Original Article
, Volume: 15( 2)Evidence for Homogeneous Adsorption of Samanea saman Extract Inhibitor on Steel Surface
- *Correspondence:
- Kesavan Devarayan, Department of Chemistry, Dhirajlal Gandhi College of Technology, Salem, Tamil Nadu, India, Tel: +91-9940389665; E-mail: dev.kesavan@gmail.com
Received: April 2, 2017; Accepted: April 12, 2017; Published: April 20, 2017
Citation: Rose K, Sukumaran M, Devarayan K, et al. Evidence for Homogeneous Adsorption of Samanea saman Extract Inhibitor on Steel Surface. Int J Chem Sci. 2017;15(2):120.
Abstract
In this study, homogeneous adsorption of Samanea saman inhibitor on the surface of steel is described. For the first time, the interaction between the aforementioned inhibitor molecules and the steel surface is evidenced using energy dispersive X-ray spectroscopy. The weight loss measurements and electrochemical studies revealed that 500 ppm of ethanolic extract of Samanea saman inhibits the corrosion of steel in 1.0 M HCl with approximately 90% inhibition efficiency. Further the adosorption of green inhibitor is found to fit Langmuir’s adsorption isotherm model.
Keywords
Corrosion; Inhibition; Samanea saman; EDS Mapping; Green inhibitor
Introduction
Steel corrosion is one of the fundamental issues which have received significant attention of both academia and industries. In general deterioration of the steel structures is a spontaneous and unavoidable process. However, it can be controlled by improving the corrosion resistance property of the steel by alloying it with suitable metals or by employing suitable inhibitors. Inhibition of corrosion of steel in acidic media using bio-based organic molecules as inhibitors was reported to be effective. Further these bio-based inhibitors are mainly derived from economical natural resources such as plants [1]. In recent years, the authors explored several plant-based organic inhibitors for inhibition of corrosion of steel in different corrosive environments [2-6].
In continuation of the efforts to find the most effective corrosion inhibitor, extract of Samanea saman (S. saman) is investigated in this study. S. saman is commonly called as rain tree and its well known for closure of leaves after sun set. S. saman is a habitat of tropical and subtropical countries such as India, Brazil, and Peru [7]. Traditionally it has been used for curing intestinal ailments, sore throat, stomach ache and other stomach related problems. S. saman is known to possess antimicrobial activity against certain human and plant pathogenic fungi and bacteria [7-10]. It is also reported that S. saman has antioxidant and antiplasmodial properties [11-13]. The phytochemical analysis of this tree revealed the presence of alkaloids, flavonoids, terpenoids, tannins, and saponins [14-16]. In this study, the ethanolic extract of the leaves of S. saman is evaluated as potential candidate for inhibition of corrosion of mild steel in 1 M hydrochloric acid (HCl) medium. The inhibition characteristics of the SS extract was evaluated by means of weight loss measurement, electrochemical studies, adsorption isotherm. The energy dispersive X-ray spectroscopy (EDS) technique was employed to understand the adsorption and distribution of the inhibitor molecules on the surface of the mild steel.
Experimental
Extraction of inhibitor
The inhibitor from the S. saman was prepared according to our previous studies [2,4]. In brief, the fresh leaves of S. saman (1 kg) were dried under shade for 7 days. Then the dried leaves were finely powdered and soaked in 95% ethanol for 3 days. Then the solvent was evaporated under vacuo to obtain green oily precipitate (30.5 g).
Weight loss studies
The steel specimens used in this study composed of C, 4.93%; Mn, 1.09%; Si, 1.78, and the remainder was Fe. At first, the steel specimens were abraded using emery sheets of different grades. Then the specimens were washed with distilled water and acetone followed by drying at room temperature. In this study, the blank solution 1.0 M HCl, was prepared by diluting concentrated HCl (Merck, AR) using double distilled water. The clean steel specimens were dipped in 1.0 M HCl for 2 hours at 298 K. The initial weight of the specimen was noted as Wo and the final weight was indicated by W. All the experiments were performed in triplicates. Similar to the blank measurements, the steel specimens were tested with blank solution containing 100, 200, 300, 400, and 500 ppm of SS extract. The inhibition efficiency (IE%), surface coverage (q), and corrosion rates (CR) were calculated using the following equations (Equations 1-3).
