Review
, Volume: 21( 2)Synthesis and Biological Evaluation of Some Substituted Phenyl Thiazolyl Naphthyl Methanone Derivatives
- *Correspondence:
- TF Abbs Fen Reji
Department of Chemistry, Manonmaniam Sundaranar University, Tirunelveli, Tamil Nadu, India
Tel: 9080416339
E-mail: abbsfen@gmail.com
Received: December 15, 2022, Manuscript No. TSIJCS-22-83706; Editor assigned: December 19, 2022, PreQC No. TSIJCS-22-83706 (PQ); Reviewed: January 02, 2022, QC No. TSIJCS-22-83706; Revised: February 17, 2023, Manuscript No. TSIJCS-22-83706 (R); Published: February 24, 2023, DOI: 10.37532/0972-768X.2023.21(2).433
Citation: Brilla C, Reji TFAF. Synthesis and Biological Evaluation of Some Substituted Phenylthiazolylnaphthylmethanone Derivatives. Int J Chem Sci. 2023;21(2):433.
Abstract
Phenylthiazolylnaphthylmethanone and their analogues serve as precursors for the synthesis of biologically active compounds. They were accounted for to have antimicrobial, analgesic, anti-inflammatory, anticonvulsant, cardiotonic, anticancer, anti-tubercular and anthelmintic activities. The Phenylthiazolylnaphthylmethanone derivatives were synthesized and characterized by FT-IR, 1H NMR, 13C NMR, Mass spectral studies and biological studies. In addition, the Lipinski rule of five, molecular docking was used to calculate binding affinities and predict binding locations for the various receptors. With an end goal to assess and plan quick, precise Density Functional Theory (DFT) strategies for the compound was finished utilizing Gaussion' 09 program using B3LYP technique with the 6-31G premise set. The antioxidant activity has been analyzed using the DPPH radical scavenging assay. Among the (2-diethylamino-4-phenylthiazol-5-yl-2-naphthyl) methanone (1b) was highly active on the SKMEL cell line.
Keywords
FT-IR; 1H NMR; 13C NMR, Mass; Lipinski rule; Molecular docking; Biological studies
Introduction
Thiazole is an aromatic five membered heterocyclics. It was first characterized by Hantzsch and Weber in 1887 [1]. Thiazoles being an integral part of many potent biologically active molecules [2]. It contains N=C=S moiety has been utilized as antiphychotics and antimalarial [3]. A large number of heterocyclic compounds containing nitrogen and sulfur are used as medicine in various therapeutic targets [4]. The thiazole nucleus is part of the vitamin B (thiamine) structure, furthermore in specific antibiotics drugs like penicillin, micrococcin and numerous metabolic results of fungi and primitive marine animal etc. [5]. The applications of thiazoles were found in drug improvement for the treatment of hypertension, inflammation, allergies, schizophrenia, bacterial, HIV infections hypnotics and more recently for the treatment of pain as fibrinogen receptor antagonists with antithrombolic activity and so new inhibitors of bacterial DNA gyros B [6]. Naphthalene is a huge hydrocarbon natural substance that gives rise to a host of replacement products used in the manufacture of dyestuffs and synthetic resins [7]. It was insoluble in water and is soluble in benzene, absoluble alcohol, ether, carbon tetrachloride, etc. [8]. Diethyl amine is targeted at therapeutic scaffolds that can act as strands of biological receptors for various biomarkers and consequently aid in drug discovery [9]. The five member pyrrolidine ring is one of the nitrogen heterocycles most commonly used by medical pharmacists to obtain compounds for the treatment of human diseases [10]. Morpholine is mainly due to its contribution to the diversity of biological functions and the improved pharmacokinetic profile of those active molecules [11].
Literature Review
The amino-1,3-thiazoles are prepared from amidines and thiouronium salts through condensation with isothiocyanates, to yield amidinothioureas and thioureidothioureas. With a base catalysed ring closure procedure, treatment of the bromo ketones led to the S-alkylated intermediate, which yielded 1,3-thiazoles [12].
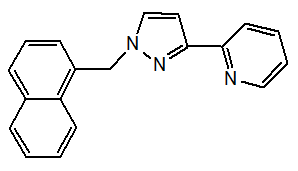
A new ligand 1-(3-(2-pyridyl) pyrazol-1-ylmethyl)naphthalene and its two metal complexes, (Cu(L)3)(ClO4)2 and (Zn(L)3)(ClO4)2(H2O)2 were synthesized and characterized. The compounds were biologically evaluated on tumor cells (HL-60 human leukaemia cells, BGC-823 human stomach cancer cells and MDA-MB-435 human mammary cancer cells) [13].
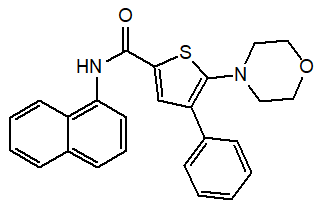
Naphthalene containing compound 5-morpholin-4-yl-4-phenyl-thiophene-2-carboxylic acid naphthalene-1-ylamide showed significant anti-inflammatory activity [14].
Experimental details
The reagents and solvents utilized were bought from sigma Aldrich; Hi-media research centers Pvt. Ltd. The IR spectra were recorded on a Perkin-Elmer spectrum in 4000 cm-1-400 cm-1 range using the KBr pellet technique. The 1H, 13C NMR and Mass spectra were recorded on Bruker Avance III, 400 MHz instrument utilizing DMSO-d6 as the internal reference and a basic analysis was performed.
