Original Article
, Volume: 15( 3)A Novel Corrosion Inhibitor for Improvement of C-Steel Resistance
- *Correspondence:
- Qhatan A Yousif Department of Chemistry, College of Science, University of Al-Qadisiyah, Al-Qadisiyah, Republic of Iraq, Iraq
Tel: +9647813112362; E-mail: Qahtan.Adnan@qu.edu.iq
Received Date: June 13, 2017 Accepted Date: July 17, 2017 Published Date: July 21, 2017
Citation: Yousif QA, Al-Zhara AA. A Novel Corrosion Inhibitor for Improvement of C-Steel Resistance. Int J Chem Sci 2017;15(3):160
Abstract
Impacts from ditiocarb sodium trihydrate inhibitor on corrosion of the carbon steel in saturated-CO2 and deaerated 0.5 M H2SO4 acidic solution has been studied using potentiodynamic polarization and open circuit potential procedures. The conclusion suggests that redundancy effectiveness of the evaluated ditiocarb sodium trihydrate compound depends on the concentration and the nature of the corrosive media: the corrosion current density (icorr) decreases with incrementing ditiocarb sodium trihydrate inhibitor concentration. Increasing the ditiocarb sodium trihydrate concentration leads not only to an increase in the efficiency of the inhibitor, but a reduction in the rate of corrosion. The corrosion potential becomes slightly more positive. Polarization results indicate that this compound behaves as a mixed-type inhibitor and it works best in deaerated, acidic media. On the basis of the data, ditiocarb sodium trihydrate is recommended for use as an effective inhibitor.
Keywords
C-steel; Ditiocarb sodium trihydrate; Corrosion inhibition; Mixed inhibitors
Introduction
Corrosion is a common problem encountered in the oil and gas industry, as refineries, pipelines and petrochemical plants suffer considerable corrosion problems. Internal corrosion in industrial equipment is commonly caused by exposure of the surfaces to water, hydrogen sulfide, and carbon dioxide, and also can be provoked by unchecked microbiological activity in the system [1]. The rate of corrosion is mainly governed by the flow management of the multiphase fluids encounter. For example, under lower flowing, decay is observed more commonly, whilst greater flowing indicates flow-accelerated corrosion, as well as erosion-corrosion, may occur. The degree of corrosion observed is generally associated with the amount and nature of the residues present. Low velocity permits residues to settle at the bottom, whereas, flow with greater velocity inclines towards swaying residues away from the pipeline, thus reducing the potential sites for pitting and corrosion [2]. The subject of corrosion, and its elimination, is particularly serious in the oil and gas industries, since the economic damage suffered by these industries, due to corrosion, is especially high [3].
Corrosion due to carbon dioxide has been an acknowledged problem associated with oil and gas manufacture and conveyance for many years [4], as the gas collects in the equipment following ingress. Dry carbon dioxide gas does not contribute to corrosion at the operating temperatures experienced commonly within gas and oil manufacturing systems, but when it subsequently comes into contact with both the aqueous phase and carbon steel, it promotes electrochemical reactions [5]. When dissolved in the water, carbon dioxide forms carbonic acid making the fluid acidic. Thus, corrosion due to carbon dioxide is particularly favored when there is an increase in the hydrogen scale value; at elevated temperatures, a change in the configuration of the aqueous stream, dissociation metal conditions and when acidic phases are present [6,7]. Consequently, it is the predominant form of corrosive attack encountered in gas and oil manufacture [8]. At high temperatures, scoring occurs on the iron carbide gas and oil pipes, which are used for their higher performance, and the metal begins to corrode under these circumstances. Carbon dioxide corrosion can have observed in two based forms: pitting [9] and a form of localized carbon dioxide corrosion under average-flow settings [10].
Of the different approaches used to control corrosion in the gas and oil sectors, the utilization of decaying materials is amongst the most efficient procedures [11,12]. There are many kinds of inhibitors of corrosion, and they are widely classified into cathodic, anodic or mixed corrosion inhibitors. There are also more subtle arrangements based on their chemical structure, with the greatest inhibitors being organic compounds, having one or more polar groups, which may be adsorbed strongly on the surfaces of metals [13-15]. The above inhibitors, which include examples containing moieties incorporating nitrogen (N), phosphorus (P) and/or sulfur (S) atoms, mitigate the response corrosion rate under solution-metal interface lacking the need for complicated measurement of the reaction rate. It is widely considered that organic inhibitors act through adsorption at interface of the solution-metal. The inhibitors decrease the corrosion current and the change in corrosion potential is attained by disrupting the process necessary for electrochemical procedures. The decay initiation on carbon steel under hostile acidic solution has been lengthily evaluated [16-39] and in the other studies, Eucalyptus globulus "Myrtaceae" has been investigated using surface analysis and electrochemical measurements as decay initiator for C38 steel in H2SO4 solution [40]. The inhibition in gas and oil fields is brings an additional level of complexity and needs certain ecological initiators based on the characteristics of implementation, i.e., wells, salvage units, pipelines, factories etc. Destructive compounds, alike H2S, CO2, and acids organic have the potential to deceive the error of initiation in wells [41]. Corrosion inhibition of X-70 pipeline steel in salt water saturated with carbon dioxide at 50°C with carboxyamidoimidazoline has been assessed by using electrochemical techniques [42] for its potential to inhibit the corrosion of C-steel alloy, along with other imidazoline derivatives [43]. The present work studies the inhibitory influence of ditiocarb sodium trihydrate on the corrosion of carbon steel (C-steel), which is used extensively in Iraq's oil equipment, in 0.5 M H2SO4 in dearated and saturated carbon dioxide solutions using electrochemical procedures.
