Original Article
, Volume: 9( 6)Controlled Synthesis of Water Soluble Micrometer Sized Highly Luminescent Zinc Sulfide Flowers Using Green Chemistry Approach and Their Characterization
Arun S1, Kameswara Rao V1, Shrivastava AR2, Sanjay U1 and Deepika J1
1Biosensor Development Division, Defence Research and Development Establishment, Gwalior, India
2Electron Microscope Division, Defence Research and Development Establishment, Gwalior, India
- *Corresponding Author:
- Kameswara Rao V, Scientist ‘G’ and Head of Biosensor Development Division, Defence Research and Development Establishment, Gwalior, India, Tel: +91-751-2230019; E-mail: vepakrao@yahoo.com
Received Date: October 26, 2016; Accepted Date: November 25, 2016; Published Date: November 28, 2016
Citation: Arun S, KameswaraRao V, Shrivastava AR, et al. Controlled Synthesis of Water Soluble Micrometer Sized Highly Luminescent Zinc Sulfide Flowers Using Green Chemistry Approach and Their Characterization. 2016;9(6): 108.
Abstract
In the present study, highly luminescent zinc sulfide (ZnS) flowers were fabricated using facile green chemistry route and characterized. The beauty of green-chemistry synthetic route is that it is relatively simple, easy to handle, convenient, inexpensive, low energy consumption, does not require large area, demanding no extreme pressure or temperature control and requiring no complicated apparatus/special equipment which make this technique more suitable than other existing synthesis methods. The formation of ZnS flowers was confirmed by various characterization techniques such as scanning electron microscopy (SEM), electron dispersive X-ray spectroscopy (EDAX), zeta-potential, dynamic light scattering (DLS), ultraviolet visible spectroscopy (UV-VIS) and photoluminescence spectroscopy (PL). The systematic formation of ZnS microstructures to be appearing like flowers was discussed in this paper. Using this method, it is easy to prepare three-dimensional flower like ZnS (average size ~882 nm) containing nanoparticles of size less than 4 nm. Even though the total size of ZnS flowers was in submicron range, the thickness of petals is in the nanometer range. The flowers were indeed made up of nano-ZnS particles. To the best of our knowledge, till now nobody has reported these attractive flowers like ZnS structures in the range of micrometer. The present study provides a new avenue to gain in-depth insight to the design of highly luminescent ZnS flowers.
Keywords
Green chemistry; Flowers like-ZnS; Scanning electron microscopy; Luminescence; Zeta-sizer; Nano material
Introduction
In the age of nanotechnology, various kinds of nanomaterials with variety of size and shapes were synthesized using various synthetic procedures [1]. Sometimes, the synthesized materials were not stable for longer period and tend to be agglomerated due to the high surface energy, high surface tension and the presence of dangling bonds on the surface [2]. Since, the synthesis of nano materials is very difficult due to their tedious synthetic procedure and the reproducibility will be lost. The traditional method of chemical synthesis generally require complicated instrumentation, toxic precursors, large working area, high energy consumption and need lot of time to prepare nano-scaled materials [3,4]. Hence, an easy and economic green synthesis procedure is highly desired as a new synthetic approach to design the variety of compounds. Now these days, the fundamentals of green chemistry are widely used for the synthesis of many compounds with different size and shapes [2,5,6]. Green synthesis procedure was generally used to reduce the formation of unwanted hazardous waste materials as compared to other traditional chemical/physical methods.
Zinc sulfide (ZnS) is a group (II-VI) type of binary compound and environmentally benign semiconductor material having optical band gap of 3.68 eV at room temperature i.e. 27°C [7-10]. In the nature, ZnS is found in two allotropes, one is zinc blende (ZB) form having cubic like structure and another is wurzite (WZ) form with hexagonal lattice structures [9]. The band gap of ZB and WZ phase has 3.72 eV and 3.77 eV respectively [11]. ZnS is an important material and taking point of interest due to the richness of attractive chemical, physical, electronic and optical properties which are used in various applications such as biosensors [12], gas sensors [13], photocatalysis [14], electronics [15], optoelectronics [16], UV-light sensors [17], flat panel displays [18], nanogenerators [19], p-type conductors [20], thermoelectric [21], solar cells [22], infrared windows [23], lasers [24] and field effect transistors [25]. It has been demonstrated that properties of ZnS is largely depend on the size and morphology. Hence, research in the field of ZnS based morphologies is still required to develop new devices such as biosensors, cancer detectors and ultrasensitive sensors due to their nontoxicity, non-carcinogenicity and excellent luminescence properties.
Various methods were reported for the synthesis of zinc sulfide in the micro and nanoscale range with different size and shapes. These methods include hydrothermal method [26], microemulsion method [27], solvothermal method [28], sol-gel method [29], thermal evaporation method [30], organometallic method [31], gas-phase condensation method [32], ion-complex transformation method [33,34], sonochemical method [35], solid-state method [36], electrochemical deposition method [37], liquid-phase chemical precipitation method [38], vapor deposition method [39,40], microwave irradiation method [41,42], screen-printing method [16] and molecular beam epitaxy [43]. But, these methods were generally avoided due to several limitations such as requirement of well trained personnel, sophisticated instrumentation, toxic chemicals, non-aqueous toxic solvents, costly equipment operation, complex process control, high vacuum conditions, require inert conditions e.g. Argon atmosphere or long reaction time, high temperature, high pressure and vigorous conditions [7,44,45]. In most of the cases, nanoparticles prepared by these methods were not uniform, not stable for long period, and easily agglomerated [26]. Hence, need of green chemistry or soft chemistry synthetic approach was realized. Now, it is possible to synthesize a large variety of compounds with different shapes and size by changing the reaction conditions i.e. the nature of solvents, concentration of the starting materials and the choice of suitable capping/stabilizing agent [5,10]. The capping agent protects the nanoparticles from agglomeration by reducing their surface tension and hence provides them stability for long periods under atmospheric conditions at room temperature. There are various reports on the synthesis of ZnS nanoparticles with different capping agents [10,46].
