Original Article
, Volume: 15( 4)Preparation, Characterization and Biological Studies on Pyridoxylidene-Cephalexin Schiff Base Complexes of Aryltellurium (IV)
- *Correspondence:
- Sapana Garg, Department of Chemistry, Maharshi Dayanand University, Rohtak, Haryana, India, Tel: 9896091443; E-mail: sapanagarg1511@gmail.com
Received: August 10, 2017; Accepted: August 20, 2017; Published: August 28, 2017
Citation: Deepak A, Chauhan S, Verma KK, et al. Preparation, Characterization and Biological Studies on Pyridoxylidene-Cephalexin Schiff Base Complexes of Aryltellurium (IV). Int J Chem Sci. 2017;15(4):182
Abstract
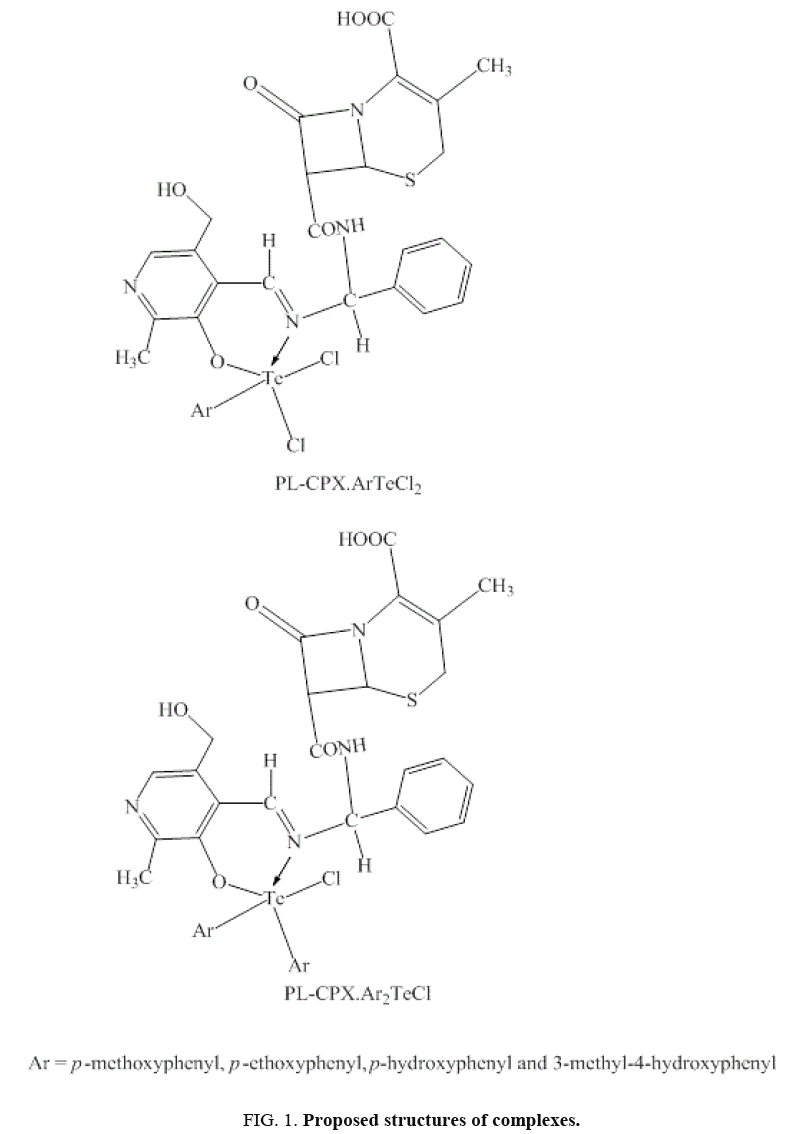
A novel Schiff base pyridoxylidene-cephalexin (HPL-CPX) synthesized from pyridoxal and cephalexin, form complexes with aryltellurium (IV) trichlorides and diaryltellurium (IV) dichlorides of the type PL-CPX.ArTeCl2 and PL-CPH.Ar2TeCl (where Ar=p-methoxyphenyl, p-ethoxyphenyl, p-hydroxyphenyl and 3-methyl-4-hydroxyphenyl). They have been characterized by elemental analyses, molar conductance, IR and 1H NMR spectroscopy. The spectral studies predict that the coordination of Schiff base to tellurium in a uninegative bidentate manner with-N, -O donor sites of the azomethine-N and deprotonated phenolic-O to give six membered chelate rings with penta coordinated tellurium centre having Ψ-trigonal bipyamidal geometry. The synthesized ligand, and its aryltellurium complexes was screened for its antimicrobial activity against various bacterial and fungal strains.
Keywords
Pyridoxal; Cephalexin; Aryltellurium (IV); Diaryltellurium (IV); Antibacterial; Antifungal activities
Introduction
Cehalexin is a beta-lactam antibiotic within a class of first generation cephalosporin [1,2]. It was first reported by Brotzu in 1948 and appeared to inhibit the synthesis of bacterial cell wall and act against both gram positive and gram-negative bacteria.