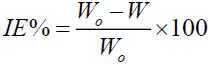 (1)
(1)
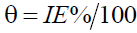 (2)
(2)
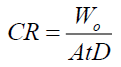 (3)
(3)
Where, CR-corrosion rate (mm/y), W0-weight difference (g) before and after weight loss measurements; A-exposed area of the specimen, t-immersion time (h), and D-density of the specimen (g/cm3).
Further the results obtained from weight loss studies were used to plot different adsorption isotherms such as Langmuir adsorption isotherm [17]. In addition, thermochemical parameters such as Kads and ΔGads (kJ/g).
Electrochemical experiments were performed using a conventional three electrode cell assembly consisting of working electrode, platinum electrode as counter, and a saturated calomel electrode as the reference. The steel specimen with an exposed area of 1 × 1 cm2 was used as a working electrode. The electrochemical impedance spectroscopy (EIS) and Tafel polarization measurements were performed using CH6043b according to our earlier studies [2,4].
Surface analyses and elemental mapping
The surface of the steel specimens was observed under a JSM-5900 scanning electron microscopy (SEM) and elemental mapping of the specimen surface was carried out using X-ray spectral analysis (EDS) (Table 1).
| C (ppm) | Surface Coverage | IE% | CR (mm/y) | Kads (kJ/g) | DGads (kJ/g) |
|---|---|---|---|---|---|
| 0 | - | - | 2.718 | ||
| 100 | 0.709 | 70.899 | 0.791 | 24.4 | -18.0 |
| 200 | 0.757 | 75.661 | 0.662 | 15.5 | -16.9 |
| 300 | 0.794 | 79.365 | 0.561 | 12.8 | -16.4 |
| 400 | 0.836 | 83.598 | 0.446 | 12.7 | -16.4 |
| 500 | 0.894 | 89.418 | 0.288 | 16.9 | -17.1 |
Table 1. Corrosion parameters for inhibition of steel corrosion in 1.0 M HCl by SS extract at 298 K.
Results and Discussion
Weight loss studies
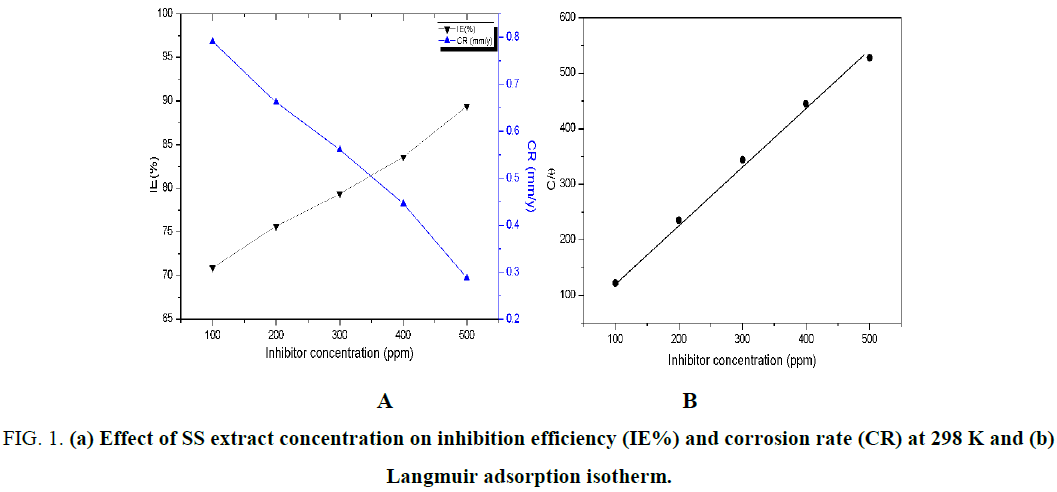
The weight loss measurements of steel specimens both in presence and absence of SS inhibitor in 1.0 M HCl were shown in Figure 1 and Table 2. The concentration of S. saman inhibitor was 100, 200, 300, 400, and 500 ppm. The rate of corrosion in blank solution was 2.718 mm/y. The addition of 100 ppm of S. saman inhibitor has dramatically decreased the corrosion rate ~3.4 folds. Further experiments with increase in S. saman inhibitor concentration gradually decreased the corrosion rate with an inhibition efficiency of 89.4% at 500 ppm concentration. It should be noted that use of S. saman inhibitor concentration beyond 500 ppm had almost no significant influence on inhibition of corrosion. This phenomenon can be attributed to the saturation of the interaction between inhibitor molecules and the steel surface.