In our present preparation method, we had followed eco-friendly green-chemistry route of the synthesis of microstructures of ZnS flowers using nontoxic and non-hazardous precursors such as zinc nitrate (used as zinc metal ion source) and sodium sulfide (used as sulfide ion source). In the literature, various nanomaterials of ZnS were synthesized with different morphologies such as nanowires [47], Nano rods [48], Nano flowers [5], nanoparticles [49], ribbons [50], Nano sheets [51,52], nanotubes [53] and Nano belts [54]. These kinds of morphologies exhibit different optical behaviour such as fluorescence, luminescence and absorbance due to the inherent dependency of these techniques on the size, shape, variety of precursor used and the synthetic route followed for the preparation of these materials [55]. There is a great challenge to obtain clear ZnS structures using a simple procedure of synthesis. Different synthesis schemes follow different growth mechanism which is depending upon various kinetic and thermodynamic factors [56,57]. Slight change in the growth conditions may change the morphology and shape. Hence, the main reason of morphological variation or uncertainty in shape or size is influenced by the experimental parameters. Therefore, it was necessary to understand the importance of growth mechanism. In this paper, we had tried to explain the morphological derivation and evolution of the new shape with some experimental parameters.
The basic idea behind this approach is to obtain the high quality of synthesized ZnS flowers with mild, easily controllable, well-repeatable and facile method. In the literature, various flower like morphologies were reported but they are not pure ZnS and composed of other compounds. In this report, we had synthesized pure ZnS flowers without using any other compound. Different techniques were utilized in this study for characterization purpose. Luminescence properties of the microstructures of ZnS flowers were characterized by photoluminescence (PL) spectroscopy. Morphological and shape determination was performed by scanning electron microscope (SEM). Optical absorbance was taken from ultraviolet-visible (UV-Vis) absorption spectrometer. Zeta-potential was measured to determine the surface charge and stability of the synthesized ZnS flowers in the aqueous medium. Dynamic light scattering (DLS) technique was studied to determine the average size of the ZnS flowers. Electron dispersive X-ray spectroscopic (EDAX) analysis was performed to check the purity of the compound and the elemental composition. The effect of pH on the morphology, luminescence intensity and zeta-potential of ZnS flowers was studied and systematically discussed. To the best of our knowledge, it is the first report on these high quality flower like-ZnS microstructures with interesting morphology and unique optical properties.
Research Significance
In the scientific area of microscopic research, there is a great demand of nanomaterials having variety of shapes. Novel properties of such nanomaterials can give an advantage to various research groups. Different morphologies were reported for zinc sulfide. Amazing flower like pictures of zinc sulfide was obtained by the method described in this research paper. The aim of this research to design the attractive nanomaterials which is useful for the development of various applications. Green-chemistry was also involved here so that one can easily synthesize this kind of material. These microscopic images were clearly shown that the obtained material having very good quality. As well this material can be synthesized at very large scale. The material obtained here can be utilized for various applications.
Experimental Procedures
Reagents and apparatus
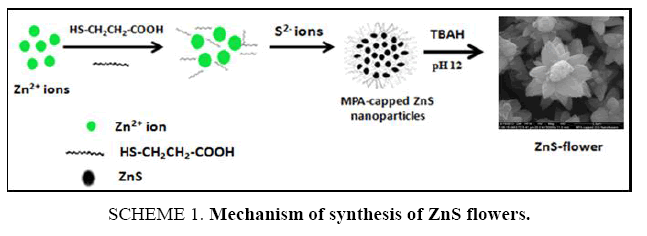
Zinc nitrate tetra hydrate Zn(NO3)2.4H2O, sodium sulfide (Na2S), 3-mercaptopropanoic acid (MPA) and tetra butyl ammonium hydroxide [(CH3CH2CH2CH2)4NOH] was procured from Sigma Aldrich, St Louis, MO. All the chemicals mentioned above were of analytical reagent (AR) grade and used without further purification ( Scheme 1). Ultrapure triple distilled Millipore water (TDW) was used to prepare all aqueous solutions throughout the experiments. The reaction was performed at room temperature.
The SEM/EDAX measurement was performed by (Quanta 400ESEM The Netherlands) and operated at an accelerating voltage of 20.0 kV. UV-VIS spectrum is taken by Implen nanophotometer (serial no.1257). Photoluminescence spectra were performed using a PerkinElmer LS55 fluorescence spectrometer in the range from 200 nm to 500 nm. Zeta-potential and dynamic light scattering (DLS) analysis of the ZnS flowers was measured by Malvern Instruments (Malvern, UK) Zetasizer Nano-ZS. Magnetic stirrer controller (Model No.TH100) and a pH meter (Eutech Instruments, Singapore) were utilized in this study. Triple distilled deionized water was obtained from water purification system (Millipore, USA).