Pyridoxal is a close analog of pyridoxine (also known as vitamin B6) [3] and this makes the Schiff bases with cephalexin and their aryltellurium complexes attractive candidates for their biological activity [4]. Due to their structural varieties and very unique characteristics and because of their excellent donor abilities and chelating agents [5-9], the Schiff bases are the most versatile studied ligands in coordination chemistry [10,11]. Intramolecular hydrogen bonding between OH hydrogen and C=N nitrogen atom of Schiff base determines the biological properties [12] like antiviral [13-15], antifungal [16-18], antibacterial [19-21] and anticancer [22-26].
Also, the aryltellurium (IV) chlorides act as Lewis acids [27-43] and form complexes with several N?, O? and S? donor bases. In view of this we herein report some new complexes of aryltellurium (IV) trichlorides, ArTeCl3 and diaryltellurium (IV) dichlorides, Ar2TeCl2 with pyridoxylidene-cephalexin Schiff base (HPL-CPX).
Experimental
Materials and methods
All the chemicals used were of analytical reagent grade. All preparations were carried out under an atmosphere of dry nitrogen and the solvents used were purified and dried by standard method [44,45]. p-Methoxyphenyltellurium (IV) trichloride [46,47], bis (p-methoxyphenyl) tellurium (IV) dichloride [47,48], p-ethoxyphenyltellurium (IV) trichloride [49], bis (p-ethoxyphenyl) tellurium dichloride [49] p-hydroxyphenyltellurium (IV) trichloride [50], bis (p-hydroxyphenyl) tellurium (IV) dichloride [50], 3-methyl-4-hydroxyphenyltellurium (IV) trichloride [51] and bis (3-methyl-4-hydroxyphenyl) tellurium (IV) dichloride [51] were prepared by the reactions of TeCl4 with anisole, phenetole, phenol, o-cresol respectively, by the methods reported in the literature [46-51].
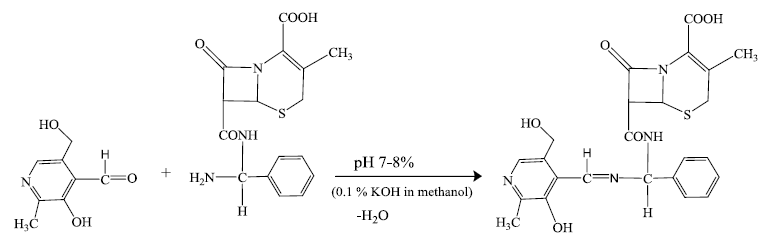
Preparation of pyridoxylidene-cephalexin Schiff base (HPL-CPX) [52]
Equimolar quantity of saturated methanolic solution of cephalexin drug and pyridoxal were mixed thoroughly. To this mixture 0.1% methanolic KOH was added to adjust the pH of the solution between 7-8 and was refluxed for 1 h. A clear dark yellow colored solution was obtained. The Schiff base ligand was isolated by crystallization after volume reduction by evaporation. The crystalline product was dried under vacuum and kept in desiccator over P4O10 until further use. Yield=75%, M.pt.(decomp.)=184°C-186°C. Analyses (Calculated) C24H26N4O6S: C(57.82), H(5.26) and N(11.24); Found: C(57.71), H(5.19) and N(11.09).
Preparation of pyridoxylidene-cephalexin complexes of aryltellurium (IV) trichlorides and diaryltellurium (IV) dichlorides
Aryltellurium (IV) trichlorides, ArTeCl3 and diaryltellurium (IV) dichlorides Ar2TeCl2 (Ar=p-methoxyphenyl, p-ethoxyphenyl, p-hydroxyphenyl and 3-methyl-4-hydroxyphenyl), when reacted with monosodium salt of HPL-CPX i.e., NaPL-CPX in equimolar ratio, yield PL-CPX.ArTeCl2 and PL-CPX.Ar2TeCl type complexes.
Sodium salt of the ligand was prepared by reacting equimolar (1:1) quantity of sodium metal and Schiff base in methanol. The solvent was distilled off to isolate the sodium salt. Then a methanolic saturated solution of 2 mmol of aryltellurium (IV) trichloride or diaryltellurium (IV) dichloride was added dropwise to suspension of 2 mmol of sodium salt of Schiff base in about 50 mL benzene under reflux. The reaction mixture was further refluxed for 3 h-4 h, cooled and precipitated sodium chloride was filtered off. The filtrate was then concentrated to about one third of original volume under reduced pressure and cooled in an ice bath to obtain colored product. This was filtered, washed with benzene+methanol (1:1) and dried in vacuum desiccator over P4O10.
Physical studies
Conductance studies were performed under dry condition at 25 ± 2°C in DMSO with a dip type conductivity cell on microprocessor based conductivity bridge type MICROSIL.