Figure 1: (a) Effect of SS extract concentration on inhibition efficiency (IE%) and corrosion rate (CR) at 298 K and (b) Langmuir adsorption isotherm.
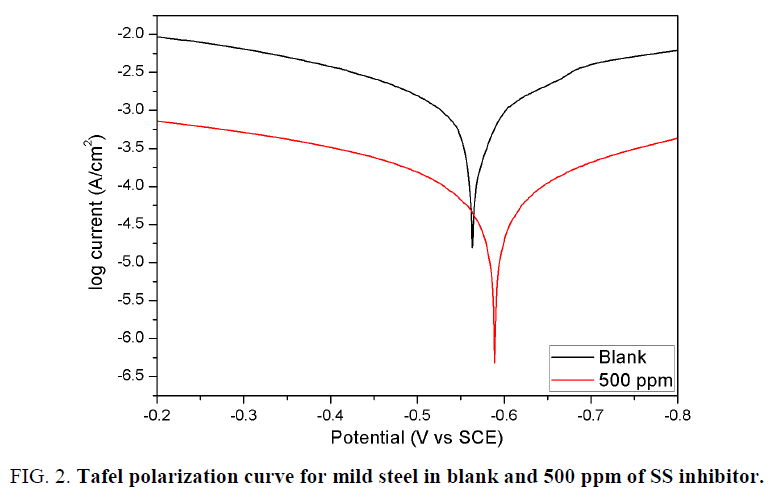
| Medium | Concentration (ppm) | bc (mV/decade) | ba (mV/decade) | -Ecorr (mV vs SCE) | Icorr (µA/cm2) | ? | IE% |
|---|---|---|---|---|---|---|---|
| Blank | - | 5.687 | 4.292 | 0.562 | 673.3 | - | - |
| S. saman | 500 | 5.212 | 5.598 | 0.589 | 51.9 | 0.923 | 92.3 |
Table 2. Tafel polarization parameters of steel specimen in presence and absence of SS inhibitor in 1.0 M HCl at 298
It has been widely accepted that the complex corrosion inhibition process proceeds via either physical or chemical adsorption or sometimes both. The mode of adsorption determines the nature of the surface protection. Thus, the knowledge of adsorption of inhibitor molecules on steel surface is important. In this view, the weight loss data were subjected to different adsorption isotherms such as Langmuir, Frumkin, Temkin, and Freundlich adsorption isotherms. However, linear relationship was observed only for Langmuir adsorption isotherm with a correlation co-efficient of 0.9971 (Figure 2). Generally, Langmuir adsorption isotherm involves in monolayer adsorption, without any interaction between the adsorption molecules which are governed by physical forces. It is obvious that the SS tree leaves comprises of alkaloids and flavonoids which has aromatic rings and electronegative atoms such as nitrogen, sulphur, and oxygen in their chemical structure. Thus, the Langmuir adsorption isotherm suggested for protection of steel surface by inhibitor molecules predominantly via physical adsorption.
Further the weight loss data were subjected to calculation of thermochemical parameters such as equilibrium constant (Kads) and the free energy of adsorption (ΔGads) using the following equations (Equations 4 and 5). The results were shown in Table 1.
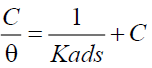 (4)
(4)
 (5)
(5)
where R is the gas constant, T is the temperature, and the value of 55.5 is the concentration of water in the inhibitor solution. In any adsorption process, if the ΔGads is lesser than -20.0 kJ/g then the inhibition mechanism proceeds via physical adsorption of the inhibitor molecules on the surface of steel. If the ΔGads is greater than -20.0 kJ/g then the inhibition is ascertained to the action of chemical adsorption. In the present study, the ΔGads values were ranging from -16.4 kJ/g to -18.0 kJ/g which indicated that the inhibition proceeds predominantly via physical adsorption mechanism. The Kads values determined for all the tested concentrations were from 12.7 kJ/g to 24.4 kJ/g (Table 1), which indicated that the corrosion inhibition by S. saman extract proceeds via electrostatic interaction with steel surface.
