Original Article
, Volume: 15( 2)Simultaneous Estimation of Mometasone Furoate and Salicylic Acid in Topical Formulation by UV-Visible Spectrophotometry
- *Correspondence:
- Choudhari VP, MAEER’s, Maharashtra Institute of Pharmacy, Kothrud, Pune, Maharashtra, India, Tel: 9049086245; E-mail: vicharevijaya11@gmail.com
Received: March 07, 2017; Accepted: May 09, 2017; Published: May 11, 2017
Citation: Vichare VS, Choudhari VP, Reddy MV. Simultaneous Estimation of Mometasone Furoate and Salicylic Acid in Topical Formulation By UV-Visible Spectrophotometry. Int J Chem Sci 2017;15(2):129.
Abstract
A UV-Visible spectrophotometric method was developed for simultaneous estimation of Mometasone furoate and Salicylic acid in topical formulation. Absorbance correction method was used for estimation. Since both drugs are present in vast concentration difference, estimation was done by standard addition technique. Methanol was used as a solvent. From overlain spectra, wavelengths 300 nm and 250 nm were selected for analysis. The % drug content in marketed formulation was found to be 97.54 and 103.85 for Mometasone furoate and Salicylic acid respectively. The developed method was validated as per ICH guidelines. The method was found to be linear in the concentration range of 1-10 μg/ml and 5-50 μg/ml for Mometasone furoate and Salicylic acid respectively. Accuracy of the method was determined by recovery studies. Precision of method was estimated by repeatability and intermediate precision studies. From results of validation parameters, it was found that, the developed method is accurate, precise and specific. Hence, it can be used successfully for routine quality control analysis, for simultaneous estimation of Mometasone furoate and Salicylic acid in topical formulation.
Keywords
Mometasone furoate; Salicylic acid; UV-Visible spectrophotometry; Validation
Introduction
Mometasone furoate [MF] ( Figure 1) is chemically 9, 21-dichloro-17α-[(2-furanylcarbonyl) oxy]-11β-hydroxy-16 α-methylpregna-1,4-diene-3,20-dione, a high potency glucocorticoid. It has anti-inflammatory, immunosuppressive, vasoconstriction and antiproliferative actions. It is effective for treatment of skin diseases like eczema, dermatitis and psoriasis. Corticosteroids are thought to act by the induction to phospholipase A2 inhibitory proteins, collectively called lipocortin’s. It is postulated that these proteins control the biosynthesis of potent mediators of inflammation [1,2]. It is official in IP, BP and USP [3-5].
Salicylic acid [SA] (Figure 2) is chemically 2-hydroxy benzoic acid, has antiseptic, antifungal and keratolytic properties. It is used to treat warts, psoriasis, corns and other skin conditions. It works by softening and loosening dry, scaly, or thickened skin so that it falls off or can be removed easily [1,2]. It is official in IP, BP and USP [3-5]. Combination of Mometasone furoate and Salicylic acid is available in market as an ointment and used for the treatment of skin inflammation, skin diseases, acne, skin redness and other conditions.
Literature survey revealed few UV-Visible spectrophotometric methods for estimation of MF in combination with other drugs like Mupirocin, Formoterol, Terbinafine etc., [6]. Also, there are few stabilities indicating HPLC methods reported for MF and SA in combination.
There is a single ratio derivative UV-Visible spectrophotometric method reported for simultaneous determination of MF and SA. This method involves calculation of ratio absorbance’s and conversion to first order derivative [7]. It is based on standard addition technique. The method reported is tedious and time consuming. So, the study was aimed to develop simple and rapid UV-Visible spectrophotometric method for simultaneous estimation of MF and SA.
Experimental Procedure
Instrumentation
Shimadzu-1800, Japan UV-Visible Spectrophotometer with 10 mm matched quartz cells was used. All weighing was done on Shimadzu model AUX 220 electronic balance and Ultra Sonicator.