Infrared spectra (4000 cm-1 to 400 cm-1) were recorded in KBr pellets on Alpha Bruker FT-IR spectrometer and far IR (400 cm- 1 to 50 cm-1) was obtained in polyethylene pellets on a F.T. Infra-Red Spectrophotometer Model RZX (Perkin Elmer) at SAIF, Panjab University Chandigarh. Proton magnetic resonance spectra were recorded in DMSO-d6 using TMS as an internal reference on BRUKER AVANCE II 400 NMR spectrometer at Sophisticated Analytical Instrumentation Facility, Punjab University Chandigarh. The antimicrobial screening was carried out by tube dilution method.
Results and Discussion
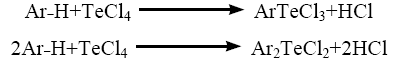
TeCl4 when heated with anisole [46?48], phenetole [49], phenol [50], o-cresol [51] (Ar?H) appears to undergo Friedel-Crafts type condensation reaction whereby TeCl3+ unit attacks a position para to the methoxy/ethoxy/hydroxy groups in the aromatic rings, thus resulting in the formation of aryltellurium (IV) trichlorides and diaryltellurium (IV) dichlorides.
Preparation of pyridoxylidene-cephalexin Schiff base (HPL-CPX) by the reaction of cephalexin drug and pyridoxal can be represented by following equations.
Sodium salt of pyridoxylidene-cephalexin Schiff base (NaPL-CPX) reacts with aryltellurium (IV) trichlorides and diaryltellurium (IV) dichlorides in 1:1 molar ratio to yield the corresponding aryltellurium (IV) complexes.

All the tellurium (IV) complexes are colored, crystalline solids, stable at room temperature and non-hygroscopic in nature. The complexes have been analyzed for their tellurium, chlorine, carbon, hydrogen and nitrogen contents and the data along with their physical properties and yields are presented in Table 1.
| Compound No. |
Complex(Ar) | Empirical formula (Formula Wt.) |
Colour(yield, %) | M. Pt. (°C) dec. |
Analyses % found (calculated) | ΛM at ca. 10-3 M S cm2mol-1 in DMSO |
||||
|---|---|---|---|---|---|---|---|---|---|---|
| C | H | N | Te | Cl | ||||||
| Schiff base | HPL-CPX | C24H24N4O6S (496.54) | Dark yellow (75) | 184-186 | 57.32 (58.05) |
5.15(4.87) | 11.03(11.28) | - | - | - |
| 1 | (PL-CPX).ArTeCl2 (p-methoxyphenyl) |
C31H30Cl2N4O7STe (801.16) |
Green (90) |
134-136 | 46.25 (46.47) |
3.99 (3.77) |
6.87 (6.99) |
15.77 (15.93) |
8.73 (8.85) |
34.68 |
| 2 | (PL-CPX).ArTeCl2 (p-ethoxyphenyl) |
C32H32Cl2N4O7STe (815.19) |
Light brown (87) |
150-152 | 46.98 (47.15) |
4.11 (3.96) |
6.77 (6.87) |
15.50 (15.65) |
8.57 (8.70) |
35.80 |
| 3 | (PL-CPX).ArTeCl2 (p-hydroxyphenyl) |
C30H28Cl2N4O7STe (787.14) |
Light green (85) |
120-122 | 45.51 (45.78) |
3.76 (3.59) |
7.01 (7.12) |
16.03 (16.21) |
8.87 (9.01) |
56.14 |
| 4 | (PL-CPX).ArTeCl2 (3-methyl-4-hydroxyphenyl) |
C31H30Cl2N4O7STe (801.16) |
Brown (80) |
116-118 | 46.18 (46.47) |
3.92 (3.77) |
6.85 (6.99) |
15.83 (15.93) |
8.69 (8.85) |
52.58 |
| 5 | (PL-CPX).Ar2TeCl (p-methoxyphenyl) |
C38H37ClN4O8STe (872.84) |
Light yellow (85) |
130-132 | 52.05 (52.29) |
4.35 (4.27) |
6.27 (6.42) |
14.51 (14.62) |
3.95 (4.06) |
42.25 |
| 6 | (PL-CPX).Ar2TeCl (p-ethoxyphenyl) |
C40H41ClN4O8STe (900.89) |
Light brown (82) |
128-130 | 53.11 (53.33) |
4.73 (4.59) |
6.17 (6.22) |
14.07 (14.16) |
3.85 (3.94) |
65.80 |
| 7 | (PL-CPX).Ar2TeCl (p-hydroxyphenyl) |
C36H33ClN4O8STe (844.79) |
Dark yellow (84) |
124-126 | 50.89 (51.18) |
4.15 (3.94) |
6.55 (6.63) |
14.94 (15.10) |
4.07 (4.20) |
45.38 |
| 8 | (PL-CPX).Ar2TeCl (3-methyl-4-hydroxyphenyl) |
C38H37ClN4O8STe (872.84) |
Brown (75) |
122-124 | 52.08 (52.29) |
4.39 (4.27) |
6.23 (6.42) |
14.49 (14.62) |
3.90 (4.06) |
39.76 |
Values of ΛM reported [53,54]for 1:1 electrolytes in DMSO=50-70 S cm2 mol-1
Table 1: Analytical data, molar conductance and physical properties of pyridoxylidene-cephalexin Schiff base complexes of tellurium (IV).