Experimental
Chemicals and solutions
C-steel was used as the working electrode to investigate the corrosion inhibition in dearated (passing N2) and saturated carbon dioxide acidic solution at 298.15 K in both the nonattendance and attendance of ditiocarb sodium trihydrate inhibitor. The chemical compositions of carbon steel obtained from Iraq's oil fields were confirmed by analysis using optical emission spectroscopy (OES) (PMI MASTER Pro 2 Oxford, UK) and the data are shown in Table 1. The samples were cut to dimensions (1 cm × 1 cm and thickness 0.5 cm) and polished to mirror finish by using emery paper and followed by immersion in aqueous alumina suspensions to make the surface very soft. Then, the surface of carbon steel was cleaned using hot benzene (N.B., caution this is a cancer suspect agent) and acetone with distilled water and finally dried by passing a flow of nitrogen gas.
| Elements | C | Si | Mn | Cr | Mo | Ni | Cu | Fe |
|---|---|---|---|---|---|---|---|---|
| Wt.% | 2.18 | 3.1 | 1.36 | 0.1 | 0.12 | 0.21 | 0.59 | Balance |
Table 1. Chemical composition of carbon steel by optical emission spectroscopy.
The working C-steel specimen retrieved from carbon steel pipes were covered using epoxy, as to establish a cross-sectional vicinity, 1 cm2, as was under was interaction of the acid compound. A consistent temperature was maintained for all instances, 298.15 K through implementing thermostat bath circulator (ex HYSC company, Korea). All solutions were established freshly using distilled water mixed with sulfuric acid (obtained through Scharlau company). Solutions of 0.5 M H2SO4 were established through mixing of analytical reagent grade 97% H2SO4 in the water.
Corrosion inhibition study
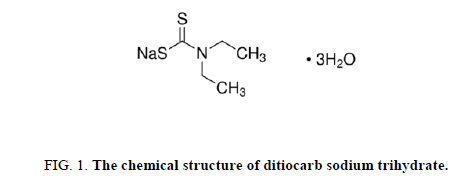
The inhibitory action of ditiocarb sodium trihydrate (which supplied from SIGMA-ALDRICH Company, Figure. 1) towards the corrosion of C-steel in dearated and saturated carbon dioxide acidic solutions under nonattendance as well as attendance of various concentrations by ditiocarb sodium trihydrate at over range from (1 × 10-3 M to 3 × 10-3).
Electrochemical measurement
The electrochemical measurement assortment utilized was a standard five necked Pyrex glass cells (250 ml), housing three-electrodes, including a working electrode (carbon steel), platinum electrode as auxiliary, furthermore a saturated Calomel electrode (Hg | Hg2Cl2 (s) | KCl) as a reference. The remaining two necks, allow the gas to enter and exit. The working electrodes with a square surface of 1 cm2 were held using epoxy resin. The auxiliary electrode constituted a platinum foil and coated with black platinum to increase a large surface area and confer high catalytic activity, constructed the Saturated Calomel electrode constituting a precise Luggin capillary tube placed under vicinity to the functioning electrode surface for the purpose of mitigating Ohmic potential drop (I × R). Then, the working electrode, platinum electrode and cited electrode using precise Luggin capillary were immersed in the saturated carbon dioxide solution and connected by Potentiostat/Galvanostat/ZRA Interface 1000 which obtained from the GAMRY company, USA) for measuring the open circuit potential (OCP) and obtained on the potentiodynamic curves, After that, using an Echem Analyst (version 6.23) to estimate the electrochemical parameters for Tafel polarization curves at a polarizing value ± 100 mV under respect towards free corrosion potential.
Results and Discussion
Open circuit potential
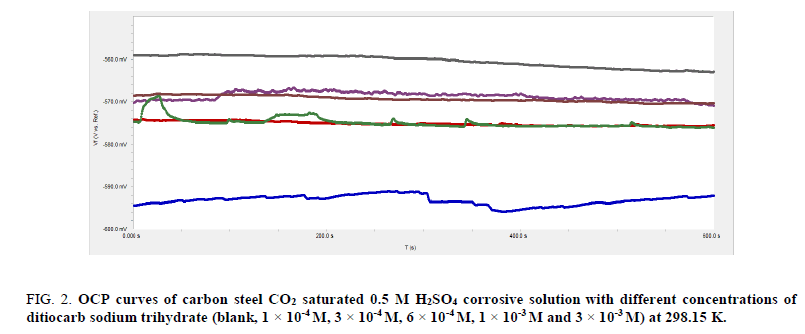
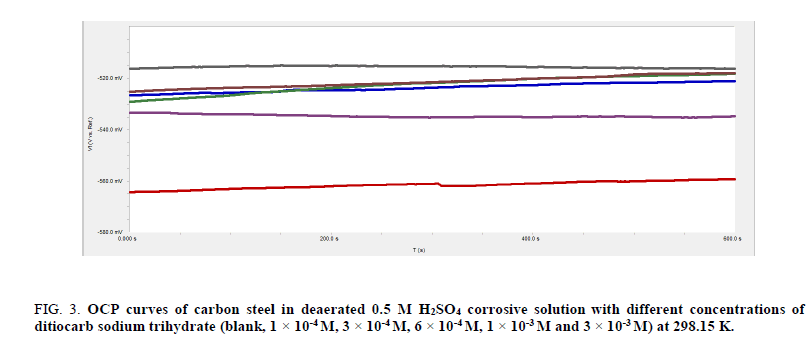
The variation of open circuit potential (OCP) against time provides an idea of cathodic and anodic procedures of corrosion reaction [44]. Fluctuation of OCP as a task of immersion time has to potential to be used as display the development processes of the shielding films [45], i.e., the open circuit potential is the steady state potential at which a carbon steel equilibrates in a given test solution and there is no external source polarizing or shifting the potential from the equilibrium value. The study was performed by immersing the carbon steel in deaerated or in carbon dioxide 0.5 M sulfuric acid solution and measuring the potential with time. The OCP was estimated when the potential becomes constant with respect to time [46]. The changes in OCP of carbon steel under nonattendance as well as attendance of various concentrations (1 × 10-4 M to 3 × 10-3 M) of ditiocarb sodium trihydrate inhibitor in saturated CO2 0.5 M H2SO4 and deaerated at 298.15 K are show in Figure. 2 and 3, respectively.
Figure 2: OCP curves of carbon steel CO2 saturated 0.5 M H2SO4 corrosive solution with different concentrations of ditiocarb sodium trihydrate (blank, 1 × 10-4 M, 3 × 10-4 M, 6 × 10-4 M, 1 × 10-3 M and 3 × 10-3 M) at 298.15 K.
