Original Article
, Volume: 10( 1)Novel Chalcones of 3-[4-(4-[4-Acetylphenoxy]-6-([Nitrophenyl]amino)-1,3,5-Triazin-2-yl)oxy)Phenyl]-1-(2,4-Dichlorophenyl)Prop-2-en-1-one for Biological Applications: Synthesis, Characterization and Antimicrobial Studies
- *Correspondence:
- Aboobuckersithique M, , PG and Research Department of Chemistry Islamiah College Vaniyambadi, Coimbatore, India, Tel: 04174235206; E-mail: mvragav444@yahoo.com
Received: January 09, 2017; Accepted: January 17, 2017; Published: January 20, 2017
Citation: Bagyaraj E, Moorthi K, Aboobuckersithique M. Novel chalcones of 3-(4-(4-[4-acetylphenoxy]-6-([nitrophenyl]amino)-1,3,5-triazin-2-yl)oxy)phenyl)-1-(2,4-dichlorophenyl)prop-2-en-1-one for Biological Applications: Synthesis, Characterization and Antimicrobial Studies. Chemxpress. 2017;10(1):116.
Abstract
A series of bioactive chalcones are synthesized using 3-(4-(4-[4-acetylphenoxy]-6-([nitrophenyl]amino)-1,3,5-triazin-2-yl)oxy)phenyl)-1-(2,4-dichlorophenyl)prop-2-en-1-one (ACP) and different substituted aromatic aldehyde under solvent free conditions. Synthesized chalcones are characterized and tested for their antimicrobial activity against gram positive and gram negative bacteria’s. The synthesized chalcones are characterized by IR, 1H-NMR and UV-visible spectroscopic techniques. The antimicrobial activities of the chalcones are determined using minimum inhibitory concentration (MIC) method. Synthesized chalcones shows high activity against tested bacteria’s especially the chalcone containing chlorine moiety shows very high activity against tested gram positive bacteria Staphylococcus aureus at 7.81 μL/ml.
Keywords
Solvent free; Chalcone; MIC; Antimicrobial activity
Introduction
Chalcone belong to a class of α, β unsaturated aromatic ketones, it forms the central core for a variety of important biological compounds. Chalcones exhibits antibacterial, antifungal, antitumor and anti-inflammatory properties. They are also intermediates in the biosynthesis of flavonoids, which are substances widespread in plants and with an array of biological activities. Chalcones are also intermediates in the Augers synthesis of flavones. Chalcone can be prepared by an aldol condensation between a benzaldehyde and an acetophenone in the presence of a catalyst. In vitro anti-plasmodial activity of phenylated chalcone derivatives of hops (Humulus lupulus) and their interaction with haemin. [1] Chalcone coating on cotton cloth-an approach to reduce attachment of live microbe [2]. That chalcones have a broad spectrum of activity (they are active against Gram +ve and Gram -ve bacteria). Thus the coated cloth has potential applications in hospital environments and can be used as bed covers, curtains, linens and dressings. However the long term stability and was ability of these coated cloths needs to be assessed in field conditions.
Synthesized chalcones using super acid as a catalyst. In his paper, he reported various chalcones using acetophenones and benzaldehydes. [3,4] reported about the new synthetic route for the synthesis of chalcones using earth clay at a pH 12.5/PEG- 400 as a catalyst. It is a practical approach using PEG as a catalytic due to the fact that, it is commercially available, low cost, recyclable, nonionic solvent. In most cases, the reaction proceeded smoothly to produce the α, β unsaturated ketone (chalcone). It has been observed that bleaching earth clay pH 12.5/PEG-400 is an efficient catalytic system for synthesis of α, β-unsaturated ketones (chalcones). In this article we have synthesized cyanuric chloride based chalcones using DHP (14), 3- (4-(4-[4-acetylphenoxy]-6-([4-nitrophenyl]amino)-1,3,5-triazin-2-yl)oxy)phenyl)-1-(2,4-dichlorophenyl)prop-2-en-1-one and substituted aromatic aldehydes. The antimicrobial activities of synthesized chalcones were tested on two different bacteria’s.
Experimental Details
Materials
2, 4-dichloroacetophenone and 4-aminoacetophenone were purchased from Merck and used as such. 4-hydroxybenzaldehyde and Cyanuric chloride were purchased from Aldrich chemical and used as such. All the solvents were purchased from S.D. Fine chemicals and used as such. Benzaldehyde, 4-nitrobenzaldehyde, 4-bromobenzaldehyde, 4-chlorobenzaldehyde, para N, N-dimethyl benzaldehyde and 4-aminobenzaldehyde were received from SD fine chemicals.