Synthesis of MPA-capped flower like-ZnS
SCHEME 1. Mechanism of synthesis of ZnS flowers: Mercaptopropanoic acid (MPA) functionalized zinc sulfide flowers were synthesized with slight modification in the following procedure reported earlier [58]. We had used tetrabutylammonium hydroxide (TBAH) in place of tetrapropylammonium hydroxide to adjust the pH during synthesis. Tetrabutylammonium hydroxide (TBAH) has larger cation as compared to tetrapropylammonium hydroxide and the basicity will be different. These basicity differences play a key factor to produce such a new morphology. The possible growth mechanism of synthesis of ZnS flowers was shown in SCHEME 1. First, zinc nitrate (0.04 M, 20 ml) solution A and sodium sulfide (0.02 M, 20 ml) solution B was prepared separately in TDW. Mercaptopropanoic acid (MPA) (0.64 mmol, 36 ml) was prepared in TDW and stirred for 5min solution C. In the next step, 2 ml of solution A was dropped slowly in the solution C with constant stirring for 10min. Then this mixture was titrated with tetrabutylammonium hydroxide (TBAH). Adjusted the pH to 12 and stirred for 10 min followed by rapid addition of 4 ml of solution B. Then, wait for 5 min before adding another 6ml of solution A. pH 12 was maintained by adding TBAH with constant stirring for 5 min. The flower like-ZnS micro particles were obtained in good dispersion in aqueous phase under magnetic stirring.
Thus, obtained MPA-capped zinc sulfide flowers were clear and colorless. The final volume of the ZnS flowers solution was 50 ml. This room temperature synthesis route was more promising for large-scale production and low-cost. The oxidation and agglomeration of the ZnS flowers were prevented at the same time using MPA as a stabilizer and the energy consumption during the preparation procedure was reduced. For comparison, MPA-capped zinc sulfide were also synthesized at different pH conditions using the same procedure as mentioned.
Results and Discussion
Scanning electron microscopy (SEM)-characterization
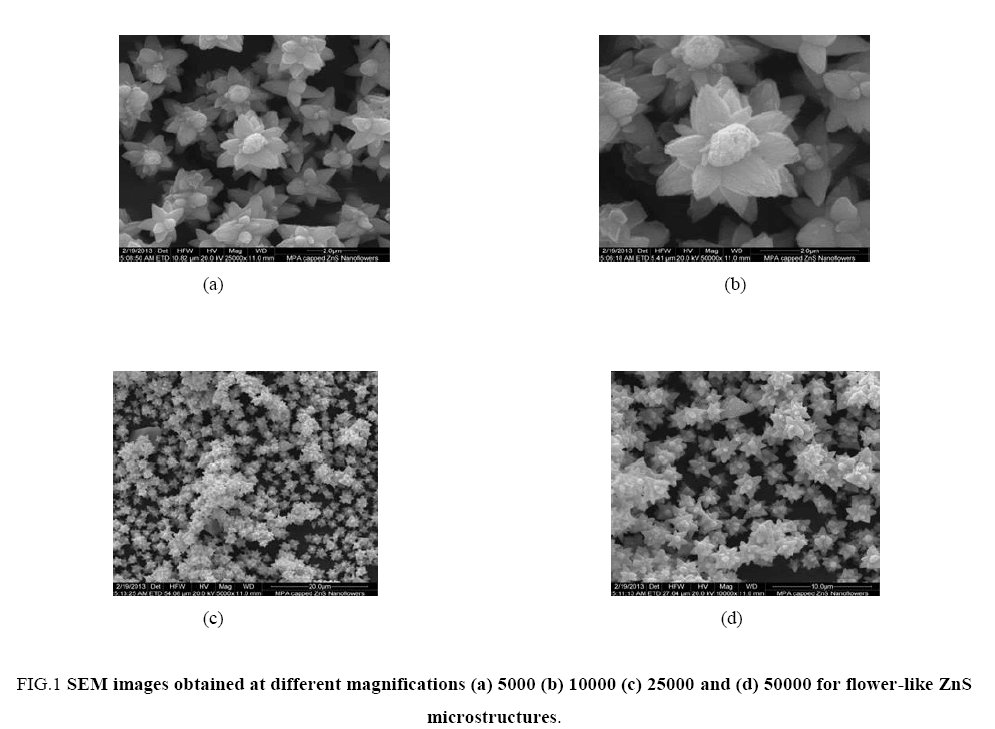
Micrometer size ZnS molecules were systematically arranged in a flower like fashion to give these interesting morphologies. The ZnS flowers are uniformly distributed in their aqueous solution at pH 12 and no agglomeration was observed. Images of flower like-ZnS microstructures which were obtained at different magnifications such as 5000, 10000, 25000 and 50000 are shown in Figure 1(a), 1(b), 1(c) and 1(d) respectively. As clearly seen in the images we can state that at pH 12 the shape of the MPA-capped ZnS attain flower like geometry which may appear due to the intrinsic characteristic property of zinc sulfide in the presence of bifunctional capping agent during their controlled growth conditions. Since, the capping agent i.e. 3-mercaptopraponoic acid was covalently bind with ZnS nanoparticles on their surface through its thiol moiety and disulfide bond formation takes place. Hence, the capping agents play a very important role to control the shape and size of the growing ZnS nanoparticles through a simple mechanism of charge transfer [10]. It is difficult to precisely control the morphology and shape of the synthesized ZnS flowers and still it is the most challenging problem.