Figure 3: OCP curves of carbon steel in deaerated 0.5 M H2SO4 corrosive solution with different concentrations of ditiocarb sodium trihydrate (blank, 1 × 10-4 M, 3 × 10-4 M, 6 × 10-4 M, 1 × 10-3 M and 3 × 10-3 M) at 298.15 K.
From the open circuit, potential curves shown in Figure. 2 and 3, it can been seen that the stable position was achieved under ten minutes introduction of carbon steel into the compound. In the absence of the ditiocarb sodium trihydrate inhibitor, the OCP drifts towards the noble direction during the initial 400s of immersion. Lacking ditiocarb sodium trihydrate, potential of the opened circuit turns negative given the dissolution of air that was present on the carbon steel prior to introduction into the compound [47]. Subsequently, an intermediate stage of the potential is observed in the range from -580.0 mV to -590.0 mV in saturated carbon dioxide and 525.0 mV to -530.0 mV in deaerated 0.5 M H2SO4 solution with respect to the saturated Calomel electrode then unit steady state. This disparity of OCP with time reveals the corrosivity of the sulfuric acid on the C-steel electrode. This indication explains passing in a potential of high frequency and low intensity, with an increase in their intensity towards the cathode direction, and the slow decay towards the anodic direction, in the special case of the material suffering from pitting corrosion [48,49]. If the anodic process of the corrosion process includes CO2 corrosion in solution and pitting corrosion in deaerated solution, then a starting period can be anticipated prior to formation of the pit, therefore the first property of the potential turns positive of the pitting overall potential, follow that the OCP turns further in decline [50]. This observation indicates that in the absence of inhibitor the OCP of the saturated carbon dioxide is lower than in deaerated 0.5 M H2SO4 solution. Simultaneously, a progress in the value of the potential creates an increment in the rate of corrosion alongside fastened development of the film.
The addition of ditiocarb sodium trihydrate inhibitor (1 × 10-4 M) to saturated carbon dioxide and deaerated 0.5 M H2SO4 solution turns towards balanced state potential property towards decline, without impacting any fluctuations in the characteristics of E-t curve, which indicates that catalyze fastened film dissolution. Thus, the shifts increment alongside incrementing concentration of ditiocarb sodium trihydrate inhibitor. The incrementing turn towards fluctuation of OCP in the presence of the ditiocarb sodium trihydrate inhibitor indicates that ditiocarb sodium trihydrate has influenced the anodic reaction of carbon steel corrosion [51]. The OCP for carbon steel in saturated carbon dioxide 0.5 M sulfuric acid solution is 560.0 mV lower than the deaerated condition, which is 518.0 mv. The changes of the OCP to more positive direction could be deliberate in regard to the establishment of shielding layer of inhibitor on surface of the carbon steel [52]. Such conclusion could be comprehended on the basis of establishment of a steady Fe (II)-complexes alongside sulfur atom [53]. Thus, the protective layer becomes thicker at the higher concentration (3 × 10-3 M) of the ditiocarb sodium trihydrate inhibitor.
Potentiodynamic polarization curves
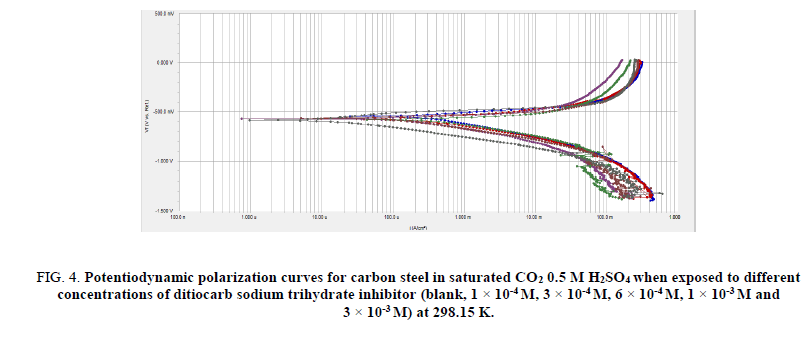
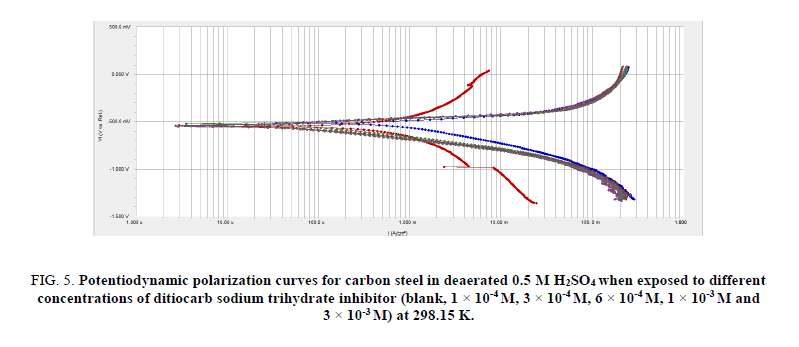
Figures. 4 and 5 depict the influence of ditiocarb sodium trihydrate inhibitor concentration (1 × 10-4 M to 1 × 10-3 M) towards carbon steel’s polarization curves in the saturated and deaerated 0.5 M H2SO4 solution at 298.15 K. The potentiodynamic polarization measurements have been conducted scanning volume of 0.5 mVs-1. Inspection of Figures. 4 and 5 reveal that either cathodic or anodic curves are turned to reduce present values under the attendance of ditiocarb sodium trihydrate inhibitor compared with the case in which the ditiocarb sodium trihydrate inhibitor is absent. This conclusion points towards the increase of ditiocarb sodium trihydrate mitigates the anodic mitigates and too obstructs the hydrogen evolution reaction for the corrosion process. Tafel lines constituting almost equal slopes were also acquired from the analytical data from the potentiodynamic scans, pointing towards the hydrogen fluctuation reaction was activation-administered [54]. This performance reflects the inhibitory action of ditiocarb sodium trihydrate. The range in the shifts in present density increments alongside incrementing ditiocarb sodium trihydrate inhibitor concentration. Therefore, the electrochemical structures, such as current corrosion density (icorr), corrosion potential (Ecorr), anodic Tafel slope (βa), and cathodic Tafel slope (βc), were obtained by Tafel extrapolation at the corrosion potential (Ecorr) and the parameters are listed in Tables 2 and 3.