Conductance Studies
Molar conductance (ΛM) data for the complexes in DMSO are compiled in Table 1. The ΛM value at ca. 10-3 M for aryltellurium (IV) complexes in DMSO lie in the range 34.68 S cm2 mol-1 to 65.80 S cm2 mol-1 which predict the weak electrolyte to 1:1 electrolyte [53,54] type behavior of these complexes in DMSO, probably due to ionization into ArTeCl.PLCPX+/Ar2Te.PL-CPX+ and Cl– in DMSO. The higher ΛM values for some complexes may be due to steric factors and donor behaviour of DMSO to result in probable dissociation into solvated cation and PL-CPX– along with Cl– in DMSO.
Infrared spectra
The IR data of Schiff base and its tellurium (IV) complexes are listed in Table 2. The spectra of Schiff base complexes are quite complex and an attempt has therefore been made to identify the donor sites by comparing the spectra of complexes with parent ligand and ArTeCl3/Ar2TeCl2.
| Compound | Methanolic (–CH2OH) ν(O?H) |
Phenolic ν(O?H) |
Azomethine ν(C=N) |
ν(C?O) | ν(Te?N) | ν(Te?O) |
|---|---|---|---|---|---|---|
| HPL-CPX | 3205 mb | 2878 b | 1672 m | 1221 s | - | - |
| 1 | 2934 mb | - | 1675 mb | 1296 s | 416 m | 272 w |
| 2 | 3100 mb | - | 1680 mb | 1290 s | 420 m | 277 w |
| 3 | 3058 mb | - | 1675 mb | 1329 s | 426 s | 280 w |
| 4 | 3124 mb | - | 1674 mb | 1305 s | 417 s | 290 w |
| 5 | 3172 mb | - | 1677 s | 1298 s | 419 s | 298 w |
| 6 | 3224 mb | - | 1675 s | 1281 s | 412 m | 275 w |
| 7 | 3159 mb | - | 1671 mb | 1274 s | 416 m | 280 w |
| 8 | 1285 mb | - | 1669 s | 1371 s | 424 m | 287 w |
s=sharp, b=broad, mb=medium broad, sh=shoulder, w=weak
Table 2: Important infrared absorption bands (cm-1) of Schiff base and complexes.
In IR spectra of ligand shows a broad band around 3200 cm-1 indicates the presence of methanolic OH. This band is also observed in the Schiff base tellurium complexes, as methanolic group(-CH2OH) attached to pyridoxal ring does not participate in coordination [4,55,56]. A broad weak band in Schiff base in the range of 2800 cm-1 to 2900 cm-1 is assigned to an intramolecular hydrogen bond [57,58] between OH hydrogen of phenolic group and nitrogen of azomethine group. This band disappear on complexation with tellurium atom and shows that the phenolic group of pyridoxal moiety is involved in bonding [59]. Also, an intense ligand band at 1221 cm-1 (phenolic –C-O) in free ligand has shifted to higher frequency side in complexes, further proved that the phenolic group coordinates to tellurium atom [60,61] after deprotonation.
The vibration of the azomethine group of Schiff base observed 61-63 at 1672 cm-1, shift to lower or higher side [62-65] by ± 10 cm-1 in the Schiff base tellurium complexes, indicating coordination through azomethine nitrogen. The two new bands appear in range 272 cm-1 to 298 cm-1 and 412 cm-1 to 426 cm-1 assigned due to ν (Te?O) 66-69 and ν (Te?N) mode 70 of vibration [65-70].
Thus, IR data predict the monobasic bidentate nature of the Schiff base (HPL-CPX) involving azomethine nitrogen atom and phenolic oxygen after deprotonation giving rise to six membered chelate rings with the penta coordinated tellurium centre.
1H NMR spectra
In order to identify the solution structure of Schiff base (HPL-CPX) and its complexes, 1H NMR spectra were recorded in DMSO-d6 and are given in Table 3. The 1H resonance of the phenolic group at 8.65 δ ppm in Schiff base due to presence of intramolecular hydrogen bonding [71-73] disappear on complexation indicating the involvement of phenolic oxygen in the coordination after deprotonation [64]. The signal due to the methanolic proton is observed at around 5.37 δ ppm which remains intact in the complexes, this confirm that it does not take part in bonding [73]. The azomethine protons [51,63,72] which resonate as a singlet at 8.12 δ ppm in parent Schiff base is shifted towards downfield side in the complexes. This clearly demonstrate [61,72] the coordination of azomethine nitrogen to tellurium.
| Compound | Phenolic-OH | Methanolic-OH | Azomethine-H | N-substituted methine proton |
|---|---|---|---|---|
| HPL-CPX | 8.655 m | 5.669 s | 8.123 d | 8.482 d |
| 1 | - | 5.166 s | 8.160 d | 8.396 s |
| 2 | - | 5.225 s | 8.123 d | 8.301 s |
| 3 | 8.132 s* | 5.331 s | 8.226 d | 8.321 s |
| 4 | 8.495 s* | 5.724 s | 8.131 d | 8.011 s |
| 5 | - | 5.337 s | 8.163 d | 8.189 s |
| 6 | - | 5.330 s | 8.145 d | 8.160 s |
| 7 | 9.891 s* | 5.333 s | 8.147 d | 8.201 s |
| 8 | 9.911 s* | 5.235 s | 8.144d | 8.159 s |
s=singlet, d=doublet, m=multiplet. *Due to phenolic OH of ArTe and Ar2Te moieties.