Electrochemical studies
The Tafel polarization results for measurements both in presence and absence of the inhibitor were shown in Table 2 and Figure 2a. It should be noted that the Icorr values for solution with 500 ppm of inhibitor was about 13 times lower than the blank solution. The cathodic and anodic slopes were not significantly changed which indicated that the mechanism of steel dissolution is unchanged [18]. Further the Ecorr difference between the Tafel curves of measurements in presence and absence of the inhibitor was lesser than ± 85 mV. The above results indicated that S. saman extract inhibits both cathodic and anodic reactions. Hence SS inhibitor can be classified as a mixed type of inhibitor [19].
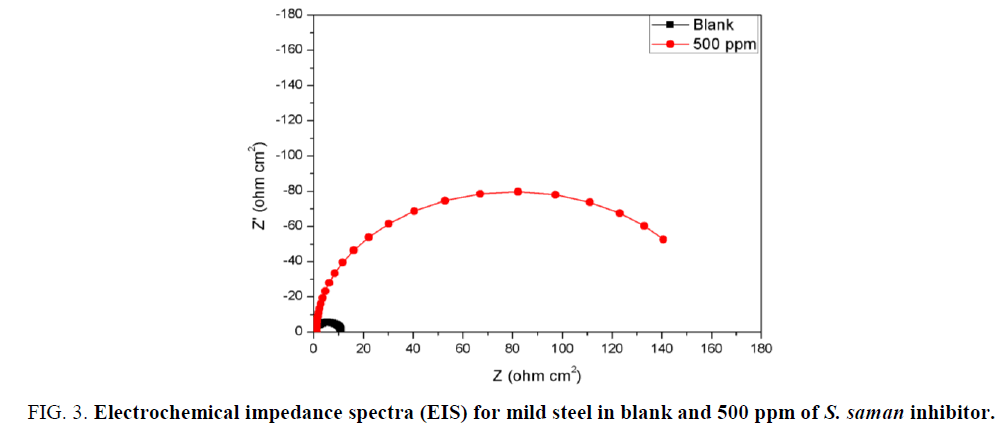
The EIS curves (Nyquist plot) for the measurements both in presence and absence of S. saman inhibitor are shown in Table 3 and Figure 2b. The depressed-semicircle natured of curves was observed for the measurements both in presence and absence of the inhibitor. The R’ct for blank measurement was 11.1 ohm/cm2. And Rct=175.4 ohm/cm2 for measurement using 500 ppm of SS inhibitor. In addition, the double-layer capacitance (Cdl) was also calculated (EQUATION 8).
| Medium | Concentration (ppm) | Rct (? cm2) | fmax | Cdl (µF cm-2) 10-3 | ? | IE% |
|---|---|---|---|---|---|---|
| Blank | 0 | 11.1 | 5.38 | 267.4 | - | - |
| S. saman | 500 | 175.4 | 79.3 | 11.5 | 0.937 | 93.7 |
Table 3. EIS parameters of steel specimen in presence and absence of S. saman inhibitor in 1.0 M HCl at 298 K.
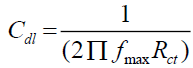 (6)
(6)
Where fmax is the frequency at which the imaginary component of the impedance (-Z”) is maximal. The Cdl values for the measurement with inhibitor solutions was 20 folds lesser than the blank measurement (Table 3). This is attributed to the decrease in the dielectric constant of the steel in presence of the inhibitor molecules (Figure 3).
Figure 3: Electrochemical impedance spectra (EIS) for mild steel in blank and 500 ppm of S. saman inhibitor.