Synthesis of target compound
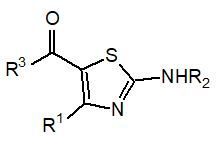
The reaction between benzoyl thiourea (20 mmol) using a distinct ring closure method of methylene-carbonyl condensation and (2-bromoacetyl) naphthalene (20 mmol) were mixed and heated ethyl alcohol [15]. After the reaction gets completed the mixture is left for cooling. When the mixture reached in 20° it was poured into cool water (50 mL) and added water and neutralization of NaHCO3. The target compound was recrystallized using ethyl alcohol (Figure 1) [16].
Computational details
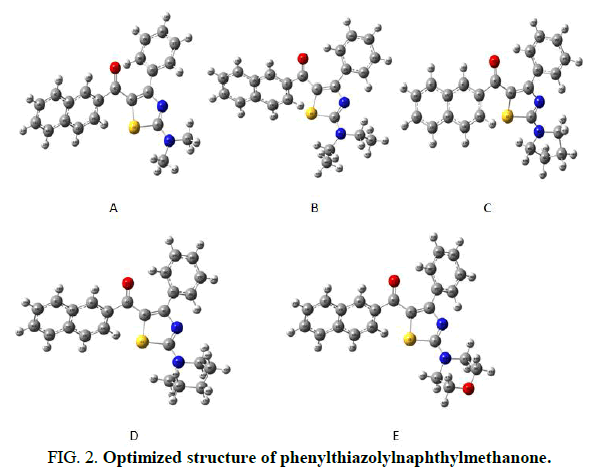
A branch of chemistry which is solving chemical problems using computer simulation is called computational chemistry. A quantum mechanical modeling method, investigating the electronic structure of molecules is Density Functional Theory (DFT) and the determination of the properties of a many electron system using this method is based on electron density (Table 1). The DFT calculations of phenylthiazolylnaphthylmethanone has used Gaussian 09 program package at the B3LYP (Becke-3Lee Yang Parr) level with a standard 3-21G basic set (Table 2) [17]. Molecular geometry optimized structural and electronic parameters were used to the vibrational frequency and DFT calculation level to confirm the structure as minima. Gauss view 5.0.9 is used in the molecular visualization program, the vibrational frequency assignment and other parameters were made. Every one of the calculations was done for the optimized structures in the gas phase (Figure 2).
| Compound | R2 |
|---|---|
| 1a | Dimethylamine |
| 1b | Diethylamine |
| 1c | Pyrrolidine |
| 1d | Piperidine |
| 1e | Morpholine |
TABLE 1. Table showing compound and R2.
| Parameters (a.u) | 1a | 1b | 1c | 1d | 1e |
|---|---|---|---|---|---|
| Total energy (a.u) | -1431.80 | -1510.42 | -1501.46 | -1540.57 | -1584.38 |
| Dipole moment (Debye) | 5.4457 | 5.5669 | 6.2280 | 5.7924 | 3.7721 |
| HOMO | -0.2299 | -0.2279 | -0.2293 | -0.2303 | -0.2311 |
| LUMO | -0.0273 | -0.0258 | -0.0243 | -0.0265 | -0.0301 |
| HOMO-LUMO (ΔE) | 0.2026 | 0.2021 | 0.2050 | 0.2038 | 0.2010 |
| Ionisation potential (I) | 0.2299 | 0.2279 | 0.2293 | 0.2303 | 0.2311 |
| Electron affinity (A) | 0.0273 | 0.0258 | 0.0243 | 0.0265 | 0.0301 |
| Electronegativity (χ) | 0.1286 | 0.1268 | 0.1268 | 0.1284 | 0.1306 |
| Hardness (η) | 0.1013 | 0.1010 | 0.1025 | 0.1019 | 0.1005 |
| Softness (S) | 4.9358 | 4.9504 | 4.8780 | 4.9067 | 4.9751 |
TABLE 2. Calculated electronic parameters.
Mulliken population analysis
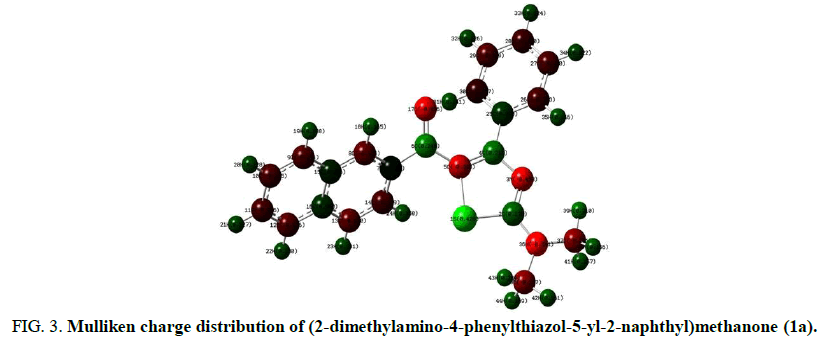
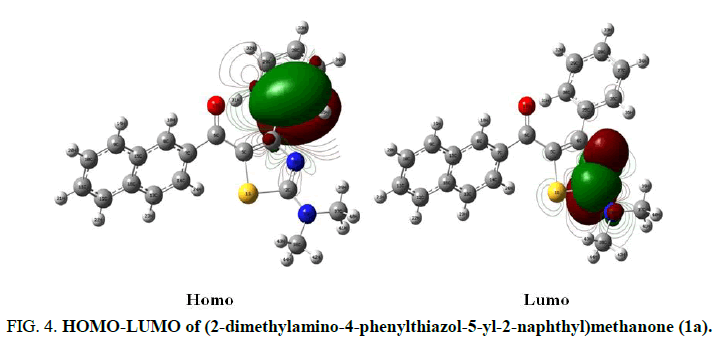
Mulliken population analysis is a quantum chemical calculation that provides charge distribution in atoms from calculations carried out by the methods of computational chemistry [18]. Mulliken charges are particularly based on a Linear Combination of Atomic Orbitals molecular orbital (LCAO) method (Figure 3). The HOMO is nucleophilic or electron donating and the LUMO is electrophilic or electron accepting. The energy gap between HOMO and LUMO of a neutral system, i.e. the difference between the Highest Occupied Molecular Orbital (HOMO) and Lowest Unoccupied Molecular Orbital (LUMO) is related with polarisability, chemical reactivity and kinetic stability [19]. For example HOMO-LUMO of (2-dimethylamino-4-phenylthiazol-5-yl-2-naphthyl)methanone (1a) (Figure 4).
