Original Article
, Volume: 11( 6)Experimental Studies on the Interaction Between Aqueous Solutions of Polymer and Surfactant
- *Correspondence:
- Kamil M , Department of Petroleum Studies, Z.H. College of Engineering and Technology, A.M.U. Aligarh-202 002, U.P., India, Tel: 09359500486; E-mail: mkamilamu@gmail.com
Received: October 31, 2016; Accepted: November 19, 2016; Published: November 28, 2016
Citation: Husain T, Kamil M. Experimental Studies on the Interaction Between Aqueous Solutions of Polymer and Surfactant. Chem Technol Ind J. 2016;11(6):110.
Abstract
The purpose of this study was to investigate the interaction between water soluble polymer (Xanthan gum) with an anionic surfactant, sodium dodecyl sulphate (SDS), and a cationic surfactant, cetyltrimethylammonium bromide (CTAB), at two temperatures 310.15 K and 318.15 K by using conductivity measurement. The behavior of surfactant-polymer interaction was found to be dependent on both surfactant and polymer concentrations. After the critical aggregation concentration (Cac), interaction between the water-soluble polymer and surfactants was started and above the critical micelle concentration (Cmc), normal micelle formation started and the polymer was saturated by surfactant with no further change of conductivity of the solution for particular concentrations of the polymer solution. Both Cac and Cmc increase with polymer concentration. The free energies of aggregation, ΔGagg, micellization, ΔGmic, and transfer, ΔGt associated with the binding interaction of the surfactants and polymers were calculated. The negative values of the ΔGt confirm the feasibility of interaction between the surfactant and polymer.
Keywords
Polymer; Surfactant; Conductivity; Aggregation; Cac; Cmc
Introduction
Polymers and surfactants mixtures are common in many industrial formulations such as food, pharmaceutical, cosmetics, and oil industries. It is known that solutions containing polymers and surfactants can give rise to molecular interactions that may affect their rheological and physicochemical properties. Interaction between the polymer and surfactant has been extensively studied for its widespread applications in industries. Specific interests on the polymer and ionic/cationic surfactant systems are because of its characteristic physicochemical properties at different possible combinations. Surfactants (usually referred as surface active agents) have the ability to lower the interfacial tension between in aqueous solution and some other phase. They are amphipathic molecules that consist of a non-polar hydrophobic portion, usually a straight or branched hydrocarbon or fluorocarbon chain containing 8-18 carbon atoms, which is attached a polar or ionic portion (hydrophilic). The hydrophilic portion can be ionic, nonionic or zwitterionic, and accompanied by counter ions in the last two cases. The cooperative action of the dispersion and hydrogen bonding between the water molecules tends to squeeze the hydrocarbon chain out of water and these chains referred as hydrophobic. The basic principles of the polymer-surfactant interactions are discussed by Kwak, [1] Goddard and Anantha padmanabhan [2], and others [3-5]. Nedjhioui et al. [4] also found the same behavior of xanthan gum solutions in the presence of other surfactant. The presence of alkali in a solution significantly influences the surface and interfacial tensions. It is well known that solutions containing both polymers and surfactants can give rise to molecular interactions that may affect their rheological and physicochemical properties. Such interactions also display features that depend on polymer and surfactant electrical charges and hydrophobicity, polymer conformation and the presence of additives such as salts. It is generally accepted that the hydrophobic character of both polymer and surfactant is responsible of interactions. The nature of these interactions has been investigated for several decades and is extensively documented [6-14]. Significant variations of the physicochemical and rheological properties of these systems are observed. The polymer-surfactant interactions study can be done in two ways. In the first one, the polymer is considered as being the substance influenced by the surfactant, in the second way, the surfactant is considered as being the substance influenced by the polymer. In the first case, the surfactant is adsorbed on the polymer sites that disturb the formation of the surfactant micelles. Alternatively, in the second case the association of surfactant molecules with macromolecules facilitates the phenomenon of micellization. The examination of the evolution of the physicochemical and rheological properties of such systems, according to the chemical nature and component concentrations, makes it possible to establish relations between these factors and the system responses such as surface tension, viscosity and conductivity. Minatti and Zanette [15] showed the variation of CAC and polymer saturation point (PSP) of polyethylene oxide and sodium dodecyl sulfate (SDS) system at different NaCl concentrations. Fishman and Elrich [16] studied the interactions of aqueous poly (N-vinylpyrrolidone) with SDS. Correlation of electric conductance and viscosity measurements with equilibrium dialysis measurements was presented by them. Hemar et al. [17] studied the changes in droplet sizes, surface coverage and creaming stability of emulsions formed with 30% (w/w) soya oil, and aqueous solution containing 1% or 3% (w/w) sodium caseinate and varying concentrations of xanthan gum. It appears that creaming stability of emulsions made with mixtures of sodium caseinate and xanthan was more closely related to the structure and rheology of the emulsion itself rather than to the rheology of the aqueous phase. Nilsson et al. [18] found that addition of hydrophilic polymers in the range 0 ppm to 10,000 ppm has little effect on surfactant bulk properties. In some cases, added hydrophilic polymer can reduce the saturation value for surfactant adsorption due to competitive adsorption. Surfactant-polymer interactions, for non-associating systems, have little effect on surfactant/polymer core floods. On a larger scale, polymer will of course provide mobility control and a somewhat higher capillary number due to the increased viscosity. Han et al. [19] presented a brief review of the present status after 1990 of pilot and field test results of polymer flooding, Xanthan gum flooding and combined chemical flooding enhanced oil recovery (EOR) techniques, and their future in China. Khan et al. [20] studied the interaction between water-soluble polymers and anionic surfactants by surface tension and conductivity measurements. SDS and sodium dodecylbenzene sulfonate (SDBS) were used as surfactant while polyacrylamide (PAA), commercial grade partially hydrolyzed polyacrylamide (PHPA), and xanthan gum were used as water-soluble polymer. Thus mostly the studies are limited to the anionic surfactants only.
Therefore, we can say that numerous studies have been reported on different polymers and anionic surfactants, but limited work has been reported on interaction of xanthan gum with the SDS, and CTAB surfactants at different temperatures. Thus, present study [21] describes the interaction between the polymer (xanthan gum) and anionic surfactant SDS, cationic surfactant CTAB at various concentrations by measuring the conductivity of the solution and to see the effect of hydrophobicity and the molecular interactions at different temperatures.
Materials and Methods
The polymer xanthan gum, (avg. mol. wt=25000, Himedia, India was used in the present study. Two surfactants, cetyltrimethylammonium bromide, CTAB (≥99.0%, Merck, Germany) and SDS, SDS (mol. wt=288.38, Merck, Germany, Purity ≥90.0%) were used as received. These products were used because of their wide application in industry [11-14].
The molecular structures of SDS, CTAB surfactants and polymer are shown in Figure 1.
Preparation of solutions
The aqueous solutions with different concentrations of surfactant and polymer were always freshly prepared using a magnetic stirrer to form a consistent homogeneous solution at a low rotation per minute to avoid any mechanical degradation. Appropriate quantity of surfactant and polymer were dissolved carefully in distilled water for about 15 min. A wide range of concentrations approximating the Cac and Cmc of SDS, CTAB were chosen for the present study. The polymer (xanthan gum) was used at 100, 700, and 1200 ppm concentrations. These ranges of compounds were chosen to correspond with those used for industrial applications [4,20,21].
Conductivity measurements
In the present investigation, the conductivity of the solution was measured by a digital conductivity meter (Systronics Conductivity-TDS Meter 308, range 0.1 μS to 100 mS, accuracy ± 1% of F.S. ± 1 digit, India). The instrument was properly calibrated with KCl solutions of known concentration (0.1, 0.01, 0.001) prior to taking the readings. The conductivity measurement runs were carried out by adding progressively concentrated surfactant stock solution into the thermostatted solvent (demineralized double distilled water of specific conductivity 1 to 2 × 10-6 S·cm-1 or solvent containing different ppm of the solution of the Xanthan gum. The conductivity runs were carried out at two different temperatures 310.15 K, and 318.15 K. The temperature was maintained in a thermostatted water bath with thermal stability of ± 0.1 K. 25 ml of the polymer solution consisting of an appropriate amount of xanthan gum in water was taken in the conductivity cell at the desired temperature. Then a known amount of stock solution of surfactants made with the same polymer solution was added. In this way, the precise conductance of polymer+surfactant mixture over the complete mole fraction range in aqueous xanthan gum.
The plots of specific conductance (κ) versus (surfactant) were used to calculate CAC and CMC of surfactants as explained earlier.