Figure 1: SEM images obtained at different magnifications (a) 5000 (b) 10000 (c) 25000 and (d) 50000 for flower-like ZnS microstructures.
Energy dispersive X-ray spectroscopy (EDAX)-characterization
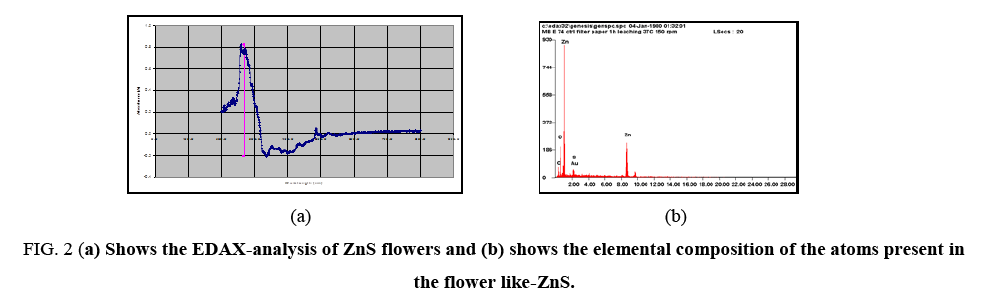
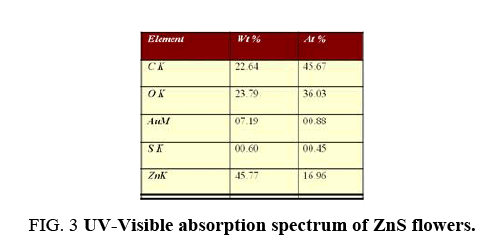
Compositional analysis of flower like-ZnS was done by electron dispersive x-ray (EDAX) spectroscopy as shown in Figure 2a. EDAX-analysis of flower like-ZnS shows that Zn and S were present in the atomic ratio of 16.96% and 0.45% respectively ( Figure 2b). The results indicated that elemental Zn species were abundant on the surface of the ZnS flowers. In the X-ray energy-dispersive spectroscopy (EDAX) spectrum, carbon and oxygen peak was present which indicates the presence of carboxylic group on to the surface of MPA-capped ZnS flowers. The strong peak of Zn was clearly present in the EDAX-analysis and no other peaks from impurities were detected, confirming the high purity of the synthesized ZnS-flowers. The Au signal in the EDAX-spectrum was attributed to the gold grid for SEM imaging.
Figure 2: (a) Shows the EDAX-analysis of ZnS flowers and (b) shows the elemental composition of the atoms present in the flower like-ZnS.
Ultraviolet-visible (UV-Vis)-characterization
UV–Vis spectroscopy is a useful technique to monitor the optical properties. The optical property of flower-like ZnS was quite different as compared to the bulk ZnS due to the difference in the band-gap energies. The change in the properties of small size particles is observed due to the increase in the surface to volume ratio and quantum confinement effect. UV–Visible absorption spectrum of ZnS flowers were shown ( Figure 3) optical density (O.D.) or absorbance around 0.822 at 268.0 nm wavelength. The absorption wavelength of the ZnS nanomaterials was changed with small change in their size and shape using different surface capping agent. There are various reports in the literature such as mercaptoacetic acid capped-ZnS QDs size (~4 nm) shows absorption band around 295 nm due to 1S3/2 – 1Se transition [10]. Thioglycolic acid-capped ZnS nanoparticles (size~ 4.5 nm) dispersed in ethanol shows UV-Vis absorption maximum at around 323 nm [59]. The absorption spectra of MPA-capped ZnS QDs approx size (around 4 nm to 5 nm) was reported around 315 nm [58]. Hence, the size of ZnS-nanoparticles which are the part of flowers shall less than 4 nm.