Figure 4: Potentiodynamic polarization curves for carbon steel in saturated CO2 0.5 M H2SO4 when exposed to different concentrations of ditiocarb sodium trihydrate inhibitor (blank, 1 × 10-4 M, 3 × 10-4 M, 6 × 10-4 M, 1 × 10-3 M and 3 × 10-3 M) at 298.15 K.
Figure 5: Potentiodynamic polarization curves for carbon steel in deaerated 0.5 M H2SO4 when exposed to different concentrations of ditiocarb sodium trihydrate inhibitor (blank, 1 × 10-4 M, 3 × 10-4 M, 6 × 10-4 M, 1 × 10-3 M and 3 × 10-3 M) at 298.15 K.
| Conc. (M) | icorr µA/cm2 |
Ecorr mV |
βa mV/decade |
βC V/decade |
|---|---|---|---|---|
| 0 | 285.0 | -545.0 | 67.20 | 134.3 |
| 1 × 10-4 | 146.0 | -571.0 | 51.20 | 87.80 |
| 3 × 10-4 | 135.0 | -571.0 | 47.00 | 119.6 |
| 6 × 10-4 | 128.0 | -580.0 | 60.50 | 165.3 |
| 1 × 10-4 | 51.50 | -564.0 | 32.90 | 77.20 |
| 3 × 10-4 | 7.970 | -581.0 | 60.30 | 65.60 |
Table 2. Parameters of carbon steel corrosion in saturated CO2 0.5 M H2SO4 when exposed to different concentrations of ditiocarb sodium trihydrate inhibitor at 298.15 K.
| Conc.(M) | icorr µA/cm2 |
Ecorr mV |
βa mV/decade |
βc mV/decade |
|---|---|---|---|---|
| 0 | 386.0 | -512.0 | 52.40 | 127.6 |
| 1 × 10-4 | 210.0 | -560.0 | 160.7 | 165.0 |
| 3 × 10-4 | 32.60 | -551.0 | 77.60 | 83.50 |
| 6 × 10-4 | 29.50 | -522.0 | 36.90 | 79.70 |
| 1 × 10-4 | 14.00 | -539.0 | 40.30 | 54.70 |
| 3 × 10-4 | 9.180 | -543.0 | 39.30 | 51.10 |
Table 3. Parameters of carbon steel corrosion in deaerated 0.5 M H2SO4 when exposed to different concentrations of ditiocarb sodium trihydrate inhibitor at 298.15 K.
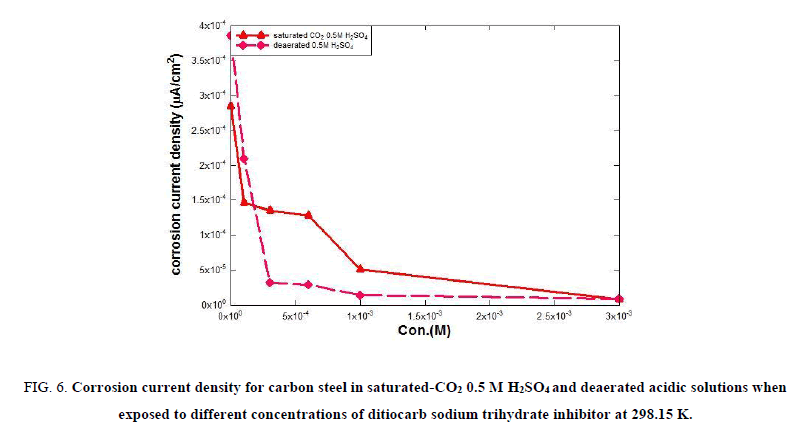
Tables 2 and 3 show that in the absence of inhibitor the corrosion current density icorr is high: 285.0 μA/cm2 for carbon steel in saturated carbon dioxide 0.5 M H2SO4 solution and 386.0 μA/cm2 in deaerated solution; the increase in corrosion current density icorr results from the increasing electrons pumps by anodic reaction for the corrosion reaction. In 0.5 M H2SO4 compounds, the anodic response of decay is the mitigation of Fe2+ ions inside the compound, and the cathodic reaction is the issue of hydrogen ions to constitute hydrogen gas, which initiates and increases the corrosion rate [55]. Nevertheless, the addition of ditiocarb sodium trihydrate inhibitor causes mitigations along the levels of corrosion current density the extent of corrosion with incrementing volumes of ditiocarb sodium trihydrate inhibitor. The value reaches 7.970 μA/cm2 in saturated carbon dioxide acidic solution and 9.180 μA/cm2 in deaerated solution at the optimum concentration 1 × 10-4 M. Generally, the reduction in corrosion current density for carbon steel samples is greater in deaerated solutions than in aerated 0.5 M H2SO4 solutions as show in Figure. 6. This is perhaps because the formation of the blockade film on carbon steel surface in deaerated solution is thicker than in the saturated-CO2 in the 0.5 M H2SO4 acidic solution.
Figure 6: Corrosion current density for carbon steel in saturated-CO2 0.5 M H2SO4 and deaerated acidic solutions when exposed to different concentrations of ditiocarb sodium trihydrate inhibitor at 298.15 K.
It has been stated that if the dislocation in Ecorr in the presence of inhibitor is greater than ± 85 mV when compared with Ecorr in the absence of inhibitor, then the inhibitor could be assessed as a property cathodic or anodic variation [56-58]. Under the current research, though a slight nonconformity in Ecorr is observed, the average dislocation is lower to 85 mV, indicating that ditiocarb sodium trihydrate could be a mixed-type inhibitor. The corrosion potential Ecorr values shifted towards the direction of slightly more progressive properties, and such conclusion recommends that anodic and the hydrogen evolution reactions are promoted; hence the ditiocarb sodium trihydrate inhibitor might be classified as mixed-type inhibitor [59]. The anodic Tafel slopes (βa) and the cathodic Tafel slopes (βc) are nearly slight altered upon addition of ditiocarb sodium trihydrate inhibitor and are somewhat greater than that predicted for H2 evolution according to the Volmer-Tafel mechanism [60]. The vicissitudes in either the anodic and cathodic Tafel slopes detected on introduction of ditiocarb sodium trihydrate inhibitor suggests that both responses are mitigated [61]. This can be interpreted by considering that the C-steel electrode is covered with iron oxide and the presence of the oxide film can evidently affect the surface reduction process, by forcing a barrier to undergo charge transfer through the oxide film. Thus, the values of the anodic Tafel slopes (βa) and the cathodic Tafel slopes (βc) are nearly equal in uninhibited and inhibited solutions. This explains that the inhibitor molecules might be adsorbed on the C-steel surface electrode and obstructive the reaction corrosion sites of the carbon steel surface lacking any influence on the anodic response instrument [62,63].