Results and Discussion
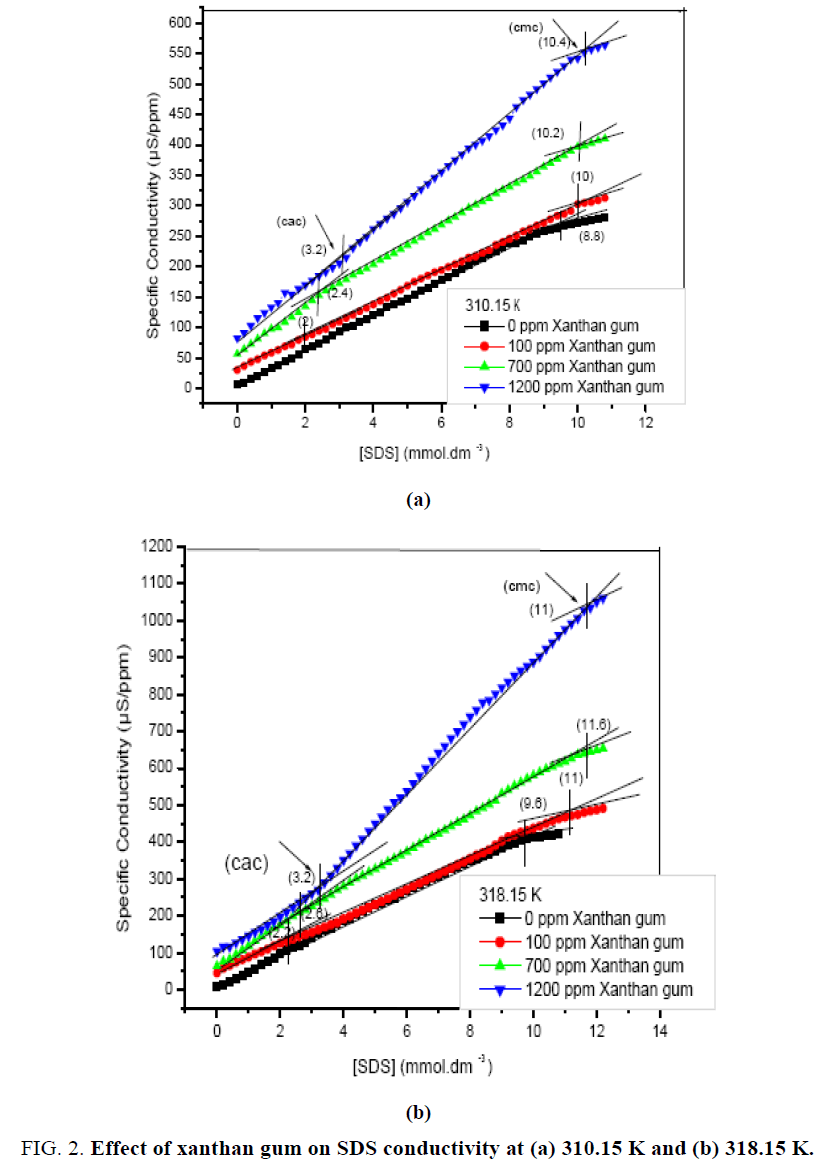
The interaction of polymer and surfactants has been observed from the conductivity measurements. Conductivity of the aqueous solutions with and without polymer is nothing but the dissociated cations and anions of the polymer and surfactant. Therefore, conductivity of the solution is related to the dissociation and thus an interaction between the polymer and surfactant. Figure 2 (a and b) represents specific conductivity as a function of SDS concentration, in the absence and presence of polymer at different temperatures. The figure shows that the conductivity increases with increase in concentration of surfactant. A break point (Cmc) is also clearly identified in the conductivity plot in the absence of polymer at 310.15 K and 318.15 K respectively. The conductivity also increases with rise in temperature. The concentration at the turning point of the curve is Cmc. Above this concentration, the slop of the conductivity vs. surfactant concentration decreases, since the free SDS ions contribute more to the solution conductivity as compared with the same ions in micelles, for both temperatures 310.15 K and 318.15 K.
