Original Article
, Volume: 15( 2)Synthesis and Physico-Chemical Properties of Transition Metal Complexes with 2,4-Dichloro-6-{(hydroxyimino) [1-(4-methylphenyl)-1H-Pyrazol-4-yl] methyl} Phenol
- *Correspondence:
- Nagawade AV Department of Chemistry, Ahmednagar College, Ahmednagar (MS) India
Tel: 9766454212; E-mail: avnagawade@gmail.com
Received Date: May 02, 2017 Accepted Date: May 23, 2017 Published Date: May 26, 2017
Citation: Dhokale NT, Karale BK, Nagawade AV. Synthesis and Physico-Chemical Properties of Transition Metal Complexes with 2,4- Dichloro-6-{(hydroxyimino) [1-(4-methylphenyl)-1H-Pyrazol-4-yl] methyl} Phenol. Dhokale NT, Karale BK and Nagawade AV. Int J Chem Sci. 2017;15(2):138.
Abstract
Four new complexes were synthesized by the reaction of 2,4-dichloro-6-{(hydroxyimino) [1-(4-methylphenyl)-1H-pyrazol-4-yl] methyl} phenol ligand with transition metal ions Manganese (Mn2+, Complex-I), Iron (Fe2+, Complex-II), Cobalt (Co2+, Complex-III) and Nickel (Ni2, Complex-IV). The metal complexes have been characterized with the help of elemental analysis, IR spectroscopy, UV-Visible, thermal analysis and molar conductance measurements. The elemental analysis data exhibited the formation of complex with 2:1 (L:M) ratio. The Schiff bases are bidentate coordinating through the imine nitrogen and phenolic oxygen of salicyloyl pyrazoleoximes. Based on analytical and spectral data, four-coordinate geometry was assigned for all complexes. The electronic absorption spectra suggest the square planer geometry for the complexes. The molar conductivity data showed the non-electrolytic nature of the complexes.
Keywords
Complexes; Elemental analysis; Thermograms; Conductance; Electrolytes
Introduction
Metals have an esteemed place in medicinal chemistry. Transition metal represents the d block elements which includes group 3 to 12 on the periodic Table. Their d shells are in incompletely filled. This property of transition metal resulted in the formation of coordination complexes. The complexes are having advances in inorganic chemistry and offer better opportunities to use metal complexes as therapeutic agents [1].
Metal complexes play an essential role in agriculture, pharmaceutical and industrial chemistry. Tridentate Schiff bases and their transition metal complexes exhibits good antibacterial activity against E. coli, S. aureous, B. substilis and B. pumpilis [2]. Some heterocyclic Schiff bases can act as antibacterial agents [3-5]. The halogenated [6], Azetidinenes [7], P-anisidene [8] derived complexes also shows good antibacterial activity. Complexes play a vital role in catalysis [9], biological system, polymer, dyes. Furthermore, some uses as antifertility and enzymatic agent. Metal complexes show antifungal [10], antioxidant [11], anti-inflammatory [12], analgesic [13], anti-tumor cytotoxic activity, synergistic action on insecticide and act as plant growth regulator [14-16]. Some of the synthesized transition metal complexes showed improved antimicrobial activity compared with the free ligand after complexation [17]. Metal complexes are used in polymer, dyes [18-20] industry. The complexes of isoflavone with Mn2+ and Ni2+ showed greater antitumor activity [21].
A number of scientists in the past have tried to find some correlation between chemical structure and physiological or biological properties. It is now well-known fact that the activity of a compound mainly depends on heterocyclic moiety present in the particular compound, the nature of the substituent and the position of the substituents in these compounds. All literature review reveled that compound containing pyrazole moiety, fluorinated compounds showed good biological applications.
Due to the emergence of new fungal pathogens 1-(3, 4 difluorophenyl-4-(2-hydroxybenzoyl)-1H-pyrazole), in order to broaden its antifungal [22] activity and increase its potency it has great importance to synthesize the novel transition metal complexes with 2,4-dichloro-6-{(hydroxyimino) [1-(4-methylphenyl)-1H-pyrazol-4-yl] methyl} phenol.