FIG 3: Mulliken charge distribution of (2-dimethylamino-4-phenylthiazol-5-yl-2-naphthyl)methanone (1a).
Vibrational analysis
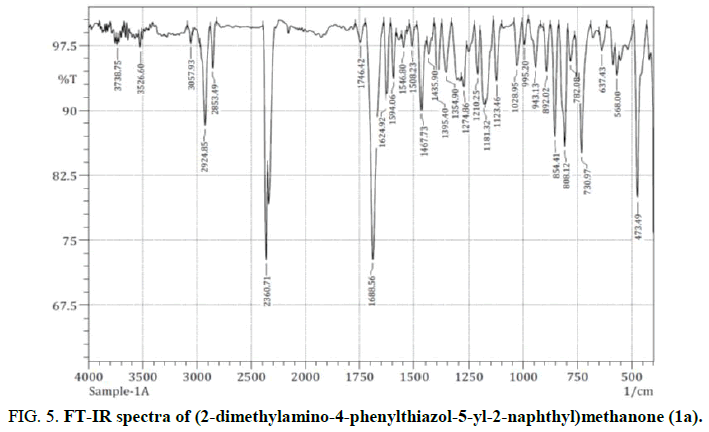
Investigations of radiation stimulated adsorption, radiolysis of hydrocarbons on metal surfaces and radiation hydrogenation of these surfaces using IR spectroscopy (reflection-absorption IR spectroscopy). Because of the alteration in the dipole moment, the absorption of Energy (E) that coincides with the vibrational frequency (V) would produce molecular vibration. Stretching (where the bond length and distance between the two atoms are both influenced) and bending (where the angle between the two bonds is varied) are the two basic vibrational modes [20]. The (2-dimethylamino-4-phenylthiazol-5-yl-2-naphthyl)methanone (1a) was described below. The FT-IR spectra of the molecules phenylthiazolylnaphthylmethanone (1a-1e) were observed. Figure 5 depicts the FT-IR spectra of (2-dimethylamino-4-phenylthiazol-5-yl-2-naphthyl)methanone (1a).
C=O vibrations
The oxidation and reduction potential, as well as the number of rings, are multiple valued functions of the C=O frequency. The C=O stretching vibration was detected between 1600 cm-1 and 1750 cm-1. The amazing carbonyl group’s conjugation shows a C=O vibration at 1688 cm-1.
C-N vibrations
The involvement of the C-N ligands in these synthetic system’s vibrational relaxation and by comparison, learn more about the behavior of the enzyme. The 1385 cm-1 ± 85 cm-1 frequency range of the C-N stretching vibration. In accordance with B3LYP analysis, the predicted wave numbers for the band detected at 1354 cm-1 and 1244 cm-1. The stretching mode C=N (aromatic) first appears in the range 1490 cm-1-1570 cm-1. The fundamental modes identified as C=N stretching modes at 1546 cm-1 in the IR spectra respectively.
C-S vibrations
Generally, the C-S stretching vibration turned into mentioned at 750 cm-1-600 cm-1. In the prevailing work, the C-S stretching vibration of (2-dimethylamino-4-phenylthiazol-5-yl-2-naphthyl)methanone (1a) compound is 730 cm-1 respectively. From theoretically computed by way of B3LYP/6-311G technique using the 1a compound is C-S stretching vibration is 698 cm-1.
C-C vibrations
The C-C fragrant stretching vibration attributes bands in determining FT-IR spectrum, causing the spectral range from 1600 cm-1-1200 cm-1. The (2-dimethylamino-4-phenylthiazol-5-yl-2-naphthyl)methanone (1a) compound is a C-C vibration band specified at 1435 cm-1 and 1354 cm-1.
C-H vibrations
The fragrant of aromatic and aliphatic C-H stretching vibration observed within the frequency range of 3100 cm-1-3000 cm-1 and 3200 cm-1-2850 cm-1. The bands captured at 3057 cm-1, 2924 cm-1 successively in IR spectrum had been assigned for aromatic and aliphatic C-H stretching vibration of our (2-dimethylamino-4-phenylthiazol-5-yl-2-naphthyl)methanone (1a) compound. The theoretically calculated C-H stretching vibrations vary between 3100 cm-1, 3075 cm-1, 3066 cm-1, 3033 cm-1 by B3LYP/6-311G strategies. The C-H in plane bending vibration appears in the region 1300-1000 cm-1. The C-H in plane bending vibration in our compound (1a) determined at 1274 cm-1, 1210 cm-1 inside the FT-IR spectrum. The C-H out plane bending vibration arises in the region 900 cm-1-675 cm-1. In strong band discovered at 854 cm-1, 808 cm-1 in IR spectrum is assigned to C-H out plan bending vibration.