Surface analyses
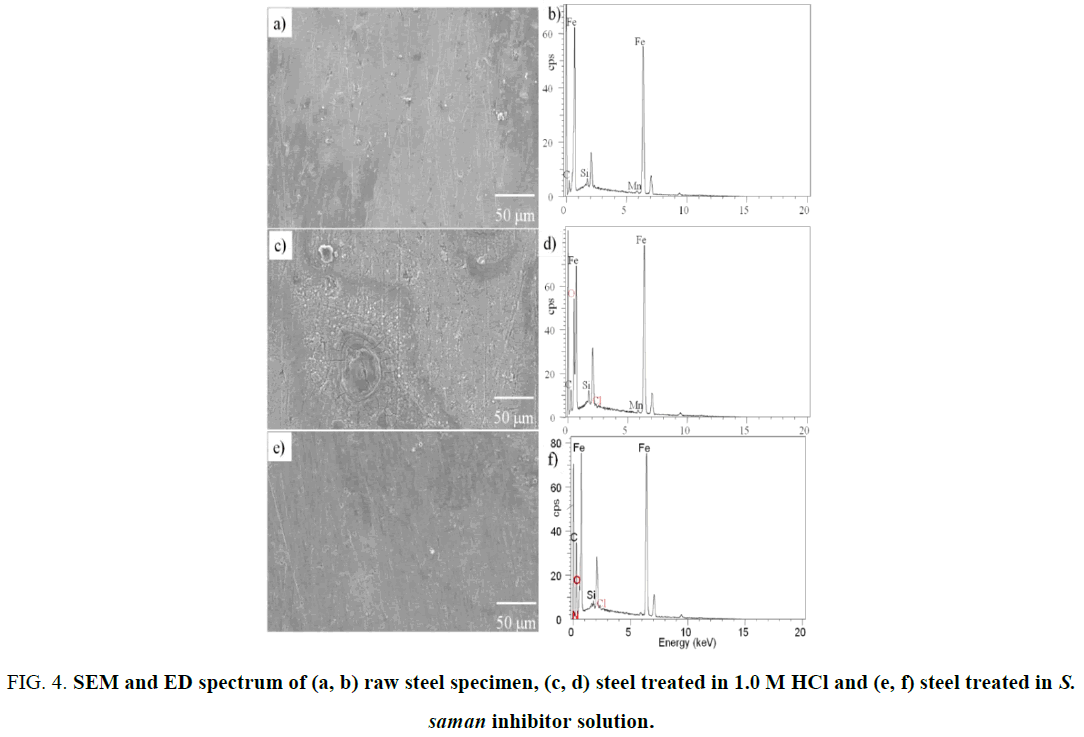
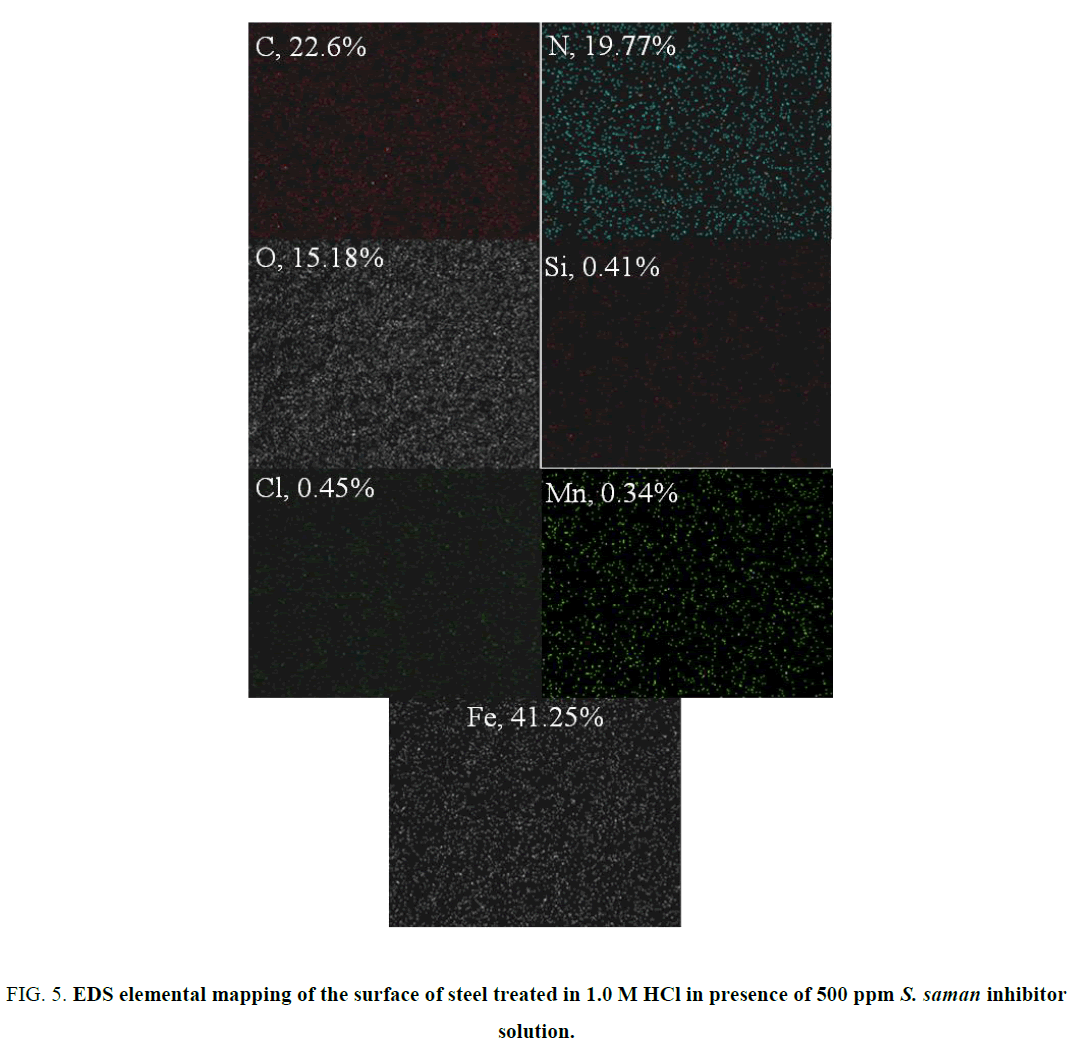
Figure 4a to 4f exhibits the SEM morphologies and ED spectra of the abraded steel, acid-treated steel, and steel treated with 500 ppm of inhibitor in 1.0 M HCl. The elemental composition of the above specimens was given in Table 4. The abraded specimen possessed some scratches that were caused during manual polishing (Figure 5a). It is obvious from the SEM images that the acid-treated specimen was highly corroded one with rough surface (Figure 5c). The specimen treated with S. saman inhibitor exhibits a smooth surface (Figure 5e).
Figure 4: SEM and ED spectrum of (a, b) raw steel specimen, (c, d) steel treated in 1.0 M HCl and (e, f) steel treated in S. saman inhibitor solution.
FIG. 5. EDS elemental mapping of the surface of steel treated in 1.0 M HCl in presence of 500 ppm S. saman inhibitor solution.
| Sample | %Composition | ||||||
| Fe | O | C | Mn | Si | Cl | N | |
| Fresh steel specimen | 92.2 | 0 | 4.93 | 1.08 | 1.79 | 0 | 0 |
| Blank | 63.03 | 27.02 | 4.39 | 0.87 | 1.55 | 3.14 | 0 |
| 1.0 M HCl + S. saman extract | 41.25 | 15.18 | 22.6 | 0.34 | 0.41 | 0.45 | 19.77 |
Table 4. Elemental composition of steel specimens.
The surface of the inhibitor-treated specimen was subjected to elemental mapping using EDS. The results are shown in Table 4 and Figure 5. It should be noted from Table 4 that neither the fresh specimen nor the acid-treated specimen contains the element ‘N’. However, the inhibitor-treated specimen exhibited 19.77% of N, which is the evidence for adsorption of inhibitor molecules on the surface of the steel.
Conclusion
The present study describes the corrosion inhibitive nature of ethanolic extract of Samanea saman. 500 ppm of inhibitor showed a maximum of 92% of inhibition efficiency against corrosion of steel in 1.0 M HCl. The Langmuir adsorption isotherm and thermochemical parameters revealed that the inhibition proceeds via physical adsorption of inhibitor molecules on the surface of the steel. The inhibitor was found to inhibit both anodic and cathodic reactions. The elemental mapping of the inhibitor-treated steel using energy dispersive X-ray spectroscopy indicated the homogeneous adsorption of the inhibitor on the surface of the steel.