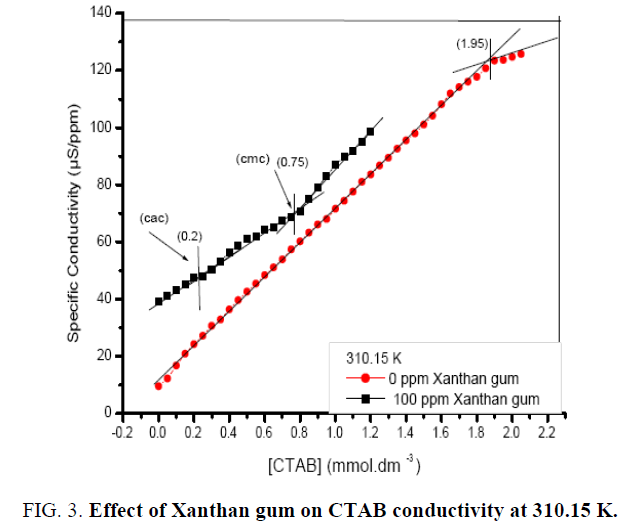
In the presence of polymer, the plots are shifted upward showing higher values of the conductivity at different concentrations of the polymer. The higher conductivity of the surfactant solutions in the presence of polymer is probably due to the higher mobility of the sodium ion of the surfactant under complexation with polymer compared with its relatively strong ion paring with SDS micelles in the absence of the polymer as shown in Figure 3 and 4. In addition, the polymer-surfactant complex may consist of the surfactant micelles bound to the polymer, enabling higher mobility of the sodium ions from one micelle to another. Evidence of the formation of the polymer-surfactant complexes may be observed from the characteristic behavior of the conductivity plots within the concentration range of Cac and Cmc [20].
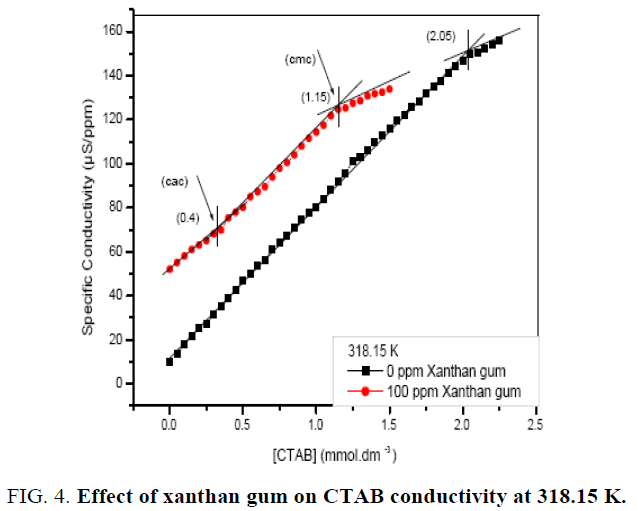
The Cmc values in the presence of polymer molecule are higher than the values in the absence of the polymer molecule, which indicates aggregation with the surfactant prior to micellization. For the different concentration of polymer, Cac and Cmc increases as the concentration of polymer increases. Further the interaction of polymer with a cationic surfactant (CTAB) may also be observed from the conductivity measurements. Figure 3. represent specific conductivity as a function of the (CTAB), in the absence and presence of polymer. The figure shows that conductivity increases with increase in temperature and also with increase in concentration of CTAB in the solution. The break point (Cmc) is also clearly identified in the conductivity plot in the absence of polymer as shown in above figures at 310.15 K. In the presence of polymer, the plots are shifted upward showing higher values of the conductivity with the different concentrations (100 ppm, 700 ppm, and 1200 ppm) of the polymer. In most cases, the charge on the polymer is fixed either positive or negative, so that possible interactions with surfactants of a given charge type can reasonably well defined. When opposite charges are present, however, the expected high degree of electrostatic interaction is usually found to occur. In aqueous solution, the result of surfactant binding by electrostatic attraction is normally a reduction in the viscosity of the system, a loss of polymer solubility, at least to the point of charge of reversal, and a reduction in effective concentration of the surfactant. Similar trend is observed at 318.15 K as shown in Figure 4. At high temperature both Cac and Cmc are slighter higher.
When polymer interacts with surfactant then polymer-surfactant complex formation starts and then a state is reached at which this aggregate with the charged polymeric chain of the polymeric compound xanthan gum. After progressively adding this surfactant in the aqueous solution a turning point in the curve called Cmc is reached and beyond a concentration of 100 ppm precipitation of the xanthan gum take place.