Reagents and chemicals
Pure samples of MF and SA were kindly supplied as a gift sample by Nulife Pharmaceuticals, Pimpri, Pune. All reagents used were of analytical grade. Momate-S ointment manufactured by Glenmark Pharmaceuticals containing MF 0.1% w/w and SA 5% w/w was purchased from local market [8].
Preparation of standard solutions
Standard stock solutions containing 1000 μg/ml of MF and 5000 μg/ml of SA were prepared separately in methanol. Appropriate dilutions were done using methanol to get 1-10 μg/ml of MF and 5-50 μg/ml of SA separately.
Preparation of laboratory mixtures
Standard stock mixture solution containing 1000 μg/ml of MF and 5000 μg/ml of SA was prepared in methanol. Appropriate dilutions were done using methanol to get 1-10 μg/ml of MF and 5-50 μg/ml of SA in mixture.
Method Development
Selection of wavelengths for analysis
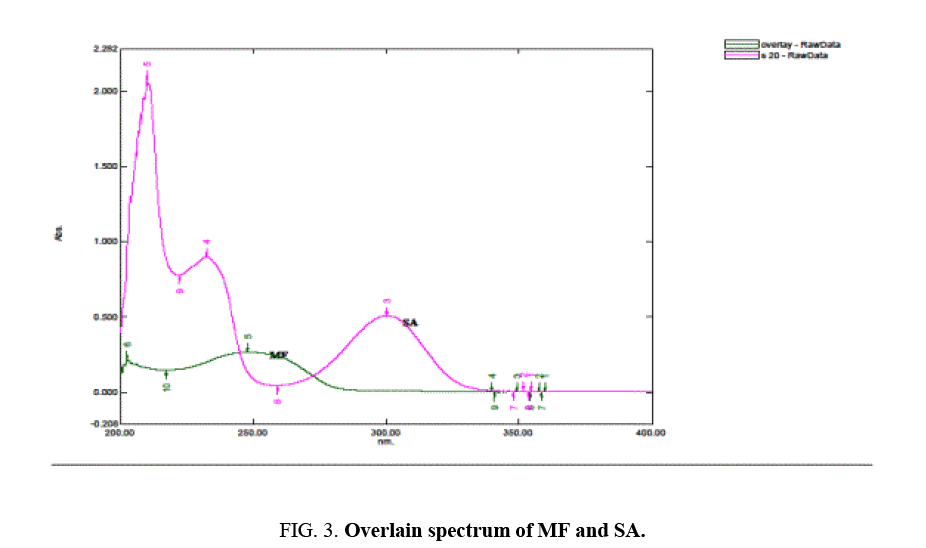
Solutions containing 4 μg/ml of MF and 20 μg/ml of SA separately were scanned in spectrum mode. An overlain spectrum ( Figure 3) was obtained and wavelengths 300 nm (Zero absorbance of MF) and 250 nm (both drugs showing absorbances) were selected for analysis. From the overlain spectra Absorption correction method was chosen for analysis.
Determination of absorptivity values
Solutions containing 1-10 μg/ml of MF and 5-50 μg/ml of SA separately were scanned and absorbances were measured at selected wavelengths. Calibration curves were obtained by plotting Ab Vs Conc. and absorptivity values were obtained.
Analysis of laboratory mixture
Laboratory mixture containing 4 μg/ml of MF and 20 μg/ml of SA was scanned and absorbances were measured at selected wavelengths. Calculations were done by following formulae,
Concentration of SA was calculated by formula,
A (300 nm) = a. b. c1 --------------------------------------------- (Formula 1)
Where
A (300 nm) = Absorbance of lab mixture at 300 nm
a = Absorbtivity of SA at 300 nm
b = Path length (1 cm)
c1 = Concentration of SA
Concentration of MF was calculated by formula,
A (250 nm) = a1. b. c1 + a2. b. c2 -------------------------------- (Formula 2)
Where,
A (250 nm) = absorbance of lab mixture at 250 nm
a1 = Absorbtivity of SA at 250 nm
c1 = Concentration of SA calculated from formula 1
a2 = Absorbtivity of MF at 250 nm
c2 = Concentration of MF
b = Path length (1 cm)
Analysis of marketed formulation
1 gm of ointment was accurately weighed. Accurately weighed 9 mg of pure sample of MF was added by standard addition technique to maintain ratio MF:SA (1:5) and dissolved in 50 ml methanol with the help of ultrasonicator for 20 min. The solution was filtered through 0.45 μm filter. Appropriate dilutions were done to get concentration of 4 μg/ml of MF and 20 μg/ml of SA. Absorbances were measured at selected wavelengths and % drug contents were calculated by above given formulae.