The inhibition efficiency may be calculated using the following equation (1) [64]:
 (1)
(1)
Where (icorr)uninh and (icorr)inh are the uninhibited and inhibited corrosion current densities. The inhibition efficiency and corrosion rate values which are parameters derived from the output of the Echem analyst program for analysis potentiodynamic scan are given in Tables 4 and 5.
| Conc. (M) | Corrosion Rate (mpy) | % IE |
|---|---|---|
| 0 | 106 | --- |
| 1 × 10-4 | 54.2 | 48.77 |
| 3 × 10-4 | 50.07 | 52.63 |
| 6 × 10-4 | 47.2 | 55.08 |
| 1 × 10-3 | 19.11 | 81.92 |
| 3 × 10-3 | 2.958 | 97.2 |
Table 4. Corrosion rate and inhibition effectiveness towards effectiveness of carbon steel in saturated-CO2 0.5 M H2SO4 compound under attendance from various volumes of ditiocarb sodium trihydrate inhibitor at 298.15 K.
| Conc. (M) | Corrosion rate (mpy) | % IE |
|---|---|---|
| 0 | 143.1 | --- |
| 1 × 10-4 | 30.19 | 45.59 |
| 3 × 10-4 | 12.10 | 91.70 |
| 6 × 10-4 | 10.95 | 92.48 |
| 1 × 10-3 | 5.190 | 96.37 |
| 3 × 10-3 | 3.409 | 97.62 |
Table 5. Corrosion rate and inhibition efficiency for carbon steel in deaerated 0.5 M H2SO4 solution under attendance of various concentrations of ditiocarb sodium trihydrate inhibitor at 298.15 K.
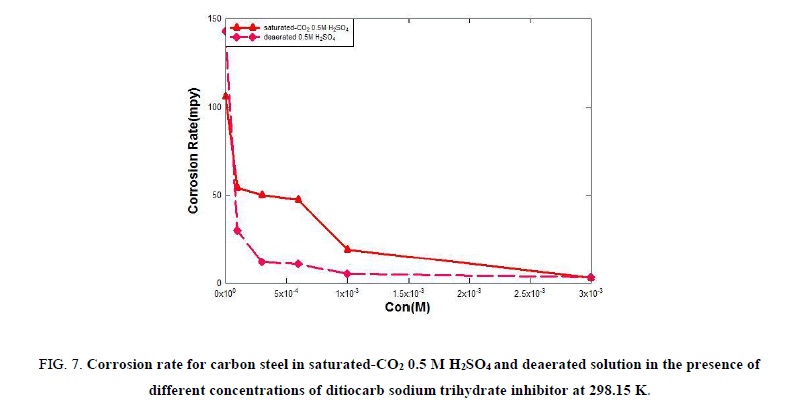
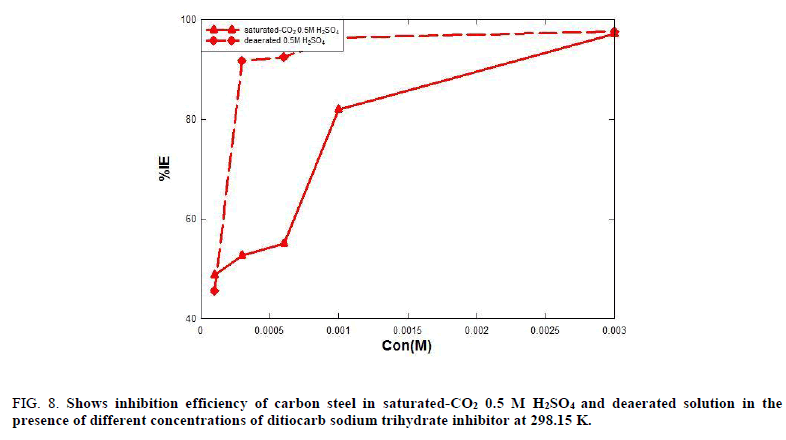
Tables 4 and 5 show that the corrosion rate values are high in the absence of the ditiocarb sodium trihydrate inhibitor, but with the addition of the ditiocarb sodium trihydrate inhibitor the corrosion rate is decreased. Thus, the corrosion rate decreases with increasing of ditiocarb sodium trihydrate inhibitor concentration, which becomes 2.958 mpy in saturated-CO2 0.5 M H2SO4 and 3.409 mpy in deaerated acidic solution at the optimum concentration (1 × 10-3 M). The reduction in the rate of corrosion with increasing concentration of the ditiocarb sodium trihydrate inhibitor in deaerated acidic solution is greater than the saturated-CO2 as shown in Figure. 7. The formation of a protective film of a significant thickness on the carbon steel surface electrode leads to an enhancement in the resistance of C-steel for corrosive media. The inhibition efficiency increased with increasing the ditiocarb sodium trihydrate inhibitor concentration. Thus, the inhibition efficiency for carbon steel in deaerated solution is greater than in saturated-CO2 0.5 M H2SO4, as show in Figure. 8.
Figure 7: Corrosion rate for carbon steel in saturated-CO2 0.5 M H2SO4 and deaerated solution in the presence of different concentrations of ditiocarb sodium trihydrate inhibitor at 298.15 K.
Figure 8: Shows inhibition efficiency of carbon steel in saturated-CO2 0.5 M H2SO4 and deaerated solution in the presence of different concentrations of ditiocarb sodium trihydrate inhibitor at 298.15 K.
The inhibition efficiency increased with increasing of ditiocarb sodium trihydrate inhibitor concentration even up 97.20% at (1 × 10-3 M) as show Figure. 4.