Photoluminescence spectral characterization of ZnS flowers
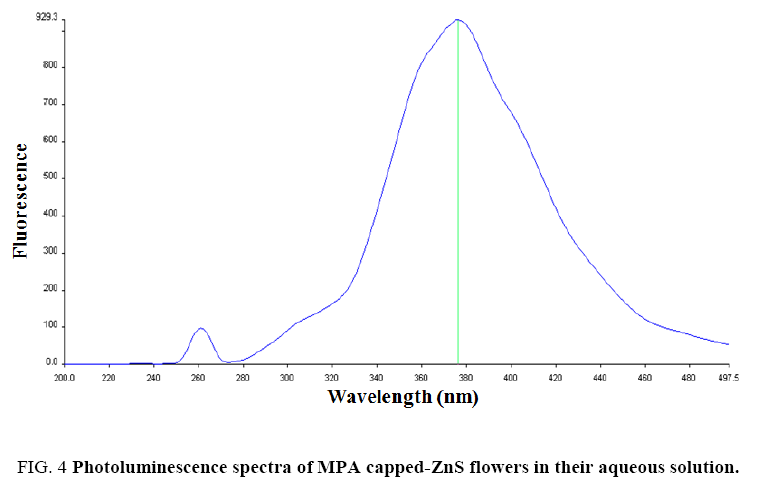
To determine the optical properties of various nanomaterials, PL-study was performed to characterize them. Luminescent properties of ZnS are very well known [8,62,63]. When ZnS crystal was excited with an external light source, a pair of electron-hole was generated. After excitation, these electron-hole pairs recombined to give emission wavelength. To show the PL-characteristics, ZnS flowers absorb high energy photons which excite the electrons to reach in the conduction band (CB) from the valence band (VB) of Zn2+ levels. These excited electrons decay non-radiatively to the surface states and then decay radiatively to the valence band and a lower energy photon was emitted [63]. The energy of excitation and emission was recorded in terms of wavelength and the number of electron-hole pairs generated was determined in terms of intensity with the help of spectrophotometer. The results indicated that highest intensity was obtained for the flower like ZnS crystals when were excited at 260 nm and the emission wavelength was observed at around 378 nm ( Figure 4). The photoluminescence emission was observed due to presence of the band edge and stoichiometric vacancies [11]. The obtained results were compared with those bulk ZnS material reported [64]. The PL-emission was observed at 450 nm for bulk ZnS by Chen et al. [64]. In case of ZnS nanoparticles, the PL-emission wavelength was decreased and reached at around 400 nm [63]. In our observation, PL-emission was achieved at about 378 nm for flower like-ZnS due to small size and presence of capping agent. The intensity of the emission peak was strong and highest for flower like-ZnS at pH 12, which further indicates that ZnS nanoparticles having good crystallinity. Hence, we can state that ZnS flowers were constructed with nanometer size ZnS particles. At pH 12 conditions, high density of carboxylate (–COO-) ions were present on the nanoparticles surface which will repel them from each other. Hence, good dispersity was observed in their dispersions. Further, increasing the pH from 12 of the ZnS flowers dispersion the luminescence intensity was very poor which could not be observed. This was due to the formation of precipitate of zinc hydroxide and there were no more flowers. In more acidic medium (less than pH 6) the luminescence emission intensity was also very small and could not be recorded. This was due to the protonation of MPA attached to the ZnS flowers and consequently removal of capping from the ZnS surface. This will make the flowers unstable and they will tend to agglomerate. Similar effect of lower and higher pH on fluorescence intensity was reported in the literature for the mercaptoacetic acid capped CdS quantum dots [65]. Photoluminescence properties of different morphologies of ZnS nanostructures were reported in the literature [66-69]. We observed flower like morphology at pH 12 only.
Dynamic light scattering (DLS) characterization
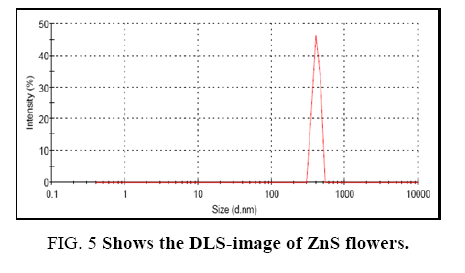
Dynamic light scattering (DLS) analysis was performed for the measurement of hydrodynamic size of ZnS-flowers at room temperature as shown in Figure 5. The hydrodynamic size was calculated from the diffusion times using Stokes–Einstein equation [60].
D= kT/3πηR
Where, D is the translational diffusion coefficient of the particles, k is Boltzmann's constant (1.38 × 10-16 erg K-1), T the temperature (°K = °C + 273), R is the hydrodynamic size of the particles and η is the viscosity of the solvent (e.g. η = 0.890 × 10-2 poise for water at 25°C). DLS is also referred to as photon correlation spectroscopy. The particle size distribution was measured at 25°C in a low-volume quartz batch cuvette. Generally, a wide dynamic range of the nanometer and micrometer sized particles can be easily detected by DLS-analysis. DLS-analysis indicated that hydrodynamic size of the ZnS-flowers was around 882 nm and the width of the flower was about 42.65 nm. However, the width of the flowers as per SEM-image was shown hundred nanometers. Hence, the width of 42.65 nm may be referred to the petals of the flower.
Effect of pH on Zeta-potential (ZP) of ZnS flowers
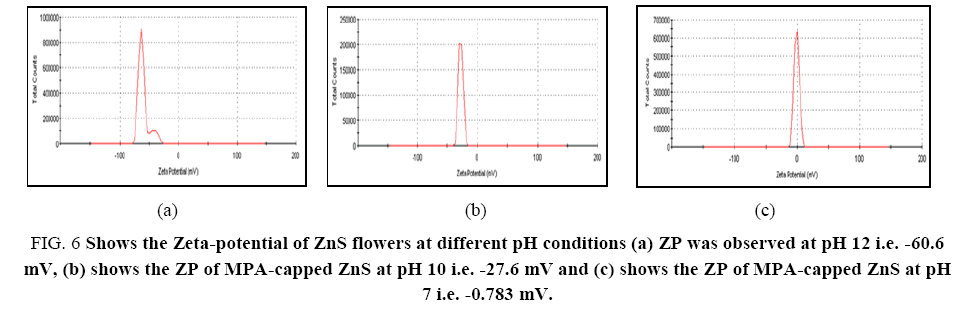
pH of the medium in which the ZnS flowers were dispersed plays a very critical role and therefore need to be studied. To study the effect of pH, ZnS flowers were dispersed in their aqueous solution and their pH was adjusted by tetra butyl ammonium hydroxide at the synthesis procedure as described earlier. The effect of pH in a range between 7 and 12 on the zeta-potential was studied and the results obtained were shown in Figure 6. Zeta-potential arises due to the presence of charge on the surface of the flowers and hence it is significantly depending on the pH of the medium in which the flowers are dispersed [61]. In basic medium, negatively charged carboxylate ions were present on the surface of the MPA-capped ZnS flowers. When the pH is increased zeta-potential is also increased due to the deprotonation of the –COOH groups present in the outer side of the flowers. The prepared ZnS microflowers are well-dispersed at pH 12 and it was suitable for the zeta-potential measurement. At pH 12, the surface of the ZnS flowers capped with mercaptopropanoic acid was fully charged. In this case, due to large electrostatic repulsion effect dispersion of the flowers was very good and resulting in -60.6 mV zeta-potential as shown in Figure 6a. We can state that only few carboxylate (-COO-) ions were present on to the surface of MPA-capped ZnS at pH 7 as compare to pH 10. Hence, at pH 10 and pH 7 zeta-potential was decreased and it was obtained at -27.6 mV (Figure 6b) and -0.783 mV (Figure 6c) respectively. The driving force for formation of flower like structure may be due to combination of various parameters such as nanoparticle size, viscosity and hydrophobic effect of tetra buty lammonium hydroxide (TBAH). The hydrophobic effect will change the contact angle and the geometry may change [70]. The selection of experimental conditions, concentration of reactants and the route of preparation used, also play an important role to control over the shape of the synthesized material. Different kinds of geometry were reported by changing the molar ratio of TBAH and its influence was clearly mentioned in the literature for the shape and size of TiO2 nanoparticles prepared by microwave-assisted hydrothermal process [71].