Method Validation
Linearity and range
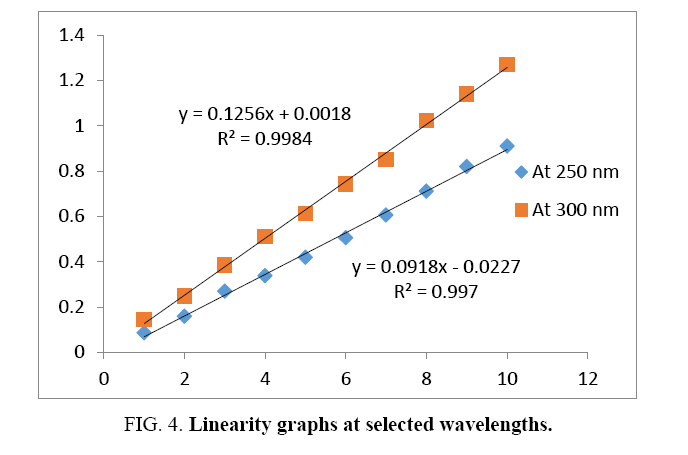
A series of dilutions of mixture containing 1-10 μg/ml of MF and 5-50 μg/ml of SA were prepared from standard stock mixture solution. All dilutions were scanned and absorbances were measured at selected wavelengths. Graphs were plotted. Regression equation and Correlation coefficients were obtained ( Figure 4).
Accuracy
Accuracy of the method was determined by recovery studies at three different levels. Known amount of pure drugs were spiked to sample at 75%, 100% and 125% level and analyzed by the developed method in triplicate.
Precision
Repeatability of the method was assured by repeating the developed procedure six times on the same day. Intermediate precision was estimated by inter-day analysis where developed procedure was repeated on three consecutive days in triplicate. Results were expressed as the standard deviation and % RSD.
Result and Discussion
Absorption correction method development
Since both drugs are present in vast concentration difference standard addition technique was applied to maintain ratio MF:SA (1:5). From the overlain spectrum, it was found that at wavelength 300 nm MF is showing zero absorbance, hence at this wavelength concentration of SA can be determined directly. So, absorption correction method was selected for analysis.
Analysis of marketed formulation
Marketed formulation was analyzed by proposed method and amount of MF and SA were found to be 97.54% w/w and 103.85% w/w respectively as shown in Table 1.
| Formulation | Drug | Label Claim | %Drug Content ± SD | %RSD |
|---|---|---|---|---|
| Momate-S | MF | 1 mg | 97.54 ± 1.79 | 1.83 |
| SA | 50 mg | 103.85 ± 0.58 | 0.56 |
Table 1: Results of analysis of marketed formulation. n=6 replicates.
Method Validation
Linearity and range
The Beer’s law was obeyed in the concentration range of 1-10 μg/ml of MF and 5-50 μg/ml of SA at selected wavelengths. The values of correlation coefficients were found near to one, indicates linearity of proposed method. The values of correlation coefficients and other parameters are summarized in Table 2.
| Parameters | At 250 nm | At 300 nm |
|---|---|---|
| Regression equation | y=0.0918 × -0.0227 | y=0.1256× + 0.0018 |
| Correlation coefficient | 0.9970 | 0.9984 |
Table 2: Linearity parameters of proposed method.