From the polarization data collected in Tables 2 to 5, it is clear that both cathodic and anodic reactions are retarded when the ditiocarb sodium trihydrate inhibitor is added to the 0.5 M H2SO4 acidic solution. From a thermodynamic perspective, as the ditiocarb sodium trihydrate inhibitor molecule contains two pairs of electrons on the sulfur atom, it is adsorbed on the surface of carbon steel, which obstructs the attack of the sulphuric acid and emphasizes that the ditiocarb sodium trihydrate is an excellent inhibitor.
Mechanism of corrosion inhibition
Firstly, the mechanism of electrochemical cathodic reaction in saturated-CO2 acidic medium [65-69] clarified in the following equations:
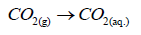 (2)
(2)
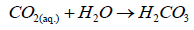 (3)
(3)
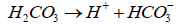 (4)
(4)
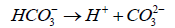 (5)
(5)
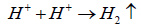 (6)
(6)
The overall of electrochemical reaction in carbon dioxide corrosion is explained in equation (7).
Fe+CO2+H2O → FeCO3+H2 ↑ (7)
Conversely, the electrochemical reaction of carbon steel in deaerated acidic medium explained in shown in equations (8) and (9) [68-70].
Fe → Fe2++2 e- (8)
2H++2 e- → H
↑ (9)
Furthermore, the inhibitory effect of the ditiocarb sodium trihydrate inhibitor in 0.5 M H2SO4 solution can be explained as follows. The chemical structure of the ditiocarb sodium trihydrate inhibitor (FIG. 1) contains a lone pair of electrons on the nitrogen atom, two lone pairs on the sulfur atom and a π-electron from double bond between the carbon and sulfur atoms. From electrochemical measurements, it was concluded that the ditiocarb sodium trihydrate inhibits the decay of carbon steel under sulphuric acid compound by adsorption at the C-Steel electrode solution interface. The possible adsorption of ditiocarb sodium trihydrate molecules on the metal surface through the electrostatic interactions [71-74] amidst charged molecules and metals. This leads to interaction of the unshared electron pairs (N, S) in the molecule inhibitor with the d-orbitals on the CSteel surface to form Fe (II) complexes [75-77], as well as the interaction of π-electrons between the carbon and sulfur atoms with the carbon steel surface. From the mentioned conclusion, the provided procedure for corrosion inhibition is suggested for ditiocarb sodium trihydrate inhibitor:
1. After C-Steel is immersed in the acidic solution, the ditiocarb sodium trihydrate (Na-DDC) complexes diffuse from the bulk of the solution towards the metal surface.
2. On the metal surface Na+-DDC complexes are converted into Fe2+-DDC complexes on the anodic sites.
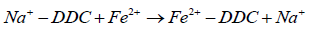 (10)
(10)
The released Na+ ions may combine with 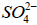 to form
to form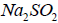 on the cathodic site.
on the cathodic site.
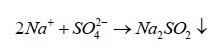 (11)
(11)
Conclusion
The open circuit potential values shift in a more positive direction with increasing ditiocarb sodium trihydrate inhibitor concentration and is discovered to mitigate both cathodic and anodic response that happen while the corrosion of carbon steel in the saturated -CO2 and deaerated 0.5 M H2SO4 solutions. Thus, the corrosion in carbon dioxide under acidic compound in non-attendance of ditiocarb sodium trihydrate inhibitor increases the dissociation of carbon steel surface via an anodic reaction. However, the addition of ditiocarb sodium trihydrate inhibitor decreases the release of the iron ions to the solution. This confirms that the ditiocarb sodium trihydrate compound is an excellent inhibitor and fulfils functions of a mixed-type inhibitor. Furthermore, the conclusion acquired through electrochemical assessments suggests the inhibitory event increments with incrementing inhibitor concentrations and the inhibition is greater in deaerated 0.5 M H2SO4 solution than in the acidic saturated carbon dioxide medium. Both the corrosion current density and corrosion rate mitigate under incrementing ditiocarb sodium trihydrate inhibitor concentration. Therefore, the inhibition effectiveness increments with incrementing ditiocarb sodium trihydrate inhibitor concentration.
References
- Rahuma MN, Ahmad I. Corrosion mitigation and inspection strategy for pipeline integrity management: An experience of Sarir Oilfield. NACE International. Corrosion Conference and Expo. 2013.
- Papavinasam S. Corrosion Handbook. 2nd ed. John Wiley & Sons. Inc. 2000.
- Santhana PS, Joseph RR. Dorothy R, et al. Corrosion problems in petroleum industry and their solution. Eur Chem Bull. 2014;3:300-7.
- Kermani MB, Harrop D. The impact of corrosion on the oil and gas industry. SPE Production Facilities. 1996;11:186-90.
- Dugstad A. The Importance of FeCO3 supersaturating on the CO2 corrosion of carbon steels, corrosion. NACE. Houston. 1992;14.
- Nalli K. Corrosion and its mitigation in the oil and gas industry. An overview: PM-Pipeliner Report. 2010.
- Gatzky LK, Hausler RH. A novel correlation of tubing corrosion rates and gas production rates. Adv in CO2 Corro. 1984;1:87.
- Kermani MB, Smith LM. CO2 corrosion control in oil and gas production: Design considerations. The Institute of Materials. Eur Federation of Corr Publications, London. 1997.
- Schmitt G. Fundamental aspects of CO2 corrosion. 1st ed. NACE, Houston. 1984;pp:10.
- Dunlop A. Hassel HL, Rhodes PR. Fundamental considerations in sweet gas well corrosion. 1st ed. NACE, Houston. 1984;pp:461-4.
- Miksic BM, Furman AY, Kharshan MA. Effectiveness of the corrosion inhibitors for the petroleum industry under various flow conditions. NACE International, Corrosion Conference and Expo. 2009.
- Rahuma MN, EL-Sabbah MB, Hamad IM. Effect of serine and methionine on electrochemical behavior of the corrosion of mild steel in aqueous solutions. Hindawi Publishing Corporation, ISRN, Corrosion: 2013;pp:1-7.
- Henschel A, Senkovska I, Kaskel S. Liquid-phase adsorption on metal-organic frameworks. Adsorption. 2011;17:219-26.