Figure 6: Shows the Zeta-potential of ZnS flowers at different pH conditions (a) ZP was observed at pH 12 i.e. -60.6 mV, (b) shows the ZP of MPA-capped ZnS at pH 10 i.e. -27.6 mV and (c) shows the ZP of MPA-capped ZnS at pH 7 i.e. -0.783 mV.
like microstructures of ZnS with attractive optical, physical and electronic properties. The highly crystalline flower like-ZnS was synthesized using green chemistry approach i.e. waste reduction, cheap raw material, less time, process safety, design of nontoxic nanomaterial and energy efficiency. The average size of the flower like-ZnS was about 882 nm observed from DLS-analysis. Various research groups are working on zinc sulfide but none has reported these kinds of SEM morphologies which are really looked like flowers of ZnS constructed with very small ZnS nanoparticles. The luminescent flowers of ZnS crystal shows the quantum confinement effect and blue shift was observed as compare to bulk ZnS. The photoluminescence was observed in the flower like ZnS due to the radiative recombination of the excited electrons from conduction band to holes in the valence band. In further studies, for application point of view such highly luminescent water soluble flower like ZnS can be used in various devices. For best performance, it is necessary to optimize the concentration of capping agent and the comparison was required with different types of capping agents in further studies. Such obtained special structures of ZnS represent good candidates for furthermore applications in various fields of nanoscale science and technology.
Conflict of Interest
The authors declare no conflict of interest.
Acknowledgments
We are thankful to the Director, Defence Research and Development Establishment for giving the permission to publish.
References
- Feigl C, Russo SP, Barnard AS. Safe, stable and effective nanotechnology: Phase mapping of ZnS nanoparticles. J Mater Chem. 2010;20(24):4971-80.
- Raveendran P, Fu J, Wallen SL. Completely “green” synthesis and stabilization of metal nanoparticles. J Am Chem Soc. 2003;125(46):13940-41.
- Taton TA. Nanoscalematerials in chemistry. In: Klabunde KJ, ed. New York: Wiley-Interscience; 2001. p. 292.
- Swihart MT. Vapor-phase synthesis of nanoparticles. Current Opinion in Colloid and Interface Science. 2003;8(1):127-33.
- Xiao N, Dai Q, Wang Y, et al. ZnSnanocrystals and nanoflowers synthesized by a green chemistry approach: Rare excitonic photoluminescence achieved by the tunable molar ratio of precursors. J Hazard Mater. 2012;211-2:62-7.
- Dai QQ, Xiao NR, Ning JJ, et al. Synthesis and mechanism of particle- and flower-shapedZnSenanocrystals: Green chemical approaches toward green nanoproducts. J PhysChem C. 2008;112(20): 7567-71.
- Barman B, Mochahari PK, Sarma KC, et al. Optical studies on some aspects of polyvinyl alcohol composite Znsnano crystalline thin films. Chalcogenide Letters. 2011;116:1391.
- Lu HY, Chu SY, Tan SS. The characteristics of low-temperature-synthesized ZnS and ZnO nanoparticles. J Cryst Growth. 2004;269(2-4):385-91.
- Shen FY, Que W, Yin XT, et al. A facile method to synthesize high-quality ZnS(Se) quantum dots for photoluminescence. J Alloy Compd.2011;509(37):9105-10.
- Wageh S, Ling ZS, Rong XX. Growth and optical properties of colloidal ZnS nanoparticles. J Cryst Growth. 2003;255(3-4): 332-37.
- Fang X, Zhai T, Gautam UK, et al. ZnS nanostructures: From synthesis to applications. Prog Mater Sci. 2011;56(2):175-287.
- Cowles CL, Zhu X. Sensitive detection of cardiac biomarker using ZnS nanoparticles as novel signal transducers. Biosensors and Bioelectronics. 2011;30:342-46.
- Luo L, Chen H, Zhang L, et al. A cataluminescence gas sensor for carbon tetrachloride based on nanosizedZnS. Anal ChimActa. 2009;635(2):183-87.