Acknowledgement
Kesavan Devarayan and Monikandon Sukumaran thank College of Fisheries Engineering, Tamil Nadu Fisheries University for their encouragement for research. Kavitha Rose thanks the management of Dhirajlal Gandhi College of Technology for their encouragement for research. Sankar Arumugam and Kavitha Rose as well thank the management of Kandasamy Kandar’s College for their support for research.
References
- Kesavan D, Gopiraman M, Sulochana N. Green inhibitors for corrosion of metals: A review. Chem Sci Rev Lett. 2012;1:1-6.
- Kavitha R, Byoung-Suhk K, Rajagopal K, et al. Surface protection of steel in acid medium by Tabernaemontana divaricata extract: Physicochemical evidence for adsorption of inhibitor. J Mol Liq. 2016;214:111.
- Vivekananthan S, Sakunthala P, Kesavan D, et al. Effect of green inhibitors on acid corrosion of AISI 1022 steel. Chem Sci Rev Lett. 2013;1:200.
- Kavitha R, Monikandon S, Kesavan D, et al. Physicochemical evidence for the adsorption of extract of Jacaranda mimosifolia on steel against corrosion in acid medium. Orient J Chem. 2016;32:2147.
- Rajeswari V, Kesavan D, Gopiraman M, et al. Physicochemical studies of glucose, gellan gum, and hydroxypropyl cellulose-inhibition of cast iron corrosion. Carbohyd Polym. 2013;95:288.
- Kesavan D, Parameswari K, Lavanya M, et al. Evaluation of a green inhibitor for corrosion of mild steel. Chem Sci Rev Lett. 2014;2:415.
- Magnus KE, Seaforth CE. Samanea saman Merill: The Rain Tree: A review. Trop Sci. 1965;7:6.
- Satish S, Raveesha KA, Janardhana GR. Antibacterial activity of plant extracts on phytopathogenic Xanthomonas campestris pathovars. Lett Appl Microbiol. 1999;28:145.
- Raghavendra MP, Satich S, Raveesha KA. Antimicrobial activity of some important medicinal plant against plant and human pathogens. World J Agric Sci. 2008;4:100.
- Thippeswamy S, Praveen P, Mohana DC. Antimicrobial evaluation and phytochemical analysis of known medicinal plant Samanea saman (Jacq.) Merr. against some human and plant pathogenic bacteria and fungi. Int J Pharm Bio Sci. 2011;2:443.
- Kohler I, Jenett-Siems K, Siems K. In vitro Antiplasmodial investigation of medicinal plants from El Salvador. Z Natureforsch. 2002;57:277.
- Arulpriya P, Laitha P, Hemalatha S. In vitro antioxidant testing of the extracts of Samanea saman (Jacq.). Merr Der Chemica Sinica. 2010;1:73.
- Ferdous A, Imam MZ, Ahmed T. Antioxidant, cytotoxicity and antibacterial potential of different extract of Geodorum densiflorum (Lam.) Schltr. J Pharm Sci. 2010;3:11.
- Wiesner K, MacDonald DM, Valenta Z, et al. Pithecolobine, the alkaloid of Pithecolobium saman Benth. I. Can J Chem. 1952;30:761.
- Prasad RN, Viswanathan S, Devi JR, et al. Preliminary phytochemical screening and antimicrobial activity of Samanea saman. J Med Plant Res. 2008;2:268.
- Nnamdi OL, Egbuonu Anthony CC, Pius OU, et al. Comparative phytochemical and antimicrobial screening of some solvent extracts of Samanea saman (Fabaceae or Mimosaceae) pods. Afr J Pure Appl Chem. 2010;4:206.
- ASTM-G31-72, Standard practice for laboratory immersion corrosion testing of metals. Annual book of ASTM Standards, Philadelphia. 2004.
- Quartarone G, Bonaldo L, Tortato C. Thermodynamics and kinetics of indole oligomerization: Preliminary results in aqueous sulfuric acid. Appl Surf Sci. 2006;252:8251.
- Riggs OL. Corrosion Inhibitors, 2nd edn. Nathan C, Houston, Texas, USA. 1973;43.