Table 3: 1H NMR spectral data of Schiff base and complexes in DMSO-d6.
Thus pyridoxylidene-cephalexin act as a monobasic bidentate –N, –O chelating ligand in PL-CPX.ArTeCl2 and PL-CPX.Ar2TeCl complexes giving five coordinate tellurium having Ψ-trigonal bipyramidal geometry in these complexes as predicated from IR studies as well. The proposed structures are as below (Figure 1).
Biological activities
The pyridoxylidene-cephalexin Schiff base (HPL-CPX) and newly synthesized aryltellurium (IV) Schiff base complexes were evaluated for their antimicrobial activity in vitro against Gram+ve bacteria (S. aureus ATCC 11632 and B. cereus MTCC 7350), Gram-ve bacteria (E. coli ATCC 35218, P. aeruginosa ATCC 23564, S. typhi ATCC 15499 and P. rettgeri DRDE) and fungal strains (A. niger, A. fumigates and A. flavus) by tube dilution method [74]. Dilution of test and standard compounds were prepared double strength nutrient broth-I.P (Antibacterial) and Sabouraud dextrose broth-I.P (Antifungal) [75]. The samples were incubated at 37 ± 1°C for 24 h (bacteria), 25 ± 1°C for 7 days (A. niger), 30 ± 1°C for 15 days (A. flavus), 35 ± 1°C for 72 h (A. fumigates) respectively and results were recorded in terms of MIC values and are presented in the Table 4.
| Compound | Bacteria strains | Fungal strains | |||||||
|---|---|---|---|---|---|---|---|---|---|
| S. aureus (ATCC 11632) |
S. typhi (ATCC 15499) |
P. aeruginosa (ATCC 23564) |
E. coli (ATCC 35218) |
B. cereus (MTCC7350) |
P.rettgeri (DRDE strain) |
A. niger | A. fumigates | A. flavus |
|
| HPL-CPX | 5.0 | - | - | - | 1.25 | 2.5 | 20 | 5.0 | 1.25 |
| 1 | 5.0 | - | - | - | 1.25 | 2.5 | - | 1.25 | 5.0 |
| 2 | - | - | - | - | - | - | - | - | - |
| 3 | 1.25 | 2.5 | 1.25 | 5.0 | - | - | 5.0 | - | - |
| 4 | - | - | 5.0 | 1.25 | 0.625 | - | 20 | 5.0 | 1.25 |
| 5 | 10 | 10 | 20 | - | 5.0 | 5.0 | - | - | 2.5 |
| 6 | 1.25 | 2.5 | 1.25 | 5.0 | - | - | 20 | 5.0 | 1.25 |
| 7 | 5.0 | 10 | 5.0 | 20 | - | 0.625 | 5.0 | 2.5 | - |
| 8 | 20 | - | 20 | - | 10 | 20 | 5.0 | 1.25 | 5.0 |
Table 4: Minimum inhibitory concentration, MIC (μg/mL); (-) resistant.
The data show that the complexes of aryltellurium (IV) exhibit moderate antibacterial and antifungal activity. The antimicrobial activity shows following trend:
PL-CPX.Ar2TeCl>PL-CPX.ArTeCl2 ≈ PL-CPX Schiff base
PL-CPX.Ar2TeCl (Ar=p-hydroxyphenyl) shows substantial activity against P. aeruginosa, whereas PL-CPX.Ar2TeCl (Ar=-methoxy-4-hydroxyphenyl) exhibit appreciable activity against E. coli. Aryletellurium Schiff base complexes are moderately more effective against fungal strain than Schiff base itself.
Conclusion
Aryltellurium (IV) and diaryltellurium (IV) dichlorides upon reaction with sodium salt of Schiff base (NaPL-CPX) derived from pyridoxal and cephalexin yield new complexes of tellurium (IV). The synthesized complexes were characterized by elemental analyses, conductance measurement, IR and 1H NMR spectral studies. The analytical data suggest that the Schiff base complexes have 1:1 stoichiometry. The Schiff base (HPL-CPX) in these complexes acts as a monobasic bidentate ligand through azomethine nitrogen and phenolic oxygen after deprotonation. Based on these studies, distorted trigonal bipyramidal geometry with six membered chelate ring with tellurium centre has been suggested. The complexes have been observed to possess moderate antimicrobial activity against bacterial and fungal strains.
Acknowledgement
The authors are grateful to M. D. University, Rohtak for providing the necessary facilities. One of the authors (Deepak) is also thankful to UGC New Delhi for providing fellowship. We also thank SAIF, Panjab University Chandigarh for providing the CHN analyses and spectral data.