- Jing C, Yinfeng L, Wenbin SJ, Shanghai University (English Edition) 2003;7(3):289-93.
- Dumbrava V, Ciupina G, Prodan G. Dependence on grain size and morphology of zinc sulfide particles by the synthesis route. Rom Journ Phys. 2005;50:831-6.
- Ruda HE. The Perfume Handbook. In: Groom N, ed. London: Chapman and Hall; 1992. p. 323.
- Fang XS, Bando Y, Gautam UK, et al. ZnO and ZnSnanostructures: Ultraviolet-light emitters, lasers, and sensors. J Crit Rev Solid State Mat Sci. 2009;34(3-4):190-223.
- Bredol M, Merikhi J. ZnS precipitation: Morphology control. J Mater Sci. 1998;33(2):471-76.
- Huang X, Wang M, Willinger MG, et al. Assembly of three-dimensional hetero-epitaxial ZnO/ZnScore/shell nanorod and single crystalline hollow ZnSnanotube arrays. ACS Nano. 2012;6(8):7333-39.
- Diamond AM, Corbellini L, Balasubramaniam KR, et al. Copper-alloyed ZnS as a p-type transparent conducting material. Phys Status Solidi A. 2012; 209(11):2101-7.
- Yue Wu, Purdue University, PRF No. 65763, 1, 2011.
- Tokio N, Keisuke F, Akio K. High-efficiency cadmium-free Cu(In,Ga)Se/sub 2/ thin-film solar cells with chemically deposited ZnS buffer layers. IEEE Trans Electron Devices. 1999;46(10):2093-97.
- Akihito F, Hideo W, Ken-Ichiro S, et al. Diamond-ZnS composite infrared window. Proc. SPIE (The International Society for Optical Engineering). 2001; 206:4375p.
- Falcony C, Garcia M, Ortiz A, et al. Luminescent properties of ZnS:Mn films deposited by spray pyrolysis. J Appl Phys. 1992;72(4):1525-27.
- He JH, Yi Y, Zhang D, et al. ZnS/silica nanocable field effect transistors as biological and chemical nanosensors. J PhysChem C. 2007;111(33):12152-56.
- Chai LL, Zhu YC, Du J, et al. One-step hydrothermal method to synthesize self-encapsulated ZnSmicron core-shell spheres on a large scale. Chemistry Letters. 2005;34(10):1324-25.
- Charinpanitkul T, Chanagul A, Dutta J, et al. Effects of co-surfactant on ZnS nanoparticle synthesis in microemulsion. Science and Technology of Advanced Materials. 2005;6(3-4):266-71.
- Zhang YC, Wang GY, Hu XY, et al. Solvothermal synthesis of uniform hexagonal-phase ZnSnanorods using a single-source molecular precursor. Mater Res Bull. 2006;41(10):1817-24.
- Bhattacharjee B, Ganguli D, Chaudhuri S, et al. ZnS:Mnnanocrystallites in SiO2 matrix: preparation and properties. Thin Solid Films. 2002;422(1-2):98-103.
- Yuan HJ, Xie SS, Liu DF, et al. Formation of ZnS nanostructures by a simple way of thermal evaporation. J Cryst Growth. 2003;258(3-4):225-31.
- Malik MA, Brien P, Revaprasadu N. Synthesis of TOPO-capped Mn-doped ZnS and CdS quantum dots. J Mater Chem.2001;11(9):2382-86.
- Lopez JCS, Fernandez A. The gas-phase condensation method for the preparation of quantum-sized ZnS nanoparticles. Thin Solid Films. 1998;317(1-2):497-99.
- Yinfeng L. Shanghai University Doctoral Dissertation, 2004; p.139-150.
- Yuguang L, Yinfeng L, Liping W, et al. UV absorption and photoluminescence of nanometer-sized ZnS prepared by different routes. Conference on nano/micro engineered and molecular systems, 4th IEEE international. 2009;458-62.
- Goharshadi EK, Sajjadi SH, Mehrkhah R, et al. Sonochemical synthesis and measurement of optical properties of zinc sulfide quantum dots. Chemical Engineering Journal. 2012;209:113-17.
- Lin CH, Chiou BS, Chang CH. Preparation and cathodoluminescence of ZnO phosphor. J D Lin Mater Chem Phys. 2002;77(3):647-54.
- Kar S, Chaudhuri S. Shape selective growth of CdSone-dimensional nanostructures by a thermal evaporation process. J PhysChem B. 2006;110(10):4542-47.
- Yang H, Holloway PH. Photoluminescent and electroluminescent properties of Mn-doped ZnSnanocrystals. J Appl Phys. 2003;93:586-92.
- Mews A, Kadavanich AV, Banin U, et al. Structural and spectroscopic investigations of CdS/HgS/CdS quantum-dot quantum wells. Phys Rev B. 1996;53:242.
- Moon H, Nam C, Kim C, et al. Synthesis and photoluminescence of zinc sulfide nanowires by simple thermal chemical vapor deposition. Mater Res Bull. 2006;41(11):2013-17.
- Tiwary KP, Choubey SK, Sharma K. Structural and optical properties of Znsnanoparticles synthesized by microwave irradiation method. Chalcogenide Letters. 2013;10(9):319-23.
- Limaye MV, Gokhale S, Acharya SA, et al. Template-free ZnSnanorod synthesis by microwave irradiation. Nanotechnology. 2008;19(41):415602.