Synthesis of 4, 6-dichloro- N-(4-nitrophenyl)-1,3,5-triazine-2-amine (CNA) (SCHEME 1)
Cyanuric chloride (5 g, 0.02 mol) dissolved in acetone was added slowly to the solution of 4-nitroaniline (3.7 g, 0.02 mol) dissolved in acetone present in a conical flask at 0°C. The PH of the reaction mixture was maintained at 6 by the addition of aqueous Na2CO3 (2 M), the reaction was carried out for 3 h. After completion, the reaction mixture was poured into ice-cold water. The precipitate was separate out by filtration and washed several times by using cold water and dried in vacuum at 50°C. And recrystallized from ethanol, the molecular weight of the obtained compound was 286.07 and melting point was-272°C to 275°C. The FT-IR (cm-1) spectrum of the CNA is shown in the Figure. 1. The characteristic peak appear at 3036 (aromatic CH-stretching), 1550 (C-NO2) and 3512 (-NH). 1H NMR (500 MHz, DMSO-d6, δ): 7.4-8 (m, 4HAr H) and 4.3 (s, 1H NH).
Synthesis of 3-(4-(4-chloro-6-(naphthalene-1-yloxy)-1,3,5-triazin-2-yl)oxyphenyl)-1-(2,4-dichlorophenyl)prop-2-en-1-one (Scheme 1)
CNA (3 g mol) dissolved in acetone was added slowly to DHP (3.008 g mol in 30 mL acetone) with constant stirring for 3 hr at 35°C and periodically 10% Na2CO3 solution was added drop wise. After completion of the reaction, the mixture was poured into ice-cold water, the precipitate was separated out by filtration and washed several times by using cold water and dried in vacuum at 50°C. IR (KBr, cm-1): 3305 (N-H str.), 3070 (=CH str.), 1649 (C=O str.), 1510 (C=C str.), 1025 (C-O-C str.), 808 (C-N str., s-triazine moiety), 1080 cm-1 (C-Cl str.); 1H NMR (CDCl3, δ, ppm): 6.42 (1H, d, -CO-CH=), 8.03 (1H, d, Ar-CH=), 6.98-7.91 (17H, m, Ar-H and -NH).
Synthesis of (E)-3-(4-(4-chloro-6-((4-nitrophenyl)amino)-1,3,5-triazin-2-yl)oxyphenyl)-1-(2,4-dichlorophenyl)prop-2-en-1-one (NTP) (Scheme 1)
Synthetic route of NTP was presented in the Scheme. 1. NTA (3 g, 0.01 mol) dissolved in acetone was added slowly to DHP (2.93 g 0.01 mol in 30 mL acetone) with constant stirring for 3 hr at 35°C and periodically 10% Na2CO3 solution was added drop wise to neutralize the solution. After completion of the reaction, the reaction mixture was poured into ice-cold water, the precipitate was separated out by filtration and washed several times using cold water and dried in vacuum at 50°C. Yield: 4.4 g (82%). Mol. Wt=542.76 and m.p is 185°C to 186°C. The IR spectra (cm-1) revels the peak at 3305 is due to the presence of N-H stretching, at 3070 is due to aromatic CH stretching, at 1663 is due to the presence of C=O stretching, at 1603 and 1570 is due to C=C and Ar-NO2 stretching respectively. The peak at 1080 is due to the C-Cl stretching, at 1025 is due to C-O-C stretching and at 812 is due to C-N stretching of s-triazine moiety. further two characteristic peak of absorption, around 250 nm is due to aromatic double bond and the peak of absorption at 330 nm is due to π to π* transition of >CH=CH< in the NTP system. 1H NMR (δ, ppm), Characteristic chemical shifts present in the spectrum are, the peak at 7.3-8 is due to aromatic protons (11 H), at 6.8-6.9 is due to the -CO-CH=CH proton and the peak at 4.1 is due to the presence of Ar-NH in the NTP system.
Scheme 1: Synthesis of 3-(4-((4-(4-acetylphenoxy)-6-((4-nitrophenyl)amino)-1,3,5-triazin-2-yl)oxy)phenyl)-1-(2,4- dichlorophenyl)prop-2-en-1-one (ACP).