Accuracy
Accuracy of the developed method was determined by recovery studies and results were calculated in terms of percentage recovery. The percentage recovery was found to be in a range of 96.09-101.14 and 101.70-103.8% w/w for MF and SA respectively. The percentage RSD values were found to be less than 2 confirm accuracy of the method. Results of accuracy study are shown in Table 3.
| Drug | % Level of addition | Amount of standard added (μg/ml) | % Recovery ± SD | % RSD |
|---|---|---|---|---|
| MF | 75 | 3 | 96.09 ± 0.65 | 0.67 |
| 100 | 4 | 99.37 ± 0.78 | 0.78 | |
| 125 | 5 | 101.14 ± 0.47 | 0.46 | |
| SA | 75 | 15 | 103.71 ± 0.30 | 0.29 |
| 100 | 20 | 101.70 ± 1.50 | 1.47 | |
| 125 | 25 | 103.80 ± 1.35 | 1.30 |
Table 3: Accuracy study data. n=3 replicates.
Precision
Results of repeatability and intermediate precision studies were estimated in terms of SD and % RSD. Values of % RSD were found to be less than 2 which prove that the proposed method is precise in nature. Results of repeatability study are shown in Tables 4 and 5.
| Drug | Amount of drug (μg/ml) | %Drug Content ± SD | %RSD |
|---|---|---|---|
| MF | 4 | 96.79 ± 0.7812 | 0.81 |
| SA | 20 | 104.17 ± 0.57 | 0.55 |
Table 4: Repeatability data. n=6 replicates.
| Drug | Amount of drug (μg/ml) | %Drug Content ± SD | %RSD |
|---|---|---|---|
| MF | 4 | 98.12 ± 0.832 | 0.85 |
| SA | 20 | 102.37 ± 0.79 | 0.77 |
Table 5: Inter-day data. n=9 replicates.
Specificity
Specificity is the ability of the analytical method to measure the response in presence of interferences. The UV spectra of drugs in sample solutions were found to be identical to the UV spectra obtained from standard solutions. No interference from formulation excipients was found, proves specificity of the proposed method.
Conclusion
A simple and rapid UV-Visible spectrophotometric was developed for simultaneous estimation of Mometasone furoate and Salicylic acid in topical ointment formulation. The developed method is based on Absorption correction method by standard addition technique. The proposed method was validated as per ICH guidelines and found to be accurate, precise and specific. Hence, it can be used successfully for routine quality control analysis.
Acknowledgements
Authors are thankful to Nulife Pharmaceuticals, Pimpri, Pune for providing gift samples of Mometasone furoate and salicylic acid. Authors are also thankful to management, PES’s Modern College of Pharmacy, Moshi, Pune for providing necessary facilities for research work.
References
- Block JH, BealeJM. Wilson and Gisvold’sTextbook of Organic Medicinal and Pharmaceutical Chemistry (11thedn).2004;813:233-4.
- TripathiKD.Essentials of Medical Pharmacology(7thedn), 2014;889:895-6.
- Indian Pharmacopoeia, Government of India, Ministry of Health and family welfare. Published by Indian Pharmacopoeia Commission, Gaziabad, Vol-II 2243-2244, Vol-III 2014;pp:2705-6.
- British Pharmacopoeia, The Stationary Office on behalf of Medicines and Health Care Products Regulatory Agency (MHRA), London, UK.2008;2:1485-6.
- United States Pharmacopoeia-34 National Formulary-29, United States Pharmacopoeial Convection, Rockville. 20113:3550-1.
- Choudhari V, PhadtareS, Gaikwad H, et al.Development and validation of RP-HPLC-PDA method for estimation of mometasonefuroate, salicylic acid, methyl paraben and propyl paraben in combined topical formulation by using design of experiment.Research and Reviews: Journal of Pharmaceutical Quality Assurance.2015;1:38-48.
- VananiDR, DesaiSD, PatelKG, et al. Application of ratio derivative spectrophotometry for simultaneous, determination of mometasonefuroate and salicylic acid in semisolid dosage form. Int J Anal Bioanal Chem.2013;3:67-71.
- Guideline IH. Validation of analytical procedures: Text and Methodology. ICH Harmonised Tripartite Guidelines, International Conference on Harmonization of Technical Requirements for Registration of Pharmaceuticals for Human Use. 2005.