NMR spectral data
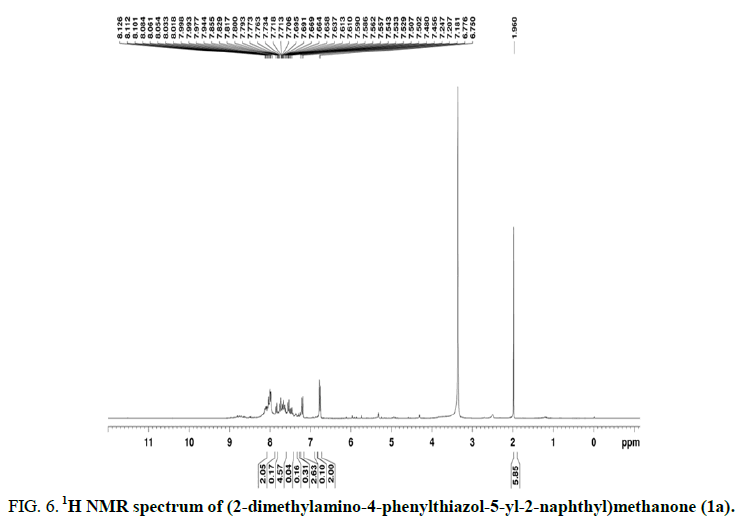
The 1H NMR (400 MHz, DMSO-d6) spectrum of the compound (2-dimethylamino-4-phenylthiazol-5-yl-2-naphthyl)methanone (1a) showed a six-hydrogen singlet at δ 1.960 ppm representing the CH proton of N(CH3)2. The two hydrogen doublet arises at δ 6.763 due to the presence of two phenyl hydrogen and three hydrogen multiplet at δ 7.181-7.247 is due to the presence of two phenyl hydrogen and H-1 of naphthalene. The hydrogen is multiplet in δ 7.610-7.691(5H) one phenyl hydrogen, H-3, H-5, H-6, H-7 of naphthalene and δ 7.894-8.084(2H) in H-4 and H-8 of naphthalene region. From the evidences, the compound wasformulated as (2-dimethylamino-4-phenylthiazol-5-yl-2-naphthyl)methanone (1a) (Figure 6).
FIG 6: 1H NMR spectrum of (2-dimethylamino-4-phenylthiazol-5-yl-2-naphthyl)methanone (1a).
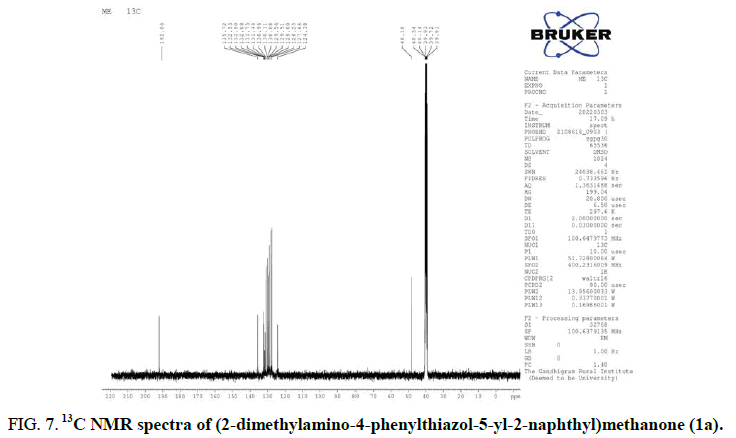
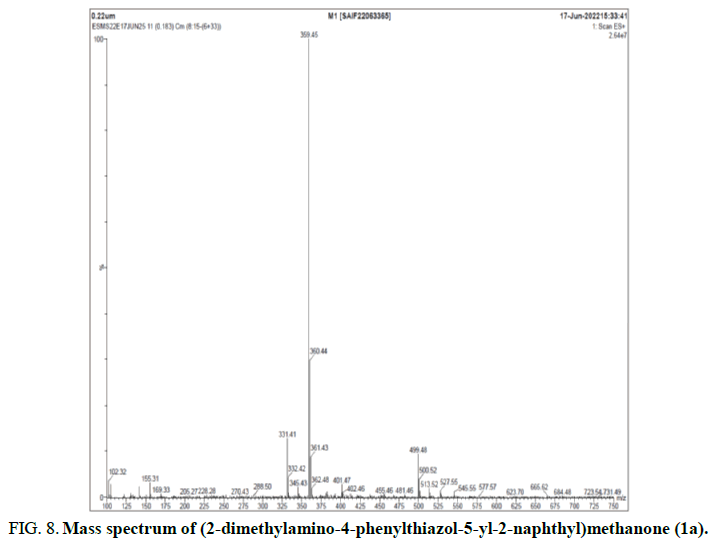
The 13C NMR spectrum suggests twenty two peaks, thus accounting for all the twenty two carbons (Figure 7). The ESI mass spectrum exhibits a MH+ peak at 359, which confirms the molecular mass of the compound to be 358 which is in accordance with the elemental analysis data (Figure 8). A similar pattern becomes determined in FT-IR, 1H NMR, 13C NMR and Mass of all the title compounds.
FIG 7: 13C NMR spectra of (2-dimethylamino-4-phenylthiazol-5-yl-2-naphthyl)methanone (1a).