Synthesis of 3-(4-((4-(4-acetylphenoxy)-6-((4-nitrophenyl)amino)-1,3,5-triazin-2-yl)oxy)phenyl)-1-(2,4-dichlorophenyl) prop-2-en-1-one (ACP) (SCHEME 1)
Synthetic route of ACP was presented in the Scheme 1. In a RB flask containing NTP (2.55 g, 0.005 mol) in acetone to this 4-hydroxyacetophenone (0.68 g, 0.005 mol) dissolved in acetone was added slowly to it with constant stirring for 6 h at 70°C. Periodically, 10% Na2CO3 solution is added to neutralize HCl evolved during the reaction. Finally the contents were poured into a crushed ice. The solid separated out and filtered and washed with water, dried and recrystallized form ethyl alcohol. The molecular weight of TAP is 642.45 g/mol. Yield: 2.5 g (79%) and the m.p is 172°C to 174°C. FT-IR (cm-1), important peaks in the spectrum are, the peak appear at 3305 is due to the N-H stretching, at 3070 is due to aromatic CH stretching, at 1675 is due to the presence of free and conjugated C=O. stretching, at 1593 is due C=C stretching, at 1028 is due to the C-O-C stretching, at 1565 is due to Ar-NO2 stretching, at 1083 is due to C-Cl stretching and 810 is due to the C-N stretching of parental s-triazine moiety. UV (nm) spectrum reveals two characteristic peak of absorption at 254 nm is due to aromatic double bond absorption and the peak of absorption at 336 nm is due to π to π* transition of >CH=CH< in the TAP system. 1H NMR (δ, ppm), characteristic chemical shifts present in the spectrum are, the peak at 6.8 to 8.1 is due to vinylic (2H) and aromatic protons (15H) and the peak at 4.1 is due to Ar-NH proton. The peak appear at 3.86 is due to the presence of -CO-CH3 in the TAP system.
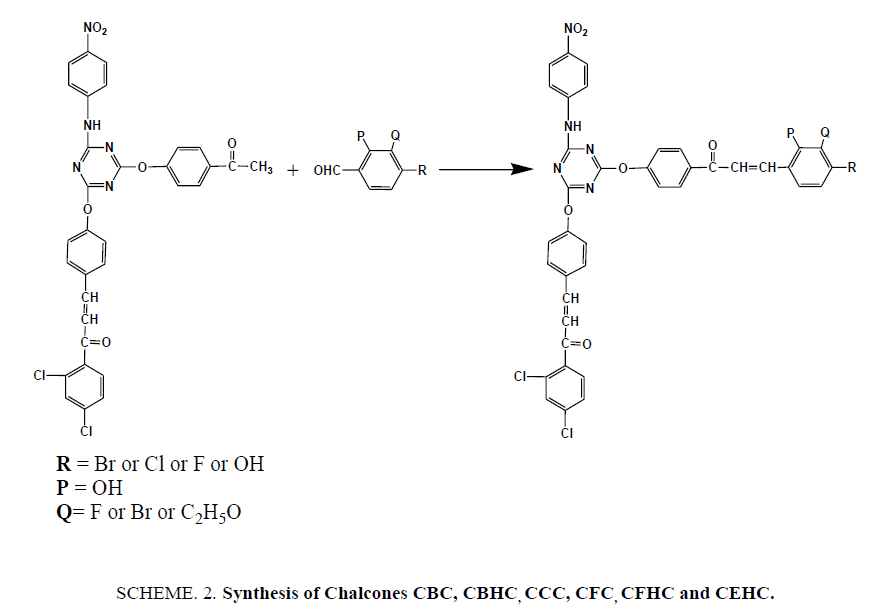
Synthesis of Triazine Containing Novel Chalcones (SCHEME 2)
Grindstone technique
Newly synthesized chalcones were prepared by grinding together equivalent amount of ACP and substituted benzaldehydes in presence of KOH in a porcelain mortar under solvent free conditions for 4-8 mins. On completion of reaction, the mixture was diluted with cold water neutralized by dilute HCl and recrystallized from acetic acid.