The values of Cac, Cmc for different surfactant-polymer combinations by the conductivity measurement are given in Table 1.
| Xanthan gum (ppm) | Temperature (K) | CTAB | SDS | ||
|---|---|---|---|---|---|
| Cac (mmol/dm3) | Cmc (mmol/dm3) | Cac (mmol/dm3) | Cmc (mmol/dm3) | ||
| 0.00 | 310.15 | - | 0.950 (0.990)a | - | 8.80 (10.0)a |
| 318.15 | - | 2.05 | - | 9.60 | |
| 100 | 310.15 | 0.2 | 0.75 | 2.0 | 10.0 |
| 318.15 | 0.4 | 1.15 | 2.2 | 11.0 | |
| 700 | 310.15 | precipitation | Precipitation | 2.6 | 11.6 |
| 318.15 | precipitation | precipitation | 3.2 | 11.6 | |
| 1200 | 310.15 | precipitation | precipitation | 4 | 10.2 |
| 318.15 | precipitation | precipitation | 3.5 | 10.5 | |
| aLiterature value (Kabir-ud-Din,* M. A. Rub, A. Z. Naqvi, J. Phys. Chem. B 114 (2010) 6354) | |||||
Table 1: Values of Cac, Cmc for CTAB and SDS in solutions containing different weight percentages of xanthan gum (determined from conductivity measurements).
The degree of micelle ionization (g) was calculated by taking the ratio between the slopes of the linear portions above and below the break point in conductivity profiles, for the determination of the Cac, Cmc and, the free energy of aggregation, ΔGagg, and the free energy of micellization, ΔGmic, the free energy of transfer, ΔGt, associated with the binding interaction between surfactant and polymer, can be calculated using the following equations:
Δ Gagg = RT (2−g1) lnXCac (1)
Δ Gmic =RT (2−g2) lnXc (2)
Δ Gt = Δ Gagg−Δ Gmic (3)
Where R is the gas constant =8.3144 J/mol K, and T is the temperature in Kelvin (K).
The degree of the micelle ionization (g) was calculated by taking the ratio between the slopes of the linear portions above and below the break point in the conductivity profiles. Hence, two values, i.e. g1 and g2, were obtained from the conductivity graph for the different temperature 310.15 K and 318.15 K. The larger value of g for the complex micelle is an indication of increased degree of ionic dissociation as a result of the interaction of the surfactants with the polymer. The highest value shows the closet fitting of the surfactant heads in SDS micelles. The closet fitting of surfactant heads increasing with the lower concentration to higher concentration of the polymer [21].
The negative values of ΔGt confirm the feasibility of interaction between the surfactants and polymer [9] (Tables 2 and 3).
| Xanthan gum Ppm | Δ Gagg | Δ Gmic | Δ Gt | Temperature K |
|---|---|---|---|---|
| 0 | -- | 8412.08 | -8412.08 | 310.15 |
| 9991.40 | -9991.40 | 318.15 | ||
| 100 | 2591 | 7540 | -6949 | 310.15 |
| 2210.78 | 9704.75 | -7493.97 | 318.15 | |
| 700 | 2889 | 8623.82 | -5734.82 | 310.15 |
| 3209.98 | 9530.0 | -6320.0 | 318.15 | |
| 1200 | 5638 | 10024.47 | -4386 | 310.15 |
| 2153.75 | 7715.32 | -5561 | 318.15 |
Table 2: Values of the Δ Gagg, Δ Gmic and Δ Gt for SDS.
| Xanthan gum ppm | Δ Gagg | Δ Gmic | Δ Gt | Temperatute K |
|---|---|---|---|---|
| 0 | - | 2910.41 | -2910.41 | 310.15 |
| 2639.39 | -2639.39 | 318.15 | ||
| 100 | -4274.78 | -296.73 | -3978.05 | 310.15 |
| -2181.41 | 595.22 | -2776.63 | 318.15 |
Table 3: Values of the ΔGagg, ΔGmic and ΔGt for CTAB.
Conclusions
On the basis of the results of the experiments which were conducted to examine the interaction between anionic and cationic surfactants and polymer by measuring the conductivity of the solutions, the following conclusions can be drawn.