References
- McPherson, Edwin M.Pharmaceutical Manufacturing Encyclopedia. Burlington, Elsevier, 3rd ed. 2007;915-17.
- Ryan CW, Simon RL, Heyningen EM. Chemistry of cephalosporin antibiotics. XIII. Desacetoxycephalosporins: The synthesis of cephalexin and some analogs. J Med Chem. 1969;12:310-13.
- Stryer L. Biochemistry. WH Freeman and Company, New York, 4th ed. 1995;631.
- Nasker S, Nasker S, Mayer-Figge H, et al. Synthesis, x-rays crystals structures, spectroscopic and cyclic volumetric studies of Cu(II) Schiff bases complexes of pyridoxal.Polyhedron. 2000;30:529-34.
- Celik Ö, Ulusoy M, Tas E, et al. Synthesis and crystallographic structure studies of N-[5-Methylisoxazoleamino-3-yl]-3,5-di-tert-bytylsalicylaldimine.Anal Sciences. 2007;23: x185-x186.
- Mahmoud MR, El-Gyar SA, Mousrafa A, et al. Ni(II) complexes of some polyfunctional N-Naphylideneamino acids.Polyhedron. 1987;6:1017-20.
- Qing-Yu H, Zheng-Hua M, Ya-Me Z. Preparation and oxygenation of Manganese(II) complexes of imines derived from salicylaldehyde and amino acid. J Coord Chem. 1990;21:199.
- Cozzi PG. Metals-Salen Schiff base complexes in catalysis: Practical aspects. ChemSoc Rev. 2004;33:410-21.
- Tarafder MTH, Jin KT, Crouse KA, et al. Coordination chemistry and bioactivity of Ni2+, Cu2+, Cd2+and Zn2+complexes containing bidentate Schiff base derived from S-benzyldithiocarbazate and the X-rays crystal structure of bis[S-benyl-β-N-(5-methyl-2-furylmethylene)dithiocarbazato]cadmium(II). Polyhedron. 2002;21:2547-54.
- Yu D, Xiaoxia H, Xiaoli S, et al. Synthesis, structural characterization and electrochemical properties of binuclear copper(II) complexes containing tetradentate Schiff base ligand.J Nat Sci. 2010;15(2):165-70.
- Kumar D, Gupta PK, Syamal A. Syntheses, magnetic and spectral studies on polystyrene supported coordination compounds of bidentate and tetradentate Schiff bases. J Chem Sci. 2005;117(3):247-53.
- Jones RD, Summerville DA, Basolo F. Synthetic oxygen carriers related to biological systems. Chem Rev.1979;17(2): 139-79.
- Holla BS, Akberali PM, Shivananda MK. Studies on nitrophenylfuran derivatives part XII. Synthesis, characterization, antibacterial and antiviral activities of some nitrophenylfurfurylidene-1,2,4-triazolo[3,4-b]-1,3,4-thiadiazines II.Farmaco, 2001; 56: 919-27.
- Jarrahpour A, Khalili D, De Clercq E, et al. Synthesis, antibacterial, antifungal and antiviral activity evaluation of some new bis-Schiff bases of Isatin and their derivatives. Molecules. 2007;12:1720-30.
- Da Silva CM, da Silva DL, Modolo LV, et al. Schiff bases: A short review of their antimicrobial activities. AdvRes. 2011;2:1-8.
- Singh H, Yadav LDS, Mishra SBS. Studies on some antifungal transition metal chelates of N-(5-phenyl-1,3,4-thiadizol-2-yl) dithiocarbamic acid. J InorgNucl Chem.1981;43:1701-04.
- Saravanan G, Pannerselvam P, Prakash CR. Synthesis and anti-microbial screening of novel Schiff bases of 3-amino-2-methyl quinazolin 4-(3H)-one. J Adv Pharm Techn Res.2010;1:320-25.
- Panneerselvam P, Nair RR, Vijayalakshmi G, et al. Synthesis of Schiff bases of 4-(4-aminophenyl)-morpholine as potential antimicrobial agents. Eur J Med Chem. 2005;40(2):225-29.
- Przybylski P, Huczynski A, Pyta K, et al. Biological properties of Schiff bases and azo derivatives of phenols.Curr Org Chem.2009;13:124-48.
- Pandeya SN, Sriram D, Nath G, et al. Synthesis, antibacterial, antifungal and anti-HIV evaluation of Schiff and Mannich bases of isatin derivatives with 3-amino-2-methylmercapto quinazolin-4(3H)-one. Pharm Acta Hely.1999;74:7-11.
- Karthikeyan MS, Parsad DJ, Poojary B, et al. Synthesis and biological activity of Schiff and Mannich bases bearing 2,4-dichloro-5-fluorophenyl moiety. Bioorg Med Chem. 2006;14:7482-89.
- Sinha D, Tiwari AK, Singh S, et al. Synthesis, characterization and biological activity of Schiff base analogues of indole-3-carboxaldehyde. Eur J Med Chem. 2008;43(1):160-65.
- Crowe AJ, Smith PJ, Atassi G. Investigations into the antitumouractivity of organotin compounds I. Diorganotindihalide and di-pseudohalide complexes. ChemBiolInteract. 1980;32:171-78.