Synthesis of 3-(4-bromophenyl)1-1-(4-((4-((E)-3-(2,4-dichlorophenyl)-3-oxoprop-1- en-1-yl)phenoxy)-6-((4-nitro phenyl)amino)-1,3,5-triazin-2-yl)oxy)phenyl)prop-2-en-1-one (CBC)
The structure of the above compound is determined using IR, NMR, UV the data’s are as follows. CBC, Mol. wt. 809.45 and m.p. is 206°C-208°C. IR (cm-1), 3030, (C-H)Ar)), 1643 (CO), 1597 (CH=CH-, str.), 1550 (N-O ), 811 (C-N, s-triazine), 845 (C-Br) and786 (C-Cl). 1H-NMR (δ, ppm): 6.8-6.9 (d, 2H, -CO-CH=CH]), 7.15-7.8 (m, 19H, Ar-H).UV (nm): aromatic (CH=CH) 224 and vinylic (CH=CH) 328. Calculated elemental analysis for molecular formula, C39H24BrCl2N5O6 (%): C, 57.87; H, 2.99; Cl, 8.76. Found: C, 57.02; H, 2.04; Cl, 8.09.
Synthesis of 3-(2-bromo-3-hydroxyphenyl)-1-(4-((4-(4-((E)-3-(2,4-dichlorophenyl)-3-oxoprop-1-en-1-yl)phenoxy)-6- ((4-nitrophenyl)amino)-1,3,5triazin-2-yl)oxy)phenyl)prop-2-en-1-one (CBHC)
CBHC, M.wt. 825.45 and m.p. 211°C to 213°C.IR (cm-1), 3520 (O-H), 3040, (C-H) Ar)), 1640 (CO), 1590 (CH=CH-, str.),1545 (N-O), 806 (C-N, s-triazine)770 (C-Cl). 1H-NMR (δ, ppm) 6.5-6.7 (d, 4H, -CO-CH=CH]),7.2-8.1 (m, 18H, Ar-H), UV(nm) aromatic (CH=CH) 230 and vinylic (CH=CH) 332. Elemental analysis calculated for the molecular formula, C39H24BrCl2N5O7 (%): C, 56.75; H, 2.93; Cl, 8.59. Found: C, 56.07; H, 2.06; Cl, 8.10.
Synthesis of 3-(4-chlorophenyl)-1-(4-((4-(4-((E)-3-(2,4-dichlorophenyl)-3-oxoprop-1- en-1-yl)phenoxy)-6-((4-nitro phenyl)amino)-1,3,5triazin-2-yl)oxy)phenyl)prop-2-en-1-one (CCC)
CCC, M.wt. 765.00 and m.p. 180°C to 181°C.IR (cm-1), 3024, (C-H)Ar)), 1643 (CO), 1597 (CH=CH-, str.),1550 (N-O ), 811 (C-N, s-triazine)786 (C-Cl). 1640 (CO), 1591 (CH=CH-, str.), 1337 (C-N), 813 (C-N, s-triazine)760 (C-Cl). 1H-NMR (δ, ppm) 6.6-6.9 (d, 4H, -CO-CH=CH]),7.15-7.80 (m, 19H, Ar-H), UV(nm) aromatic (CH=CH) 235 and vinylic (CH=CH) 330. Elemental analysis calculated for the molecular formula, C39H24BrCl2N5O7 (%): C, 61.23; H, 3.16; Cl, 13.90. Found: C, 60.08; H, 2.91; Cl, 12.90.
Synthesis of (2E)-1-(2,4-dichloroyphenyl)-3-(4-((4-(4-(3-(3-ethoxy-4-hydroxyphenyl) acryloyl)phenoxy)-6-((4-nitro phenyl)amino)-1,3,5triazin-2-yl)oxy)phenyl)prop-2-en-1-one (CEHC)
CEHC, M.wt. 790.60 and m.p. 215°C to 216°C.IR (cm-1), 3030, (C-H)Ar)), 2870 (C-H) Aliphatic) 1643 (CO), 1585 (CH=CH-, str.),1545 (N-O ), 801 (C-N, s-triazine)780 (C-Cl). 1H-NMR (δ, ppm) 6.8-6.9 (d, 4H, -CO-CH=CH]), 7.15-7.80 (m, 18H, Ar-H), UV(nm) aromatic (CH=CH) 229 and vinylic (CH=CH) 336. Elemental analysis calculated for the molecular formula, C39H24BrCl2N5O7 (%): C, 62.29; H, 3.70; Cl, 8.97. Found: C, 61.50; H, 2.95; Cl, 8.31.