Results and Discussion
(2-dimethylamino-4-phenylthiazol-5-yl-2-naphthyl)methanone (1a): Yellow solid, yield 68%, anal. Found: C, 73.69, H, 5.01, N, 7.79%. Calculated for C22H18N2OS (358.46): C, 73.71, H, 5.06, N, 7.82%. IR (KBr, vmax, cm-1): 3738, 3526, 3057, 2924,2853, 2360, 1746, 1688, 1624, 1594, 1546, 1508, 1467, 1435, 1395, 1354, 1274, 1210, 1181, 1123, 1028, 995, 943, 892, 854, 808, 782, 730, 637 cm-1; 1H NMR (400 MHz, DMSO-d6, ppm): δ 1.960 (6H, s, N(CH3)2), 6.763 (2H, J= 10.4 Hz, d, ArH), 7.181-7.247 (3H, m, 2H of ArH, H-1 of naphthalene), 7.610-7.691 (5H, m, 1H of ArH, H-3, H-5, H-6, H-7 of naphthalene), 7.894-8.084 (2H, m, H-4, H-8 of naphthalene); 13C-NMR (400 MHz, DMSO-d6, ppm) δ: 39.5, 39.7, 39.9, 40.1, 40.3, 48.1(N-CH3),124.3, 127.6 (naphthalene), 128.2, 129.0, 129.5, 129.5 (Phenyl), 130.0, 130.1, 130.9, 131.4, 131.7, 132.0, 132.5, 132.53, 135.7 (thiazole), 192.0 (C=O); MS (ESI-MS) m/?: 359 (MH+).
(2-diethylamino-4-phenylthiazol-5-yl-2-naphthyl)methanone (1b): Yellow solid, yield 66%, anal. Found: C, 74.53, H, 5.72, N, 7.22%. Calculated for C24H22N2OS (386.51): C, 74.58, H, 5.74, N, 7.25%. IR (KBr, vmax, cm-1): 3747, 3056, 2924, 2851,2310, 1747, 1688, 1625, 1593, 1536, 1467, 1394, 1356, 1306, 1273, 1240, 1211, 1172, 1123, 1075, 1027, 996, 943, 892, 853, 807, 730, 637; 1H NMR (400 MHz, DMSO-d6, ppm): δ 1.239 (6H, s, (2CH3), 2.511 (4H, s, N(CH2)2), 6.949-8.114 (12H, m, 5Hof ArH, 7H of naphthalene).
2-naphthyl(4-phenyl-2-pyrrolidin-1-ylthiazol-5-yl)methanone (1c): Yellow solid, yield 68%, anal. Found: C, 74.95, H, 5.23,N, 7.26%. Calculated for C24H20N2OS (384.49): C, 74.97, H, 5.24, N, 7.29%. IR (KBr, vmax, cm-1): 3751, 3615, 3275, 2923,2852, 2313, 1796, 1747, 1688, 1625, 1595, 1545, 1509, 1467, 1395, 1355, 1275, 1248, 1210, 1174, 1123, 1086, 1028, 996, 943,892, 854, 808, 730, 635; 1H NMR (400 MHz, DMSO-d6, ppm): δ 1.188 (4H, J= 7 Hz, t, N(CH2)2), 1.812 (4H, J= 5.6 Hz, t,(2CH2), 7.002 (2H, J= 8.4 Hz, d, ArH), 7.247-7.348 (3H, m, 2H of ArH, H-1 of naphthalene), 7.482-7.761 (5H, m, 1H of ArH, H-3, H-5, H-6, H-7 of naphthalene), 7.796-8.177 (2H, m, H-4, H-8 of naphthalene).
2-naphthyl(4-phenyl-2-piperidin-1-ylthiazol-5-yl)methanone (1d) : Yellow solid, yield 68%, Anal. Found: C, 75.33, H, 5.55,N, 7.02%. Calculated for C25H20N2OS (398.52): C, 75.35, H, 5.56, N, 7.03%. IR (KBr, vmax, cm-1): 3746, 3525, 3330, 3057,2923, 2851, 2314, 1923, 1747, 1687, 1624, 1593, 1510, 1466, 1395, 1355, 1292, 1250, 1210, 1171, 1123, 1088, 1027, 995, 943,891, 853, 807, 730, 634; 1H NMR (400 MHz, DMSO-d6, ppm): δ 1.197 (4H, J= 1.8 Hz, t, N(CH2)2), 2.345-2.701 (2H, m, CH2),3.112 (4H, J= 7.2 Hz, t, (2CH2), 6.957 (2H, J= 2.4 Hz, d, ArH), 7.269-7.304 (3H, m, 2H of ArH, H-1 of naphthalene), 7.509-7.861 (5H, m, 1H of ArH, H-3, H-5, H-6, H-7 of naphthalene), 7.898-8.076 (2H, m, H-4, H-8 of naphthalene).
(2-morpholin-1-yl-4-phenylthiazol-5-yl)-2-naphthylmethanone (1e): Yellow solid, yield 68%, anal. Found: C, 71.96, H, 5.01, N, 6.97%. Calculated for C24H20N2O2S (400.49): C, 71.98, H, 5.03, N, 6.99%. IR (KBr, vmax, cm-1): 3747, 3272, 2923, 2851,2312, 1916, 1746, 1690, 1625, 1595, 1536, 1468, 1393, 1358, 1288, 1210, 1170, 1122, 1087, 1027, 996, 943, 892, 854, 808, 730, 692, 635; 1H NMR (400 MHz, DMSO-d6, ppm): δ 1.184 (4H, J= 6.4 Hz, t, N(CH2)2), 2.590 (4H, J= 8.3 Hz, t, O(CH2)2), 7.121(2H, J= 7.6 Hz, d, ArH), 7.520-7.717 (3H, m, 2H of ArH, H-1 of naphthalene), 7.755-7.851 (5H, m, 1H of ArH, H-3, H-5, H-6, H-7 of naphthalene), 7.896-8.168 (2H, m, H-4, H-8 of naphthalene).