Material and Methods
All chemicals are pure (Sigma Aldrich, SRL, Fischer scientific etc.,) the solvents are analytical grade and purified before used. Distilled water and ethyl alcohol were always used. Melting points of the synthesized complexes were recorded by open glass capillaries method and are uncorrected. The molar conductance was measured using 0.001 M solution of complexes in DMF using digital conductivity meter (Elico cm-180). The elemental analysis data were recorded on Thermo Scientific elemental analyzer (FLASH 2000). The FTIR spectra of ligand and complexes were measured in the range 4000 cm-1 to 350 cm-1 with Shimadzu FTIR spectrophotometer. The TGA spectra were recorded using Shimadzu Thermometric Analyzer (TGA-50). The electronic absorption spectra were recorded on Shimadzu UV-Visible double beam spectrophotometer. Standard volumetric methods were used to find the concentration of metal ions [23]. The ligand 2,4-dichloro-6-{(hydroxyimino) [1-(4-methylphenyl)-1H-pyrazol-4-yl] methyl} phenol was synthesized using the method reported in our previous investigation [24].
Synthesis of complexes
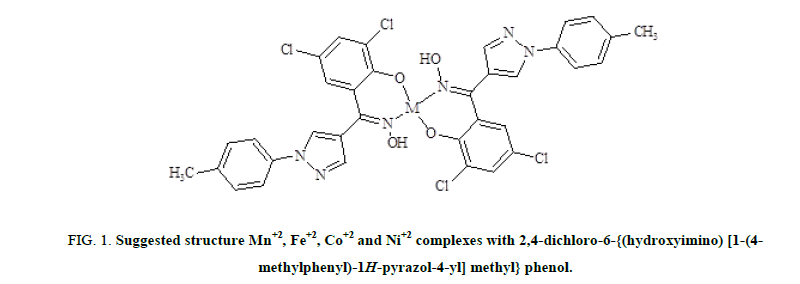
The metal sulphate (MnSO4, FeSO4, CoSO4 and NiSO4) solutions (0.001 mol) were prepared in distilled water and acidified by concentrated Hydrochloric acid. The acidic metal sulphate solution was warmed on hot water bath. The ligand solution (0.002 mol) was prepared in dry ethanol. The ligand solution was added slowly drop after drop in metal sulphate solution with continuous stirring. Slight excess of ligand solution was added to ensure the complete complexation. The resulting reaction mixture was made alkaline by treating it with alcoholic ammonia. The content was then digested on boiling water bath where colored complex precipitated out. The colored product was filtered on suction, washed first with small portion of hot distilled water and then by dry ethyl alcohol to remove excess of ligand. It was then dried under ambient conditions (Figure. 1 ).
Figure 1: Suggested structure Mn+2, Fe+2, Co+2 and Ni+2 complexes with 2,4-dichloro-6-{(hydroxyimino) [1-(4-methylphenyl)-1H-pyrazol-4-yl] methyl} phenol.
Result and Discussion
The Schiff base metal (II) complexes (I-IV) were synthesized by the stoichiometric reaction of metal sulphate and ligands in a molar ratio 1:2 (M:L). The synthesized complexes were characterized by various physical, analytical and spectroscopic techniques. The elemental analysis data was found to be in agreement with proposed formulae and supports for ML2 composition of the complexes. The complexes formed are of various colours which are different from colour of ligand indicating the formation complexes. The melting points of complexes are more than 200°C and which are different and higher than that of free ligands an evidence for complexation. The synthesized complexes are non-hygroscopic, non-deliquescent and stable at room temperature. The solubility of complexes was examined in different polar and non-polar solvents. All the complexes are soluble in DMF, DMSO but insoluble in water, acetone, chloroform, ethyl alcohol and carbon tetrachloride. The analytical data and solubility behavior of complexes suggest that all the synthesized complexes are monomers. The solution conductivities of synthesized complexes were measured at Department of Chemistry, Ahmednagar College, Ahmednagar. A simple digital conductivity meter (Elico Model cm-180) with dip type cell having platinized platinum electrodes with cell constant 0.88 was used for this purpose. The instrument and conductivity cell were calibrated using 0.01 M KCl solution at room temperature. The metal complexes were dissolved in DMF and the molar conductivities of their 0.001 M solutions were measured at room temperature. The low molar conductivity values indicate that the complexes are non-electrolyte and covalent in nature [25]. The molar conductance values are presented in Table 1.