Synthesis of (2E)-1-(2,4-dichlorophenyl)-3-(4-((4-(4-(3-(4-fluorophenyl)acryloyl)phenoxy)-6-((4-nitrophenyl)amino)-1,3,5triazin-2-yl)oxy)phenyl)prop-2-en-1-one (CFC)
CFC, M.wt. 748.54 and m.p. 204°C. IR (cm-1), 3040, (C-H)Ar)), 1649 (CO), 1590 (CH=CH-, str.),1552 (N-O ), 1120 (C-F), 811 (C-N, s-triazine)782 (C-Cl). 1H-NMR (δ, ppm) 6.8-6.9 (d, 4H, -CO-CH=CH]), 7.3-7.8.4 (m, 19H, Ar-H), UV(nm) aromatic (CH=CH) 232 and vinylic (CH=CH) 337. Elemental analysis calculated for the molecular formula, C39H24BrCl2N5O7 (%): C, 62.58; H, 3.23; Cl, 9.47. Found: C, 61.58; H, 2.98; Cl, 8.26.
Synthesis of (2E)-1-(2,4-dichlorophenyl)-3-(4-((4-(3-(3-fluro-4-hydroxylphenyl)acryloyl)phenoxy)-6-((4-nitro phenyl)amino)-1,3,5triazin-2-yl)oxy)phenyl)prop-2-en-1-one (CFHC)
CFHC, M.wt. 764.54 and m.p. 182°C to 183°C.IR (cm-1), 3340 (OH), 3037, (C-H)Ar)), 1650 (CO), 1590 (CH=CH-, str.), 1545 (N-O), 818 (C-N, s-triazine)770 (C-Cl). 1H-NMR (δ, ppm) 6.8-6.9 (d, 4H, -CO-CH=CH]),7.4-8.6 (m, 18H, Ar-H), UV(nm) aromatic (CH=CH) 241 and vinylic (CH=CH) 332. Elemental analysis calculated for the molecular formula, C39H24BrCl2N5O7 (%): C, 61.27; H, 3.16; Cl, 9.27. Found: C, 60.12; H, 2.68; Cl, 8.76.
Antibacterial Activity
Bacteria used for the determination of antibacterial activities of compounds were Gram positive; Staphylococcus aureus MTCC 29213, Gram negative; Pseudomonas aeruginosa MTCC 2488. All bacterial strains were obtained from Microbial Type Culture Collection and Gene Bank, Institute of Microbial Technology Sector 39-A, Chandigarh-160036, India. All bacterial strains were sub cultured on nutrient agar medium, incubated at 37°C for 24 hrs and stored at 4°C in refrigerator to maintain stock culture [5].
Media Used
Nutrient Agar (NA) (Gram/litre)
Peptone -5.0 g
Yeast extract -2.0 g
NaCl -5.0 g
Agar -18.0 g
Distilled water -1000 ml
pH -7.0
Nutrient broth (NB) (Gram/litre)
Peptone -5.0 g
Yeast extract -2.0 g
NaCl -5.0 g
Distilled water -1000 ml
pH -7.0
Muller Hilton agar (Gram/litre)
Beef extract powder -2.0 g
Acid Digest of Casein -17.5 g
Starch -1.5 g
Agar -17.0 g
pH -7.0
Muller Hilton broth (Gram/litre)
Beef extract powder -2.0 g
Acid digest of casein -17.5 g
Starch -1.5 g
pH -7.0
Result and Discussion
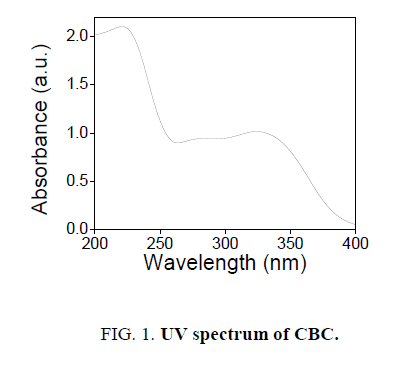
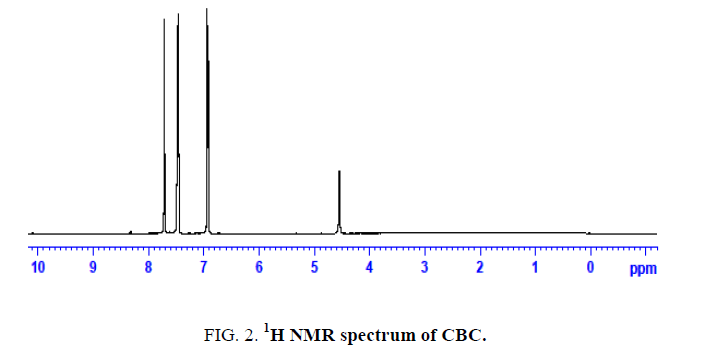
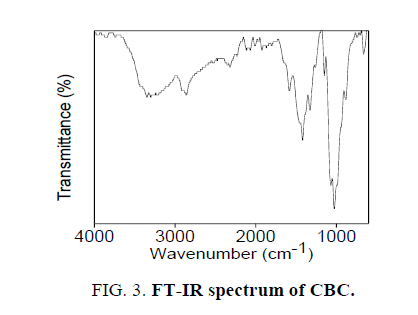
The novel six chalcones were prepared according to Scheme. 