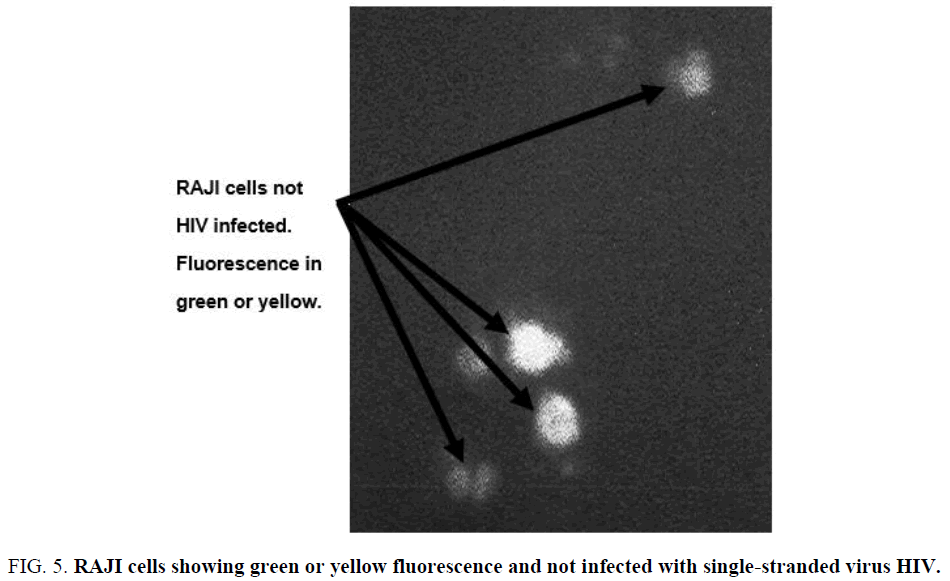
The RAJI cell line is a continuous human cell line that originates from a hematopoietic source [24]. The RAJI cell line produce an unusual strain of Epstein-Barr virus which can transform cord blood lymphocytes [24]. This is a very unique cell line that is placed through the staining methodology described (see Experimental). These cells were effectively fixed upon glass slides, fixated by Carnoy solution, and followed by acridine orange dye insertion, all done successfully. Shown in Figure 5 are RAJI cell types not infected with HIV, but showing the characteristic emission for non-infection with single-stranded RNA virus HIV which is a green fluorescence (see Figure 5). The emission signal is stark and easily discerned from adjacent matter. The use of acridine orange is effective for clinical applications. The dye is shown here to be effective for identifying the presence of the single-stranded RNA virus HIV. The dye can be injected into cells properly fixed onto glass slides utilizing L-Lysine attachment and fixation utilizing Carnoy solution (see Experimental).
The diagnosis of microbial infection is important for the timely and effective cure of the illness. Acridine orange has been shown to be useful for automated instrumental diagnostic procedures as well as manual methodologies. Here the technique, along with attachment and fixation as described, is shown to identify the presence of single-stranded RNA virus HIV. Further investigation of additional applications of the dye staining may significantly aid in the recognition of the presence of microbial pathogens.
Conclusion
The application of the dye acridine orange has been shown to have numerous advantages for clinical diagnosis of microbial infection and observation of cellular constituents. Acridine orange staining has been shown here to be useful for distinguishing HIV single strand RNA virus particles in the presence of double-strand DNA of the host cells. The single-strand HIV particles are detected in white blood cells and RAJI cells (human cell line from hematopoietic origin). Human white blood cells not infected with HIV can be distinguished from cells having proliferating HIV particles. The fluorescence is sufficiently stark as to estimate the number of viral infected cells compared to the number of cells not infected. The potential of acridine orange dye will continue to be explored for clinical applications.
Acknowledgements
This work was completed at the University of California-San Diego, Medical Center, San Diego California USA, S.D.G.
References
- Darynkiewiez Z. Methods in Cell Biology. 1990;33: 285-91.
- Behera B, Mathur P, Gupta B. Indian Journal of Medical Microbiology. 2010;28(2): 138-42.
- Meseguer M, Rafael L, Baquero M, et al. Eur. J. Clin. Microbiol. 1984;3(2):113-15.
- Mirret S, Lauer B, Miller G, et al. Comparison of acridine orange, methylene blue, and Gram stains for blood cultures. J. Clin. Microbiol. 1982;15(4): 562-66.
- Vander J, Harris C, Ellis S. Reticulocyte counts by means of fluorescence microscopy. J. Lab. Clin. Med. 1963;62: 132-39.
- Anderson E. Visual observation of deoxyribonucleic acid changes in bacteria during growth of bacteriophage. Nature. 1957;180: (4598) 1336-39.
- Von-Bertalanffy L, Masin M, Masin F. Cancer. 1958;11(5): 873-81.
- Sodeman T. The use of fluorochromes for the detection of malaria parasites. Am. J. Trop. Med. Hyg. 1970;19(1): 40-2.
- Metcalf R. The physiology of the salivary glands of Anopheles quadrimaculatus. Journal of the National Malaria Society. 1945;4(3): 223-27.
- Khatoon R, Jahan N, Khan H, et al. Evaluation of Different Staining Techniques in the Diagnosis of Trichomonasvaginalis Infection in Females of Reproductive Age Group. J. Clin. Diagn. Res. 2014;8(12): 5-8.
- Pierzynska-Mach A, Janowski P, Dobrucki J. Evaluation of acridine orange, LysoTracker Red, and quinacrine as fluorescent probes for long-term tracking of acidic vesicles. Cytometry A. 2014;85(8): 729-33.
- Crout F, Tilton R; Diagn. Rapid screening of urine for significant bacteriuria by Gram stain, acridine orange stain, and the Autobac MTS system. Microbiol. Infect. Dis. 1984;2(3): 179-84.
- Groden L, Rodnite J, Brinser J, et al. Acridine orange and Gram stains in infectious keratitis. Cornea. 1990;9(2): 122-28.
- Mascart G, Bertrand F, Mascart P. Comparative study of subculture, Gram staining and acridine orange staining for early detection of positive blood cultures. J. Clin. Pathol. 1983;36(5): 595-98.
- Lauer B, Reller L, Mirrett S. Comparison of acridine orange and Gram stains for detection of microorganisms in cerebrospinal fluid and other clinical specimens. J. Clin. Microbiol. 1981;14(2): 201-07.
- Long G, Jones T, Rickman L, et al. Acridine orange diagnosis of Plasmodium falciparum: evaluation after experimental infection. Am. J. Trop. Med. Hyg. 1994;51(5): 613-16.
- Kong H, Chung D; Korean J. Comparison of acridine orange and giemsastains for malaria diagnosis. Parasitol. 1995;33(4): 391-94.
- Bartzatt R. Acridine orange staining of bovine respiratory syncytial virus. J. Histotech. 1989;12(1): 115-17.
- Lee M, Sano K, Morales F, et al. Sensitive reverse transcriptase assay to detect and quantitate human immunodeficiency virus. J. of Clin. Microb. 1987;25(9): 1717-20.
- Bartzatt R; J. Histotech. 1987;10(2): 91-94.
- Bartzatt R; J. Histotech. 1987;10(2): 95-97.
- Lide. CRC Handbook of Chemistry and Physics. CRC Press, New York; 2004.
- Ferguson J, Mau. Absorption studies of acid-base equilibria of dye solutions Chem. Phys. Lett. 1972;17: 543-46.
- Karpova M, Schoumans J, Ernberg I, et al. Raji revisited: cytogenetics of the original Burkitt's lymphoma cell line. Leukemia. 2005;19(1): 159-61.