| M. Formula | Color | M. P. (°C) | % Yield | Found (Calcd.) % | ΛM | |||
|---|---|---|---|---|---|---|---|---|
| M | C | H | N | |||||
| Ligand (LH) | White | 200-202 | 76 | - | 56.76 (56.37) | 4.00 (3.62) |
12.02 (11.60) |
29.5 |
| [MnL2] (I) | Shiny Brown | 208-210 | 86 | 7.29 (7.07) | 52.33 (52.53) | 3.35 (3.11) |
10.75 (10.81) |
26.1 |
| [FeL2] (II) | Brown | 286-288 | 87 | 7.44 (7.18) |
52.87 (52.47) |
3.38 (3.11) |
11.05 (10.80) |
41.2 |
| [CoL2] (III) | Gray | 214-216 | 85 | 7.64 (7.54) |
52.44 (52.26) |
3.47 (3.10) |
10.91 (10.76) |
36.9 |
| [NiL2] (IV) | Green | 260-262 | 88 | 7.40 (7.51) |
51.98 (52.28) |
3.29 (3.10) |
10.54 (10.76) |
38.3 |
Table 1. Physical, analytical and molar conductance of ligand and its metal complexes.
IR spectra
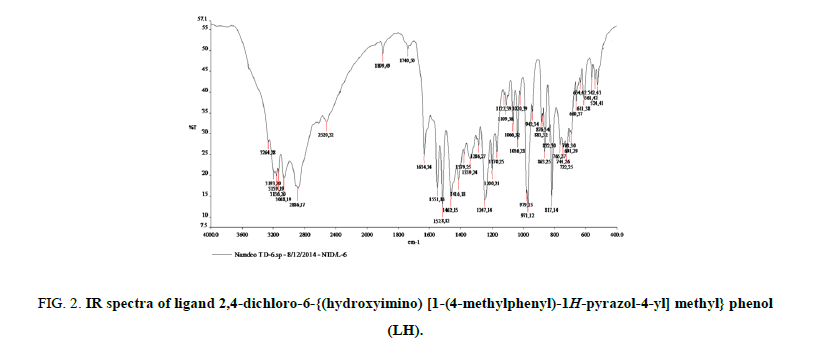
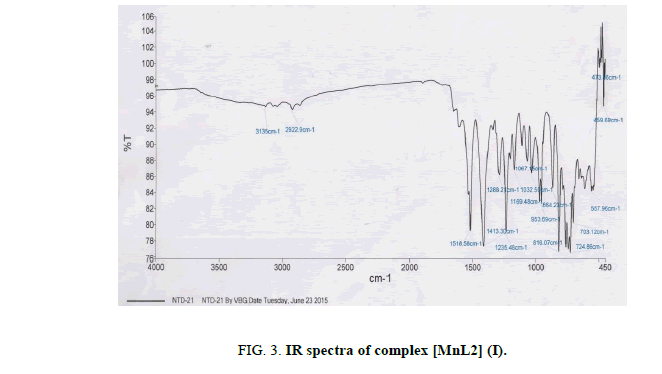
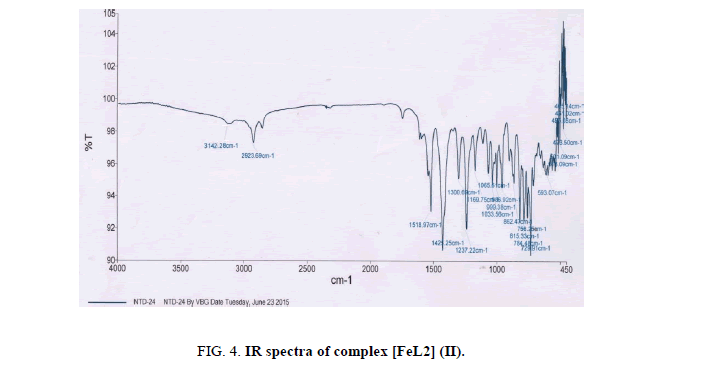
The most important bands in IR spectra of free ligand and their metal complexes are presented in Table 2 and discussed with respect to assignment of band frequencies for different groups involved in complex formation. To study the binding mode of ligand and metal, IR spectra of free ligand and their metal complexes were compared. The free ligand exhibit broad band at 3159 cm-1 assignable to hydrogen bonded ν(O-H) stretching frequency and absorption at 3264 cm-1 assignable to free ν(O-H) stretching frequency. The frequency at 3264 cm-1 due to free hydroxyl group, which was disappeared upon complexion, indicates deprotonation followed by the formation of metal oxygen bond of phenolic group. The hydroxyl stretching frequency in the range 3159 cm-1 was also shifted up to 24 cm-1 indicative of the coordination of nitrogen atom and presence of strong inter molecular hydrogen bonding between ligands to stabilize the complex. The stretching frequency due to azomethine linkage was appeared at 1515 cm-1 due to ν(C=N) and at 1247 cm-1 due to ν(N-O) in the spectra of free ligands. The small shift towards lower value in stretching frequency of azomethine group is also strong evidence for the coordination of nitrogen atom with metal ion. The absorption peaks due to ν(C-O) observed at 971 cm-1 in the spectra of free ligand which showed hypsochromic shift in the spectra of the complexes. This supports bonding of the metal ions to the phenolic -OH after deprotonation [26,27]. The shift equally confirms the participation of oxygen in the C-O-M bond. The further conclusive evidence of the coordination of phenolic -OH group and azomethine nitrogen was proved by appearance of weak bands in the range 560 cm-1 to 536 cm-1 and 465 cm-1 to 459 cm-1 assigned to ν(M-O) and ν(M-N) respectively [28,29]. These bands are only observed in the spectra of complexes not in free ligand. The spectra of free ligand and corresponding complexes are shown in Figures. 2-6.