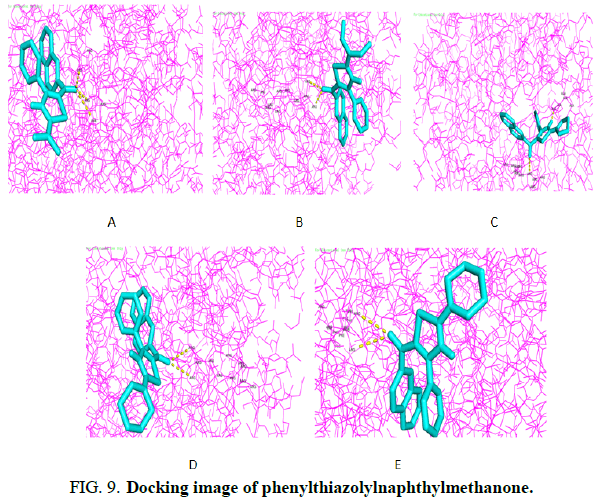
Molecular docking
Molecular docking studies were conducted. The interaction of molecules with proteins can be analyzed using the most popular molecular docology. Researchers use docking in cancer research as it provides important insights into ligand binding mechanisms, protein-ligand interactions and information on ligand positions in its target. In Phenylthiazolylnaphthylmethanone derivatives selected 107G (PDB ID) using a visual PyRx test tool (Figure 9). Hydrogen bonding interactions and docking scores of phenylthiazolylnaphthylmethanone outflow are summarized in (Table 3). The above five compounds of 1c and 1d are the most active ligand and the active site of this ligand that contains two hydrogen bond interactions. The observed 1c and 1d amino acid residues are ARG-342, ALA-584(1c) and ARG-577(1d).
| Compound | Docking score (Kcal/mol) | Residue involved in hydrogen bonding |
|---|---|---|
| 1a | -7.6 | ARG-577, ARG-577, ASN-95 |
| 1b | -7.9 | ARG-577, ARG-577 |
| 1c | -8.7 | ARG-342, ALA-584 |
| 1d | -8.7 | ARG-577, ARG-577 |
| 1e | -8.4 | PHE-224, ARG-577 |
TABLE 3. Docking score and hydrogen bonding interactions of phenylthiazolylnaphthylmethanone.
Lipinski rule of five
A qualitative concept used in drug design is druglikeness with respect to factors like ligand efficiency and lipophilic efficiency. Druglikeness is estimated from the molecular structure before the substance is even synthesized and tested biologically. Lipinski rule of five is used to evaluate lipophilic efficiency, i.e. drug likeness or determine if a chemical compound has properties of certain biological activity. The phenylthiazolylnaphthylmethanone which are obeying Lipinski rule of five for the docking studies and are listed in Table 4.
| Compound | Molecular weight <500 Dalton | HB donor <5 | HB acceptor <10 | Log P <5 | Molecular refractivity 40-130 |
|---|---|---|---|---|---|
| 1a | 358 | 1 | 3 | 3.29 | 108.59 |
| 1b | 386 | 1 | 3 | 3.31 | 117.83 |
| 1c | 384 | 1 | 3 | 3.22 | 115.71 |
| 1d | 398 | 1 | 3 | 4.15 | 120.33 |
| 1e | 400 | 1 | 4 | 4.05 | 117.30 |
TABLE 4. Lipinski rule of phenylthiazolylnaphthylmethanone.
Antioxidant studies
The newly synthesized compound was screened for his or her antioxidant potentials, for DPPH (2-dipheny-l,2-picrylhydrazyl hydrate) assay the ascorbic acid was used as reference standard. The ascorbic acid inventory solution became organized in distilled water (1 mg/ml; w/v). A 60 μM solution of DPPH in methanol was freshly organized and a 200 μl of this solution was mixed with 50 μl of test sample at various concentrations (1.56 μg/ml, 3.12 μg/ml, 6.25 μg/ml, 12.5 μg/ml, 25 μg/ml, 50 μg/ml, 100 μg/ml, 200 μg/ml, 400 μg/ml, 800 μg/ml). The plates have been saved within the dark for 15 minutes at room temperature and the decrease in absorbance was measured at 515 nm. Control was prepared with DPPH solution simplest, without any extract or ascorbic acid. 95% methanol was used as a blank.
The radical scavenging pastime was calculated with the aid of the subsequent formula:

Antioxidant activity evaluation
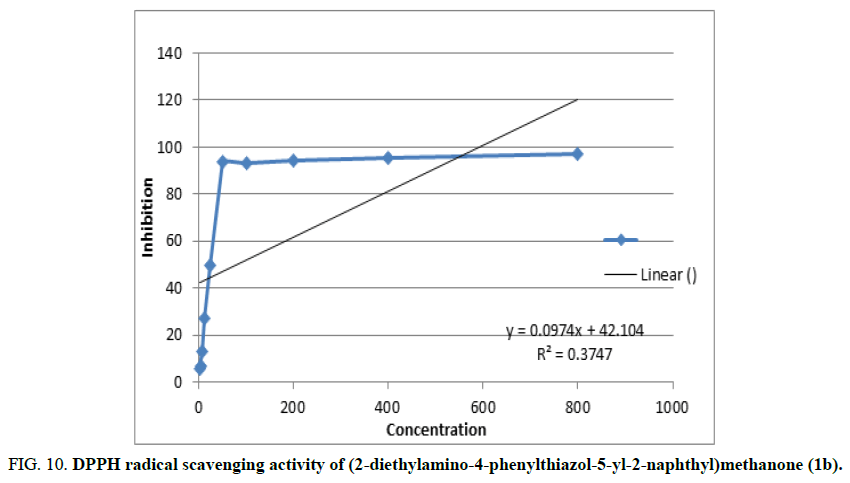
The DPPH assay was used to evaluate the free radical scavenging abilities of the phenylthiazolylnaphthylmethanone derivatives. By measuring the IC50 (μM) values in table, the antioxidant activity of the produced compounds was also ascertained (Table 5). With IC50 values of 81 μM and 89 μM, respectively, the substances (2-diethylamino-4-phenylthiazol-5-yl-2-naphthyl)methanone (1b) and 2-naphthyl(4-phenyl-2-pyrrolidin-1-ylthiazol-5-yl)methanone (1c) have excellent antioxidant activity (Figure 10). Compounds 1a, 1e and 1d have also shown moderate scavenging action, with IC50 values of 89 μM, 109 μM and 132 μM, respectively. The standard (ascorbic acid) was given an IC50 value of 90 μM.