- Wang M, Wang LF, Li YZ, et al. Antitumour activity of transition metal complexes with the thiosemicarbazone derived from 3-acetylumbelliferon. Trans Met Chem. 2001;26:307-10.
- Desai SB, Desai PB, Desai KR. Synthesis of some Schiff bases,thiazolidinones and azetidinones derived from 2,6-diaminobenzo[l,2-d:4,5-d']bisthiazole and their anticancer activities. HetrocyclCommun. 2001;7:83-90.
- Dhumwad SD, Gudasi KB, Goudar TR. Synthesis and structural characterization of biologically-active metal-complexes of N(1)-(N-morpholinoacetyl)-N(4)-phenyl thiosemicarbazide and 3,4-methylenedioxybenzaldehyde thiosemicarbazone with oxovanadium(IV), chromium(III), manganese(II), iron(III), cobalt(II), nickel(II), copper(II), cadmium(II),uranium(VI), thorium(IV) and silicon(IV). Indian J Chem.1994;33A:320-24.
- Wynne KJ, Pearson PS.Chalcogen chemistry vs. complexes of organotelluriumtrihalideswith tetramethylthiourea. Inorg Chem. 1971;10:2735-39.
- Wynne KJ, Pearson PS.Preparation of methyltrihalogeno(tetramethylthiourea) tellurium(IV) compounds: Pentaco-ordinate Tellurium. J ChemSocCommun. 1970;556-57.
- Aynsley EE, Cambell WA. Complexes of thiourea containing tellurium. J Chem Soc. 195;3290-93.
- Clark ER, Collet AJ, Naik DG.Tetraethyldithio-oxamide complexes of tellurium(IV). J ChemSoc Dalton. 1973;19: 1961-62.
- Wynne KJ, Clark AJ, Berg M. Chalcogen chemistry. Part VIII. Complexes of arylselenium and aryltelluriumtrichlorides with Pyridine, 4-Picoline and 4-Picoline-N-oxide. J ChemSoc Dalton. 1972;2370-74.
- Srivastava TN, Singh M, Singh HB. Complexes of organotellurium chlorides with N, P, O and S-donors. Indian J Chem. 1982;21A:307-12.
- Srivastava TN, Srivastava RC, Srivastava M. Complexes of organotellurium chlorides with N, O and S-donors. Indian JChem. 1982;21A:539.
- Srivastava TN, Srivastava RC, Srivastava VK. Studies on molecular adducts of hydroxyphenyl tellurium (IV) trichlorides. J Indian Chem Soc.1983;60(9):891-92.
- Garad MV. Molecular complexes of aryltellurium(IV) chlorides. Polyhedron.1985;4:1353-55.
- Verma KK, Reena. Complexes of 4-hydroxyphenyltellurlum trihalides with piperidine, dimethylformamide and thiourea. Synth React Inorg Met –Org Chem.1999;29:499-512.
- Verma KK, Dahiya R, Soni D. Synthesis and characterization of complexes of some hydroxyaryltelluriumtrichlorides with N-donor ligands. Synth React Inorg Met–Org Chem. 1999;29:1033-52.
- Verma KK, Dahiya R. Synthesis and characterization of pyridine and bipyridyl complexes of 4-hydroxyphenyltellurium trihalides. Synth React Inorg Met –Org Chem.1999;29:1299-1314.
- Verma KK, Reena. Studies on thiourea complexes of some hydroxyaryltelluriumtrichlorides. Phosphorus, Sulfur and Silicon and the Related Elements, 1999;148(1):227-34.
- Verma KK, Seema. Study on picoline complexes of p-Hdroxyphenyltellurium (IV) trihalides. Int J Chem Sci. 2008; 6(1):371-80.
- Srivastava S, Soni DK, Gupta HS. Some molecular adducts of dibenzyl tellurium(IV) derivatives with nitrogen donor molecules. J Indian Chem Soc.1996;73:255-56.
- Narwal JK, Chhabra S, Malik RK, et al. Studies on the pyrazine complexes of some diaryltelluriumdihalides. Oriental J Chem. 2013;29:1339-49.
- Chhabra S, Verma KK. Studies on 1, 10-phenanthroline complexes of some diaryltelluriumdihalides. J Chem Pharm Res. 2010;2(4):569-75.
- Vogel AI. A Text Book of Quantitative Inorganic Analysis Including Elementary Instrumental Analysis, 3rd ed. Longmans, London, 1975.
- Weissberger A. Editor. In: Technique of Organic Chemistry, Vol. 7, 2nd ed.Interscience Publishers Inc. NY, 1967.
- Morgan GT, Kellet RE. Interactions of tellurium tetrachloride and aryl alkyl ethers. Part II.J Chem Soc.1926;1080-88.
- Petragnani N, Stefani HA. Tellurium in Organic Synthesis.2nd ed. Academic Press, London (2007);pp:67-76.
- Bergman J. Tellurium in organic chemistry-I A novel synthesis of biaryl. Tetrahedron. 1972;28:3323-31.