Figure 2: IR spectra of ligand 2,4-dichloro-6-{(hydroxyimino) [1-(4-methylphenyl)-1H-pyrazol-4-yl] methyl} phenol (LH).
| Compound | νO-H | νC=N | νN-O | νC-O | νM-O | νM-N |
|---|---|---|---|---|---|---|
| Ligand (LH) | 3264, 3159 | 1551 | 1247 | 971 | - | - |
| [MnL2] (I) | 3135 | 1518 | 1235 | 953 | 557 | 459 |
| [FeL2] (II) | 3142 | 1518 | 1237 | 956 | 536 | 465 |
| [CoL2] (III) | 3142 | 1515 | 1233 | 967 | 546 | 461 |
| [NiL2] (IV) | 3150 | 1519 | 1245 | 970 | 560 | 459 |
Table 2. The significant peaks in FTIR spectra of free ligand and its complexes.
Thermal analysis
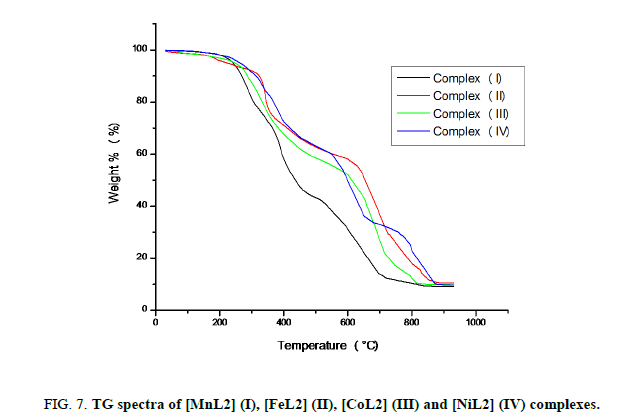
The Thermogravimetric analyses of metal complexes have been carried out on Shimadzu TGA-50 thermometric analyzer at Department of Chemistry, New Arts, Commerce and Science College, Ahmednagar. The instrument was calibrated using CuSO4·5H2O. The metal complexes were heated from 30°C to 900°C at constant heating rate 10°C per minute in air atmosphere. Alternatively, weight loss of substance was recorded as a function of time with increase in temperature at uniform rate. The weight loss in the substance was used to understand the stoichiometry, thermal stability, evolution of gases, types of water molecules and decomposition products. TGA spectra of free ligand and its transition metal complexes are shown in Figure. 7. According to Thermogravimetric data complexes (I-IV) exhibit high stability and melt with decomposition in the range 200°C to 280°C. In TGA thermograms of complexes (I-IV) does not show any significant weight loss up to 200°C indicating there is no lattice or coordinated water molecules. Further increase in temperature, the complexes decompose slowly leading to formation of air stable metal oxide as the end product at 800°C to 900°C.
Electronic spectra
The electronic spectrum gives most convincing evidence concerning the geometry of the complexes. In transition metal complexes, the absorptions in visible region is relatively weak and are associated with transitions largely localized on metal ion, transfer of electron from one atom to other atom so called charge transfer bands and d→d transitions. The absorption bands in ultra violet region are intense and associated with π→π* and n→π* transitions due to organic molecules. In the present study, the electronic absorption spectra of metal complexes showed two strong band in the region 266 nm and 335 nm due to π→π* and n→π* transitions. These bands are attributed to organic molecules and appeared to lower value than free ligands. Along with that in the absorption spectra of metal complexes, weak bands observed in the region 448 nm to 579 nm. These are associated with strong charge transfer bands and characteristics of square planar geometry for the complexes [30].