| Compound | IC50 value (µM) |
|---|---|
| 1a | 89 |
| 1b | 63 |
| 1c | 81 |
| 1d | 132 |
| 1e | 109 |
| Ascorbic acid (Std) | 90 |
TABLE 5. Antioxidant activities of phenylthiazolylnaphthylmethanone.
FIG 10: DPPH radical scavenging activity of (2-diethylamino-4-phenylthiazol-5-yl-2-naphthyl)methanone (1b).
Anticancer activity
Chemotherapy, also known as chemo or anti-cancer drugs, is a drug used to destroy, kill, reduce or reduce the growth of cancer cells. There are more than a hundred chemo medicines. The synthesized compounds with high antioxidant activities were examined for their anticancer activity against the SKMEL cell line (human skin cancer) in vitro by the MTT assay method. The anti-cancer function of compound (2-diethylamino-4-phenylthiazol-5-yl-2-naphthyl)methanone (1b) was obtained using SKMEL cancer cells (human skin cancer) controlled by different concentrations of the sample (Table 6). The mobile viability turned into expressing the use of the following components:
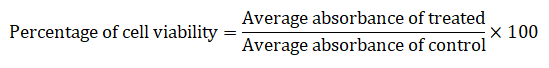
| Concentration (µg/mL) | Percentage viability |
|---|---|
| 1b | |
| 6.25 | 68.76 |
| 12.5 | 72.45 |
| 25 | 65.54 |
| 50 | 52.43 |
| 100 | 38.98 |
| IC50 | 65.91 |
TABLE 6. Anticancer studies of (2-diethylamino-4-phenylthiazol-5-yl-2-naphthyl)methanone (1b).
Evaluation of in vitro cytotoxic activity
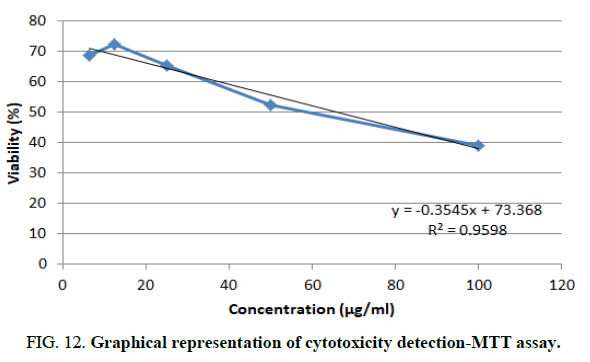
The cytotoxic activity and IC50 value of the (2-diethylamino-4-phenylthiazol-5-yl-2-naphthyl)methanone (1b) compound was calculated from the MTT assay. The morphological changes of SKMEL cell line (human skin cancer) after 24 h incubation of (2-diethylamino-4-phenylthiazol-5-yl-2-naphthyl)methanone (1b) compound is plotted in (Figure 11). A 6.25 μg/mL reduces the cell viability to 68.76% μg/mL, 12.5 μg/mL reduces the cell viability to 72.45% μg/mL, 25 μg/mL reduces the cell viability to 65.54% μg/mL, 50 μg/mL reduces the cell viability to 52.43% μg/mL and 100 μg/mL reduces the cell viability to 38.98%. In anticancer study of (2-diethylamino-4-phenylthiazol-5-yl-2-naphthyl)methanone (1b) exhibited the highest anticancer activity against SKMEL cell lines (human skin cancer) with IC50 values 65.91 μM are very good anticancer compound (Figure 12).
FIG 11: Morphological changes of SKMEL cell line in control cells.
Conclusion
In conclusion, a new series of extracts from phenylthiazolylnaphthylmethanone have been successfully synthesized compounds with high yields and are characterized by IR, 1H-NMR, 13C-NMR and mass analyzes. The computational studies, like structural optimization, vibrational assignments, Mulliken charge distribution and molecular orbital energies theoretically helped to find the arrangement of atoms in phenylthiazolylnaphthylmethanone. The electronic parameters correspond to HOMO and LUMO show the phenylthiazolylnaphthylmethanone have a low energy gap, hence they are softer and highly reactive compounds. These parameters were calculated using the Gaussian 09 software. Docking study of these phenylthiazolylnaphthylmethanone derivative shows as good oral drugs candidates. The antioxidant activity of composite compounds was determined by the DPPH activity for free radical disposal. The highest free radical scavenging activity was achieved for compound (2-diethylamino-4-phenylthiazol-5-yl-2-naphthyl)methanone (1b) and their IC50 value is 63 μM. The compound (2-diethylamino-4-phenylthiazol-5-yl-2-naphthyl)methanone (1b) displayed the highest anticancer activity in SKMEL cell line (human skin cancer) and their IC50 value is 65.91 μM.