1. The results show that there is interaction with both SDS and CTAB surfactants and xanthan gum, polymer.
2. Anionic surfactant interacts strongly with xanthan gum as compared to cationic surfactant. Conductivity of the surfactant solutions increases in the presence of polymer. However, the conductivity of xanthan gum and SDS system was found to increase more than CATB with increasing concentration of the polymer for the fixed concentration of surfactant.
3. The negative values of the ΔGt confirms the feasibility of interaction between polymers and surfactants.
References
- Kwak JCT. Polymer-surfactant systems. Dekker: New York;1998.
- Goddard ED, Ananthapadmanabhan KP. Interactions of surfactants with polymers and proteins, CRC Press Inc. BocaRaton, FL;1993.
- Avranas A, Iliou P. Interaction between hydroxypropyl methylcellulose and the anionic surfactants hexane-, octane-, and decanesulfonic acid sodium salts, as studied by dynamic surface tension measurements. J Colloid Interface Sci. 2003;258(1):102-9.
- Nedjhioui M, Moulai-Mostefa N, Morsli A, et al. Combined effects of polymer/surfactant/oil/alkali on physical chemical properties. Desalination. (2005);185:543-50.
- D’Errico G, Ciccarelli D, Ortona O, et al. Interaction between pentaethylene glycol n-octyl ether and poly (acrylic acid): Effect of the polymer molecular weight. J Colloid Interface Sci. 2007;314(1):242-50.
- Holmberg K, Jonsson B, Kronberg B, et al. Surfactants and polymers in aqueous solution, 2nd ed. John Wiley & Sons; Ltd England: 2002.
- Tadros TF. Applied surfactants, principals and applications. Wiley-VCH Verlag GmbH & Co. KGA; Weinheim: 2005.
- Capalbi A, Mesa CL. Polymer-surfactant Interactions. J Therm Anal Calorim. 2001;66(1):233-41.
- Sardar N, Kabir-ud-Din KM. Interaction between nonionic polymer hydroxypropyl methyl cellulose (HPMC) and cationic gemini/conventional surfactants. Ind and Eng Chem Res. 2012;51(3):1227-35.
- Ochoa FG, Santos VE, Casas JA, et al. Xanthan gum: Production, recovery, and properties. Biotechnology Adv. 2000;18(7):549-79.
- Lakatos-Szabo J, Lakatos I. Effect of sodium hydroxide on interfacial rheological properties of oil-water systems. Colloids Surf A. 1999;149:507-13.
- Li GZ, Mu JH, Li Y, et al. An experimental study on alkaline/surfactant/polymer flooding systems using nature mixed carboxylate. Colloids Surf A. 2000;173:219-29.
- Taylor KC, Nasr-El-Din HA. The effect of synthetic surfactants on the interfacial behaviour of crude oil/alkali/polymer systems. Colloids Surf A. 1996;108:49-72.
- Horvath-Szabo G, Czarnzecki J, Masliyah JH. Sandwich structures at oil-water interfaces under alkaline conditions. J Colloid Interface Sci. 2002;253:427-34.
- Minatti E, Zanette D. Salt effects on the interaction of poly (ethylene oxide) and sodium dodecyl sulfate measured by conductivity. Colloids Surf A. 1996;113:237-46.
- Fishman ML, Eirich FR. Interactions of aqueous poly(N-vinylpyrrolidone) with sodium dodecyl sulfate. II. Correlation of electric conductance and viscosity measurements with equilibrium dialysis measurements. J Phys Chem. 1975;79:2740-4.
- Hemar Y, Tamehana M, Munro PA, et al. Influence of xanthan gum on the formation and stability of sodium caseinate oil-in-water emulsions. J Food Hydrocolloids. 2001;15:513-9.
- Nilsson S, Lohne A, Veggeland K. Effect of polymer on surfactant floodings of oil reservoirs. Colloids Surf A. 1997;127:241-7.
- Han DK, Yang CZ, Zhang ZQ, et al. Strategies for development of clean energy in China. J Pet Sci Eng. 1999;22:183-8.
- Khan MY, Samanta A, Ojha K, et al. Interaction between aqueous solutions of polymer and surfactant and its effect on physicochemical properties. Asia-Pac J Chem Eng. 2008;3:579-89.
- Hussain T. Experimental studies on solution behavior of xanthan gum in presence of surfactants, Department of Petroleum Studies, AMU, Aligarh, 2014.