- Kuklin RN, Emets VV. Physicochemical processes at the interfaces: Form factor of the electron density of an anion specifically adsorbed on metal surface in an electrolyte. Protection of Metals and Physical Chemistry of Surfaces. 2014;50:5-14.
- Frumkin AN. Potentsialynulevogozaryada (Potentia ls of Zero Charge). Moscow, Nauka. 1982.
- Raja PB, Sethuraman MG. Studies of inhibition of mild steel corrosion by Rauvolfia serpentine in Acid Media. J Mat Engr Perf. 2010;19:761-6.
- Li L, Zhang XL. Adsorption and corrosion inhibition of Osmanthus fragran Leaves Extract on Carbon Steel. Corros Sci. 2012;63:82-90.
- Raja PB, Qureshi AK, Rahim AA, et al. Indole alkaloids of Alstoniaangustifolia var. latifolia as green inhibitor for mild steel corrosion in 1 M HCl media. J Mat Engr Perf. 2013;22:1072-8.
- Popova A, Christov M, Raicheva S, et al. Adsorption and inhibitive properties of benzimidazole derivatives in acid mild steel corrosion. Corros Sci. 2004;46:1333-50.
- Ahamad A, Quraishi MA. Bis (benzimidazol-2-yl) disulphide: An efficient water soluble inhibitor for corrosion of mild steel in acid media. Corros Sci. 2009;51:2006-13.
- Shukla SK, Quraishi MA. 4-Substitutedanilino methyl propionate: New and efficient corrosion inhibitors for mild steel in hydrochloric acid solution. Corros Sci. 2009;51:1990-7.
- Singh AK, Quraishi MA. Effect of 2, 2′ benzothiazolyl disulfide on the corrosion of mild steel in acid media. Corros Sci. 2009;51:2752-60.
- Hosseini SM, Azimi A. The inhibition of mild steel corrosion in acidic medium by 1-methyl-3-pyridin-2-yl-thiourea. Corros Sci. 2009;51:728-32.
- Ahamad A, Quraishi MA. Mebendazole: New and efficient corrosion inhibitor for mild steel in acid medium. Corros Sci. 2010;52:651-56.
- Singh AK, Quraishi MA. Effect of cefazolin on the corrosion of mild steel in HCl solution. Corros Sci. 2010;52:152-60.
- Muthukrishnan P, Jeyaprabha B, Prakash P. Corrosion inhibition and adsorption behavior of Setaria verticillata leaf extract in 1 M sulphuric acid. J Mat Engr Perf. 2013;22:3792-800.
- Oguzie EE, Enenebeaku CK, Akalezi CO, et al. Adsorption and corrosion inhibiting effect of Dacryodis edulis extract on low carbon-steel corrosion in acidic media. Int J Colloid Interf Sci. 2010;349:283-92.
- Lebrini M, Robert F, Lecante A, et al. Corrosion inhibition of C38 steel in 1 M hydrochloric acid medium by alkaloids extract from. Int J Biochem Sci. 2011;53:2443-60.
- Okafor PC, Ikpi MI, Uwah IE, et al. Inhibitory action of Phyllanthus amarus extract on the corrosion of mild steel in acidic media. Corros Sci. 2008;50:2310-7.
- El-Etre AY. Inhibition of acid corrosion of carbon steel using aqueous extract of olive leaves. J Colloid Interface Sci. 2007;50:578-83.
- Lecante A, Robert F, Blandinieres PA, et al. Anti-corrosive properties of S. tinctoria and G. ouregou alkaloid extracts on low carbon steel. Curr Appl phys. 2011;11:714-24.
- Satapathy AK, Gunasekaran G, Sahoo SC, et al. Corrosion inhibition by Justica gendarussa plant extract in hydrochloric acid solution. Corros Sci. 2009;55:2848-56.
- Oguzie EE. Evaluation of the inhibitive effect of some plant extracts on the acid corrosion of mild steel. Corros Sci. 2008;50:2993-8.
- Eduok UM, Umoren SA, Udoh AP. Synergistic inhibition effects between leaves and stem extracts of Sida acuta and iodide ion for mild steel corrosion in 1 M H2SO4 solutions. Arabian J of Chem. 2012;5;325-37.
- Hamdy A, El-Glendy NS. Thermodynamic, adsorption and electrochemical studies for corrosion inhibition of carbon steel by henna extract in acid medium. Egyptian J of Petrol. 2013;22:17-25.
- Uwah IE, Okafor PC, Ebiekpe VE. Inhibitive action of ethanol extracts from Nauclea latifolia on the corrosion of mild steel in H2SO4 solutions and their adsorption characteristics. Arabian J of Chem. 2013;6:285-93.
- El Bribri A, Tabyaoui M, Tabyaoui B et al. The use of Euphorbia falcata extract as eco-friendly corrosion inhibitor of carbon steel in hydrochloric acid solution. Materials Chem and Phy. 2013;141:240-7.
- Tebbji K, Aouniti A, Attayibat A. Inhibition efficiency of two bipyrazole derivatives on steel corrosion in hydrochloric acid media. Indian J of Chem Technol. 2011;244-53.
- Nahl´e A, Abu-Abdoun I, Abdel-Rahman I. Electrochemical studies of the effect of trans-4-hydroxy-4’-stilbazoleon the corrosion inhibition of mild steel in HCl solution. Anti-Corrosion Methods and Materials. 2007;54:244-8.
- Rekkab S, Zarrok H, Salghi R et al. Green corrosion inhibitor from essential oil of Eucalyptus globulus (Myrtaceae) for C38 steel in sulfuric acid solution. J Mater Environ Sci. 2012;3:613-27.
- Rajeev P, Surendranathan AO, Murthy ChSN. Corrosion mitigation of the oil well steels using organic. J Mater Environ Sci. 2012;3:856-6.
- Ortega-Sotelo DM, Gonzalez-Rodriguez JG. CO2 corrosion inhibition of X-70 pipeline steel by carboxyamido imidazoline. J Solid State Electrochem. 2010.
- Chen GH, Mzha OJ. Corrosion inhibition of imidazoline derivates with benzene rings on mild steel in CO2-saturated brine solution. Chem Res Chinese Uni. 2012;28:691-5.