1H NMR spectra
1H NMR spectra of selected compounds was recorded in DMSO-d6 as solvent and TMS as internal standard in the range 0 δ ppm to 16 δ ppm. However due to presence of metal ion, proton resonance was not effected and gave broad peaks indicating the formation of metal complexes.
XRD spectra
The powder X-ray diffraction of some selected synthesized metal complexes were obtained in solid form at SAIF, Punjab University Chandigarh. The powder XRD spectra were scanned on Goniometer powder diffraction PW 3050/60 with Cu-K-alpha-1 radiation (λ=1.5406 Å). The powder XRD patterns were measured in 2 theta range between 5.0084 and 89.9744 with step size 0.0170. The XRD spectra of metal complexes were used for indexing the pattern and to find out the unit dimensions and space groups. The X-ray diffractogram of all complexes showed broad peaks, which indicate polycrystalline nature [31]. Though polycrystalline nature of complexes was observed they were generally not soluble in non-polar solvents.
Conclusion
Ligand 2,4-dichloro-6-{(hydroxyimino) [1-(4-methylphenyl)-1H-pyrazol-4-yl] methyl} phenol (LH) and its four new complexes (I-IV) were synthesized. The newly synthesized Mn(II), Fe(II), Co(II) and Ni(II) complexes were characterized by spectral and elemental analysis. The formations of complexes were also confirmed by thermal methods of analysis. The physical and spectroscopic characterization of the complexes revealed that phenolic oxygen and imine nitrogen atom of oxime group are coordinate with the metal ion. The complexes are having good solubility in DMF and DMSO but insoluble in water and other organic solvents. The thermal analysis data suggested that complexes have high stability below 200°C and decomposes slowly after 200°C giving formation of sTable residue of corresponding metal oxides. The electronic absorption spectra indicate square planer geometry for the complexes. The low molar conductance value suggested the non-electrolytic nature of the complexes. The powder X-ray diffractogram indicates polycrystalline nature of the complexes.
Acknowledgment
Authors are thankful to SAIF, Punjab University, Chandigarh for providing spectral analysis facilities.
References
- Rafique S, Idrees M, Nasim A, et al. Transition metal complexes as potential therapeutic agents. Biotechnol Mol Biol Rev. 2010;5:38-45.
- Chohan ZH, Kausar S. Synthesis, characterization and biological effect of anions [NO3-, SO42-, C2O42-, or CH3CO2-] on Co (II) and Ni (II) chelates of tridentate Schiff-base Ligand. J Chem Soc. 2001;23:163-7.
- Bhusare SR, Pawar VG, Shinde SB, et al. Synthesis of some new heterocyclic Schiff bases, 4-Thiazolidinones and 2-Azetidinones as an antibacterial and antifungal agent. Int J Chem Sci. 2003;1:31-6.
- Singh K, Barwa MS, Tyagi P. Synthesis, characterization and biological studies of Co(II), Ni(II), Cu(II) and Zn(II) complexes with bidentate Schiff bases derived by heterocyclic ketone. Eur J Med Chem. 2006;41:147-53.
- Mishra V, Saksena DK, Jain MC. Synthesis and biocidal activity of isothiocyanato-Chromium (III) complexes of some newly synthesized heterocyclic Schiff bases. Synth React Inorg Met Org Chem. 1987;17:987-1002.
- Baseer MA, Jadhav VD, Phule RM, et al. Synthesis and antibacterial activity of some new Schiff bases. Orient J Chem. 2000;16:553-6.
- Mulwad VV, Shriodkar JM. Synthesis and biological activity of some new Schiff bases, Thiazolidinone and Azetidinones of 4-hydroxy coumarin. Ind J Hetero Chem. 2002;11:199-202.
- Raman N, Muthuraj V, Ravichandran S, et al. Synthesis, characterization and electrochemical behaviour of Cu(II), Co(II), Ni(II) and Zn(II) complexes derived from acetylacetone and p-anisidine and their antimicrobial activity. Ind Acad Sci Chem Sci. 2003;115:161-7.
- Beokon YN, Bulychev AG, Mallev VI, et al. Asymmetric synthesis of cyanohydrins catalysed by potassium bis [N-salicylidene-(R) tryptophanato] cobaltate complex. Chem Abstr. 2005;143:249-50.