- Chan SK, Lok SK, Wang G, et al. MBE-grown cubic ZnSnanowires. J Electron Mater. 2008;37(9): 1433-37.
- Lin M, Zhang J, Boothroyd C, et al. Hollowing mechanism of zinc sulfide nanowires in vacuum induced by an atomic oxygen beam. J PhysChem B. 2004;108(28):9631-37.
- Yuan SY, Juan Y, Qiu S. Synthesis of ZnS nanoparticles by solid-liquid chemical reaction with ZnO and Na2S under ultrasonic. Trans Nonferrous Met Soc China 20. 2010; s211-s215.
- Koneswaran M, Narayanaswamy R. L-Cysteine-capped ZnS quantum dots based fluorescence sensor for Cu2+ ion. Sensors and Actuators B. 2009;139(1):104-9.
- Chen H, Shi D, Qi J, et al. The stability and electronic properties of wurtzite and zinc-blende ZnS nanowires. Phys. Lett A. 2009;373(3): 371-5.
- Zhao QT, Hou LS, Huang R. Synthesis of ZnSnanorods by a surfactant-assisted soft chemistry method. InorgCommun. 2003;6(7): 971-3.
- Tiwary KP, Choubey SK, Sharma K. Structural and optical properties of ZnS nanoparticles synthesized by microwave irradiation method. Chalcogenide Letters. 2013;10(9):319-23.
- Kar S, Chaudhuri S. Controlled synthesis and photoluminescence properties of ZnSnanowires and nanoribbons. J PhysChem B. 2005;109(8):3298-302.
- Liang CH, Shimizu Y, Sasaki T, et al. Au-mediated growth of wurtziteZnSnanobelts, nanosheets, and nanorodsvia thermal evaporation. J PhysChem B. 2004;108(28):9728-33.
- Fang XS, Ye CH, Peng XS, et al. Large-scale synthesis of ZnSnanosheets by the evaporation of ZnSnanopowders. J Cryst Growth. 2004;263(1-4):263-8.
- Ruitao LV, Cao CB, Guo YJ, et al. Preparation of ZnS nanotubes via surfactant micelle-template inducing reaction. J Mater Sci. 2004;39:1575-8.
- Gong JF, Yang SG, Duan JH, et al. Rapid synthesis and visible photoluminescence of ZnSnanobelts. ChemCommun. 2005;(3):351-3.
- Jiahua M, Wenbin S, Weimin S. Influence of the structure and morphology of ZnS Nanoparticle on its optical Properties. ActaOpticaSinica. 2003;23(3):257-60.
- Yinfeng L, Wenbin S, Zhongyan M, et al. Kinetics of the coordination transformation for preparation of nanosizedZnS in a PVA film. Journal of Macromolecular Science. 2004;43(3):625-37.
- Chen XJ, Xu HF, Xu NS, et al. Kineticallycontrolled synthesis of wurtziteZnSnanorods through mild thermolysis of a covalent organic−inorganic network. Inorg Chem. 2003;42(9):3100-6
- Hui Li. PhD Thesis. 2008; p1-194.
- Salavati-Niasari M, Davar F, Mazaheri M. Synthesis and characterization of ZnSnanoclusters via hydrothermal processing from [bis(salicylidene)zinc(II)]. Journal of Alloys and Compounds 470(1-2), 502-506 (2009).
- Malvern instruments. Manual, p. 1-8.
- Malvern instruments. User manual, p. 1-312.
- Zhao ZG, Geng FX, Cong HT, et al. A simple solution route to controlled synthesis of ZnSsubmicrospheres, nanosheets and nanorods. J Nanotechnology. 2006;17(18):4731-35.
- Tamrakar R, Ramrakhiani M, Chandra BP. Effect of capping agent concentration on photophysical properties of zinc sulfide nanocrystals. The Open Nanoscience Journal. 2008;2(1):12-26.
- Chen L, Zhang J, Luo Y, et al. Effect of Zn[sup 2+] and Mn[sup 2+] introduction on the luminescent properties of colloidal ZnS:Mn[sup 2+] nanoparticles. ApplPhysLett. 2004;84(1):112.
- Koneswaran M, Narayanaswamy R. Mercaptoacetic acid capped CdS quantum dots as fluorescence single shot probe for mercury(II). Sensors and Actuators B. 2009;139(1):91-96.
- Tran TK, Park W, Tong W, et al. Photoluminescence properties of ZnSepilayers. J Appl Phys. 1997; 81(6):2803-9.
- Chang M, Cao XL, Xu XJ, et al. Fabrication and photoluminescence properties of highly ordered ZnS nanowire arrays embedded in anodic alumina membrane. PhysLett A. 2008;372(3):273-76.
- Zhang ZX, Wang JX, Yuan HJ, et al. Low-temperature growth and photoluminescence property of ZnSnanoribbons. J PhysChem B. 2005;109(39):18352-55.
- Yue GH, Yan PX, Yan D, et al. Synthesis of two-dimensional micron-sized single-crystalline ZnS thin nanosheets and their photoluminescence properties. J Cryst Growth. 2006;293(2):428-32.
- Bhushan B, Jung YC. The Ohio State University, USA, p. 1-46.
- Lee H, Choi M, Kye Y, et al. Control of particle characteristics in the preparation of TiO2nano particles assisted by microwave. Bull Korean Chem Soc. 2012;33(5):1699-1702.