- Christopher Brei I. The electrochemical behavior of aluminum in acid chloride solution. Corro Sci. 1992;33:20-1.
- Gao H, Li Q, Chen FN, et al. Study of the corrosion inhibition effect of sodium silicate on AZ91D magnesium alloy. Corro Sci. 2011;53:1401-7.
- Wiersma BJ. Hydrogen generation during the corrosion of carbon steel in oxalic acid. Strategic Materials Technology Department(SMTD). 2004.
- Sadawy MM, Heseinov RG, Shirinov TU. Corrosion and electr ochemical behavior of martensitic-austenitic stainless steel in hydrochloric acid solutions. Int J Pure Appl Chem. 2011;6:319-22.
- Hladky K, Dawson JL. The measurement of corrosion using electrochemical 1f noise. Corros Sci. 1982;22:231-7.
- Dawson JL, Rothwell AN, Walsh Lawson TG, et al. NACE. 1993.
- Brett Cma, Costa J. Extended Abstracts of 38th Meeting of ISE. Maastricht, Holland. 1987.
- Ahmed Al-Amiery A, Kadhum HAA. Novel corrosion inhibitor for mild steel in HCl. Materials. 2014;7:662-72.
- Amin MA, Abd El-Rehim SS, El-Sherbini EEF, et al. The inhibition of low carbon steel corrosion in hydrochloric acid solutions by succinic acid: Part I. Weight loss, polarization, EIS, PZC, EDX and SEM studies Electrochim. Acta 2007;pp:3588.
- Harms H, Volkland HP, Repphun G, et al. Action of chelators on solid iron in phosphate-containing aqueous solutions. Corros. Sci, 2003;45:1717-32.
- Ramesh Saliyan V, Airody VA. Inhibition of corrosion of mild steel in acid media by N′-benzylidene-3-(quinolin-4-ylthio) propanohydrazide. Bull Mater Sci. 2008;31;699-711.
- Milliams Dec, Waard De. Carbonic acid corrosion of steel Corrosion, 1975;31:131-6.
- Mahdavian M, Tehrani-Bagha AR, Holmberg K. Comparison of a cationic gemini surfactant and the corresponding monomeric surfactant for corrosion protection of mild steel in hydrochloric acid. J Surf Deterg. 2011;14:605-13.
- Palomar ME, Olivares-Xometl CO, Likhanova NV, et al. Imidazolium, pyridinium and dimethyl-ethylbenzyl ammonium derived compounds as mixed corrosion inhibitors in acidic medium. J Surf Deterg. 2011;14;211-20.
- Quraishi MA, Jamal D. Dianils: New and effective corrosion inhibitors for oil-well steel (N-80) and mild steel in boiling hydrochloric acid. Corros. 2000;56:156-60.
- Yurt A, Ayk?n O. Diphenolic Schiff bases as corrosion inhibitors for aluminium in 0.1M HCl: Potentiodynamic polarisation and EQCM investigations. Corros Sci. 2011;53:3725-32.
- Bockris JM, Reddy AKN. Modern Electrochemistry. Plenum Press, New York. 1974;2:2-6.
- Zohdy KM. Surface protection of carbon steel in acidic solution using ethylene diaminetetra acetic disodium salt. Int J Electrochem Sci. 2015;414-31.
- Abd El Rehim SS, Hasan HH, Amin MA. Mater Chem Phys. 2002;78:337.
- Abdel Rehim SS, Ibrahim MAM, Khaled KF. The inhibition of 4-(2′-amino-5′-methylphenylazo) antipyrine on corrosion of mild steel in HCl solution. Mater Chem Phys. 2001;70:268-73.
- Bouanis FZ, Bentiss F, Traisnel M, et al. Enhanced corrosion resistance properties of radiofrequency cold plasma nitrided carbon steel: Gravimetric and electrochemical results. Electrochim Acta. 2009;54:2371-8.
- Cde W, Lotz U. Prediction of CO2 corrosion of carbon steel. EFC, The Institute of Materials, London. 1994.
- Dean F, Powell S. Hydrogen flux and high temperature acid corrosion, 06436th ed. NAC Expo, conference. 2006.
- Nimmo B, Hinds G. Beginners guide to corrosion. NPL, Teddington. 2003.
- Hunnik EWJ, Pots BFM, Hendriksen ELJA. The formation of protective FeCO3 corrosion product layers in CO2 corrosion. Control. 1996;96:6.
- Nesic S, Lee KLJ. A mechanistic model of iron carbonate film growth and the effect on CO2 corrosion of mild steel. Corr. 2002;pp:237.
- Sherif E-SM, Erasmus RM, Comins JD. In situ Raman spectroscopy and electrochemical techniques for studying corrosion and corrosion inhibition of iron in sodium chloride solutions. Electrochim Acta. 2010;55:3657-63.
- Sherif E-SM. Effects of 5-(3-aminophenyl)-tetrazole on the inhibition of unalloyed iron corrosion in aerated 3.5% sodium chloride solutions as a corrosion inhibitor. Mater Chem Phys. 2011;129:961-7.
- Sherif E-SM. Comparative study on the inhibition of iron corrosion in aerated stagnant 3.5 wt % sodium chloride solutions by 5-phenyl-1H-tetrazole and 3-amino-1,2,4-triazole. Ind Eng Chem Res. 2013;52:14507-13.
- Qhatan A, AL-Zhara AA. Adsorption aspects, inhibitory properties and a study of surface corrosion of carbon steel in acidic media. Modern App Sci. 2016;10:82.
- Qhatan A, AL-Zhara AA. Electrochemical methods, SEM-EDX and AFM studies for assessing corrosion inhibitor of carbon steel in acidic media. ARPN of Eng. And app Sci. 2016;11:12619.
- Qhatan A, AL-Zhara AA. Evaluation of corrosion inhibition of carbon steel in 0.25 M H2SO4. J chem sci pharm res. 2016;8:64.
- Qhatan A. A study of quantum and electrochemical investigation for a sample of mild steel in HCl-CO2 system. Res J of Pharmaceutical, Biol and Chem Sci. 2016;8:1654.
- AL Zhara AA, Qhatan A. Assessment of electrochemical behaviour for X65-steel: Part A: OCP and PDP. Int J Chem Tech Res. 2017;10:477.