- Sharma RN, Kumar A, Kumari A, et al. Synthesis, characterization and antifungal studies of some as (III), Sb(III) and Bi(III) complexes with o-tolyl ammonium dithiocarbamate. Asia J Chem. 2003;15:57-61.
- Guo Z, Xing R, Liu S, et al. The synthesis and antioxidant activity of the Schiff bases of chitosan and carboxymethyl chitosan. Bio-org Med Chem lett. 2005;15:4600-3.
- Kar DM, Sahu SK, Pradhan D, et al. Synthesis, antimicrobial and anti-inflammatory activities of some novel asatinoid compounds. J Teach Res Chem. 2003;10:20-4.
- Jayashree BS, Jerald J, Venugopala KN. Synthesis and characterization of Schiff bases of 2-amino-4-(3-coumarinyl) thiazole as potential NSAIDs. Orient J Chem. 2004;20:123-6.
- Huneck S, Schreiber K, Grimmecke HD. Schiff bases and derived secondary amine as plant growth inhibitors. J plant growth regul. 1984;3:75-84.
- Xi LB, Xian LS, Fa YW, et al. Synthesis and biological activity of Schiff base of tetrazole. Chem Abstr. 2001;134:311-54.
- Wang Y, Yu X, Lu B, et al. Studies of synthesis and plant harmone on Schiff bases of tetrazole. Chem Abstr, 2002;137:109-38.
- Wang Y, Yu X, Lu B, et al. Synthesis and plant harmone activity of Schiff base ester of 5-aminotriazole-3-carboxylic acid. Chem Abstr. 2002;136:257-59.
- Mennicke W, Westphal. Mixture of 1:2 chromium complex dyes. Chem Abstr. 1986;104:111359.
- Dehnert J, Juchemann W. Azo group containing metal complex dyes. Chem Abstr. 1985;103:106288.
- Bergmann U, Hansen. Azo dyes for leather. Chem Abstr. 1985;103:7731.
- Chen X, Tang LJ, Sun YN, et al. Synthesis, characterization and antitumor activities of transition metal complexes with isoflavone. J Inorg Biochem. 2010;104:379-84.
- Gadakh AV, Pandit C, Rindhe S, et al. Synthesis and antimicrobial activity of novel fluorine containing 4-(substituted-2-hydroxybenzoyl)-1H-pyrazoles and pyrazolylbenzo[d]oxazoles. Bioinorg med chemi Lett. 2010;20:5572-6.
- Vogel’s. Text book of practical organic chemistry, 5th edn., John Wiley, New York. 1989.
- Dhokale NT, Karale BK, Nagawade AV. Synthesis and characterization of series of salicyloyl pyrazoles and their oximes. Res J Chem Sci. 2014;1:100.
- Geary WJ. The use of conductivity measurements in organic solvents for the characterisation of coordination compounds. Coord Chem Rev. 1971;7:81-122.
- Bukhari IH, Arif M, Akbar J, et al. Preparation, characterization and biological evaluation of Schiff base transition metal complexes with cephradine. Pakistan J Biol Sci. 2005;8:614-7.
- Basavaraju B, Bhojyanaik HS, Prabhakara MC. Transition metal complexes of methyl-quinolino [3,2-b] [1,5] benzodiazepine and methyl quinolino [3,2-b] [1,5] benzodiazepine: Synthesis, characterisation and antimicrobial studies. e-J Chem. 2007;4:39-45.
- Black SI, Young GB. Synthesis and spectroscopic characteristics of 2-methyl-2-phenylpropyl and dimethyl(phenyl)silyl methyl nickel(II) complexes. Polyhedron. 1989;8:558-96.
- Mohapatra BB, Saraf SK. Polymetallic complexes. Part-LXXXII. Bis-bidentate and bis-tridentate azodyedimeric complexes of Co(II), Ni(II), Cu9II), Zn(II), Cd(II) and Hg(II). J Ind Chem Soc. 2003;8:696.
- Mehta BH, Swar YA. X-Ray Crystallographic studies of Nickel (II) Palladium (II) complexes of o-hydroxy acetophenone oxime. Asia J of Chem. 2001;13:928-32.
- Hussain R, Juneja HD. X-ray diffraction studies of some chelate polymers of adipic acid. Int J Chem Sci. 2009;7:632-8.