- Khandelwal BL, Kumar K, Berry FJ.Hydroxyphenyltellurium (IV) halides.InorgChimActa. 1981;47:135-39.
- Berry FJ, Kustan EH, Roshani M, et al.1H NMR spectra of some aryltellurium compounds. J Organometal Chem.1975; 99:115-17.
- Khandelwal BL, Kumar K, Raina K. Synthesis and characterization of (methylhydroxyphenyl) tellurium(IV) halides. Synth React Inorg Met –Org Chem. 1981;11(1):65-78.
- Iqbal MS, Khan AH, Loothar BA, et al. Effect of derivation of sulfamethoxazole and trimethoprim with copper and zinc on their medicinal value. Med Chem Rev. 2009;18:31-42.
- Geary WJ. The use of conductivity measurements in organic solvents for the characterization of coordination compounds. CoordChemRev.1971;7:81-122.
- Greenwood NN, Straughan BP, Wilson AE.Behaviour of tellurium(IV) chloride, bromide and iodide in organic solvents and structures of the species. J ChemSoc A. 1968;2209-12.
- Flett, M St C. Studies of the band near 3μ in some hydroxy compounds. SpectrochimActa. 1957;10:21-37.
- Bellamy IJ. Infrared Spectra of Complex Molecules. Chapman and Hall Ltd. London, 1975.
- Baker AW, Shulgin AT. Intramolecular hydrogen bonding. II. The determination of hammett sigma constants by intramolecular hydrogen bonding in Schiff’s bases. J Am Chem Soc. 1959;81:1523-29.
- Freedman HH.Intramolecular H-bonds. I. A spectroscopic study of the hydrogen bond between hydroxyl and nitrogen. J Am Chem Soc. 1961;83:2900-05.
- Rosu T, Pahontu E, Reka-Stefana M, et al. Synthesis, structural and spectral studies of Cu(II) and V(II) complexes of a novel Schiff base derived from pyridoxalantimicrobial activity. Polyhedron. 2012;31:352.
- Biradar NS, Aminabhavi TM, Patil CS. Selenium and tellurium complexes with 2-Substituted benzimidazoles. InorganicaChimica Acta.1983;78:47-50.
- Thirumagal B, Malik S, Balasubramaniam A. The Pharmacist. 2001;27.
- Iqbal MS, Khurshid SJ, Iqbal MZ. Preparation, characterization and biological evaluation of Cu(II)-Schiff base complexes derived from anthranilic acid and aldoses. Can J Chem. 1993;71:629-33.
- Tumer M, Celik C, Koksal H, et al. Transition metal complexes of bidentate Schiff base ligands. Transition Metal Chemistry. 1999;24:525-32.
- Rudzinski WE, Aminabhavi TM. Biologically active sulfonamide Schiff base complexes of selenium(IV) and tellurium(IV). InorganicaChimica Acta.1982;67:177-82.
- Mohamed GG, Abd El-Wahab ZH. Salicylidene-2-aminobenzimidazole Schiff base complexes of Fe(III), Co(II), Ni(II), Cu(II), Zn(II) and Cd(II). Journal of Thermal Analysis and Calorimetry. 2003;73:347.
- Verma KK, Soni D.Diaryltellurium (IV) carboxylates: Synthesis viatelluroxides and their characterization. Phosphorus, Sulfur and Silicon, 2000;166:231.
- Pant BC, McWhinnie WR, Dance NS. Organotellurium carboxylates and related compounds: Structural and synthetic considerations. J Organmetal Chem.1973;63:305-10.
- Srivastava TN, Singh JD.Diorganotellurium(IV) bis(dimethyl glyoximates). Indian J Chem. 1987;26A:519-20.
- Chauhan S, Garg S, Verma KK. Studies on some salicylhydroxamate complexes ofaryltellurium (IV). ChemSci Trans. 2016;5(2):431-41.
- Kulkarani YD, Srivastava S, Abdi SHR, et al. Synthesis and reactivity of some α, α´-Bis(2-and-4-substitued benzoyl) tellurium dichlorides. Synth React Inorg Met –Org Chem. 1985;15(8):1043-59.
- Chauhan S, Garg S, Verma KK. Synthesis, characterization and antimicrobial studies of some aryltellurium (IV) complexes of 5-Chlorosalicylhydroxamate. Res J Pharm BiolChem Sci. 2016;7(2):265-74.
- Beyramabadi S Ali, Morsali A, Khoshkkholgh MJ, et al. N, N´-dipyridoxal Schiff bases: Synthesis, experimental and theoretical characterization.SpectrochimicaActa Part A. 2011;83:467-71.
- Aminabhavi TM, Rudzinski WE. Complexes of tellurium with Vanillydene-Schiff Bases. InorganicaChimica Acta.1983;76:L131-L134.
- Cappuccino JG, Sherman N.Microbiology-A Laboratory Manual. Addison Wesley, California. 1999;263.
- Pharmacopoeia of India. Volume 1. Controller of Publication, Ministry of Health Department, Government of India, New Delhi. 2007;37.