Acknowledgement
For the spectral and analytical data, the authors are grateful to CDRI Lucknow.
Declaration of competing interests
All authors declare no competing interest regarding the publication of this article.
References
- Siddiqui N, Arshad MF, Ahsan W, et al. Synthesis, characterization and antimicrobial evaluation of some new 1,3- thiazole-2,4-diamine derivatives. Acta Pol Pharm. 2010;67(3):239-246.
[Google Scholar] [PubMed]
- Pattan SR, Dighe NS, Nirmal SA, et al. Synthesis and biological evaluation of some substituted amino thiazole derivatives. Asian J Res Chem. 2009;2(2):196-201.
- Farouk Elsadek M, Mohamed Ahmed B, Fawzi Farahat M. An overview on synthetic 2-aminothiazole based compounds associated with four biological activities. Molecules. 2021;26(5):1449.
[Crossref] [Google Scholar] [PubMed]
- Gomha SM, Ahmed SA, Abdelhamid AO. Synthesis and cytotoxicity evaluation of some novel thiazoles, thiadiazoles and pyrido (2,3-d) (1,2,4) triazolo (4,3-a) pyrimidin-5(1H)-ones incorporating triazole moiety. Molecules. 2015;20(1):1357-1376.
[Crossref] [Google Scholar] [PubMed]
- Kupwade RV, Khot SS, Kulkarni MA, et al. Diethylamine Dess-Martin periodinane: An efficient catalyst-oxidant combination in a sequential, one-pot synthesis of difficult to access 2-amino-3,5-dicarbonitrile-6-sulfanylpyridines at ambient temperature. R Soc Chem. 2017;7(62):38877-38883.
- Li Petri G, Raimondi MV, Spano V, et al. Pyrrolidine in drug discovery: A versatile scaffold for novel biologically active compounds. Top Curr Chem. 2021;379(5):34.
[Crossref] [Google Scholar] [PubMed]
- Tzara A, Xanthopoulos D, Kourounakis AP. Morpholine as a scaffold in medicinal chemistry: An update on synthetic strategies. Chem Med Chem. 2020;15(5):392-403.
[Crossref] [Google Scholar] [PubMed]
- Masquelin T, Obrecht D. A new general three component solution phase synthesis of 2-amino-1,3-thiazole and 2,4-diamino-1,3-thiazole combinatorial libraries. Tetrahedron. 2001;57(1):153-156.
- Zhang H, Liu CS, Bu XH, et al. Synthesis, crystal structure, cytotoxic activity and DNA binding properties of the copper (II) and zinc (II) complexes with 1-[3-(2-pyridyl)pyrazol-1-ylmethyl]naphthalene. J Inorg Biochem. 2005;99(5):1119-1125.
[Crossref] [Google Scholar] [PubMed]
- Saeidian H, Sadeghi A, Mirjafary Z, et al. Solvent free synthesis of 2-amino-3-aryl-5-substituted thiophenes as anti-inflammatory agents using KF-Al2O3 under microwave irradiation. Int J Rapid Commun Synth Org Chem. 2008;38(12):2043-53.
- Demirayak S, Sahin Z, Ertas M, et al. Novel thiazole-piperazine derivatives as potential cholinesterase inhibitors. J Heterocycl Chem. 2019;56(12):3370-3386.
- Pearson RG. Absolute electronegativity and hardness correlated with molecular orbital theory. Proc Natl Acad Sci USA. 1986;83(22):8440-8441.
[Crossref] [Google Scholar] [PubMed]
- El-Azazy M. Introductory chapter: Infrared spectroscopy-A synopsis of the fundamentals and applications. Infrared Spectrosc. 2018;1.
- Josien ML, Fuson N, Lebas JM, et al. An infrared spectroscopic study of the carbonyl stretching frequency in a group of ortho and para quinones. J Chem Phys. 1953;21:331.
- Kaziannis S, Wright JA, Candelaresi M, et al. The role of CN and CO ligands in the vibrational relaxation dynamics of model compounds of the (FeFe)-hydrogenase enzyme. Phys Chem Chem Phys. 2011;13(21):10295-10305.
[Crossref] [Google Scholar] [PubMed]
- Khalaji AD, Chermahini AN, Fejfarova K, et al. Synthesis, characterization, crystal structure and theoretical studies on Schiff-base compound 6-((5-Bromo-pyridin-2-yl) iminomethyl) phenol. Struct Chem. 2010;21:153-157.
- Arjunan V, Rani T, Mythili CV, et al. Synthesis, FTIR, FT-Raman, UV-visible, ab initio and DFT studies on benzohydrazide. Spectrochim Acta A Mol Biomol Spectrosc. 2011;79(3):486-496.
[Crossref] [Google Scholar] [PubMed]
- Ambujakshan KR, Tresavarghese H, Mathew S, et al. Vibrational spectroscopic studies and theoretical calculations of 2-phenyl-4H-3,1-benzoxazin-4-one. Orien J Chem. 2008;24(3):865-874.
- Lipinski CA. Drug like properties and the causes of poor solubility and poor permeability. J Pharmacol Toxicol Methods. 2000;44(1):235-249.
[Crossref] [Google Scholar] [PubMed]
- Brand-Willams W, Cuvelier ME, Beret C. Use of a free radical method to evaluate antioxidant activity. LWT-Food Sci Technol. 1995;28(1):25-30.