2, under solvent free conditions. All the synthesized chalcons were characterized by UV, IR and 1H NMR techniques to confirm the structure. Figures 1-3 reveals the UV, proton NMR and FT-IR spectrum of CBC respectively was the representative of the chalcone series. The presence of HC=CH was confirmed by FT-IR (cm-1), the peak appear at 1597 and at 1643 confirms the presence of CH=CH and C-O of CBC respectively. In proton NMR (δ, ppm), the peak at 6.8-6.9 and 7.1-7.8 confirms the presence of presence of CH=CH and aromatic hydrogen’s of CBC respectively. UV spectrum of CBC reveals the two characteristic peak one at 224 was due the aromatic π-π*transition and around 328 nm was due to the π-π* transition of CH=CH. Melting points of the synthesized compounds were measured using open capillary method and were incorrect. The antimicrobial activities of the synthesized compounds were evaluated using minimum inhibitory concentration (MIC) method and the obtained MIC values were presented in the Tables 1 and 2.
Figure 2: 1H NMR spectrum of CBC.
| Polymer | Control | 250μl | 125μl | 62.5μl | 31.25μl | 15.62μl | 7.81μl |
|---|---|---|---|---|---|---|---|
| CBC | 0.314 | 0.055 | 0.074 | 0.098 | 0.163 | 0.204 | 0.276 |
| CBHC | 0.314 | 0.05 | 0.074 | 0.087 | 0.155 | 0.186 | 0.253 |
| CCC | 0.314 | 0.062 | 0.086 | 0.097 | 0.168 | 0.214 | 0.284 |
| CFC | 0.314 | 0.59 | 0.082 | 0.101 | 0.166 | 0.21 | 0.286 |
| CFHC | 0.314 | 0.038 | 0.056 | 0.075 | 0.148 | 0.181 | 0.247 |
| CEHC | 0.314 | 0.048 | 0.066 | 0.084 | 0.139 | 0.173 | 0.268 |
Table 1: MIC values of synthesized compounds on P. aeruginosa.
| Polymer | Control | 250µl | 125µl | 62.5µl | 31.25µl | 15.62µl | 7.81µl |
|---|---|---|---|---|---|---|---|
| CBC | 0.369 | 0.067 | 0.098 | 0.132 | 0.159 | 0.227 | 0.284 |
| CBHC | 0.369 | 0.072 | 0.121 | 0.148 | 0.162 | 0.203 | 0.291 |
| CCC | 0.369 | 0.056 | 0.088 | 0.118 | 0.138 | 0.179 | 0.265 |
| CFC | 0.369 | 0.073 | 0.099 | 0.137 | 0.167 | 0.269 | 0.371 |
| CFHC | 0.369 | 0.041 | 0.077 | 0.116 | 0.142 | 0.216 | 0.311 |
| CEHC | 0.369 | 0.063 | 0.101 | 0.14 | 0.17 | 0.263 | 0.319 |
Table 2: MIC values of synthesized compounds on S. aureus.
Minimum inhibitory concentrations (Mics)
The minimum inhibitory concentrations of the synthesized compounds CBC, CBHC, CCC, CFC, CFHC and CEHC were determined by dilution method (Brantner and Grein, 1994). The strains were grown in Mueller Hinton broth to exponential phase with an A560 of 0.8, representing 3 × 108 CFU/ml. Different concentration (7.8, 15.6, 31.2, 62.5, 125 and 250 μg/ml) of synthesized compounds (1 mg of compound in 1 ml of 7% DMSO) were added on to separate test tubes containing 4 ml of MH broth inoculated with 0.5 ml bacterial suspension at a final concentration of 108 CFU/ml. Each MIC was determined from five independent experiments performed in triplicates. The tubes containing 4.5 ml of bacterial inoculates and 0.5 ml of 7% DMSO were used as bacterial control, 4.5 ml of uninoculated Mueller Hinton broth and 0.5 ml 7% DMSO served as a blank. The tubes were incubated at 37°C for 18 h; inhibition of bacterial growth was determined by measuring the absorbance at A560 nm.
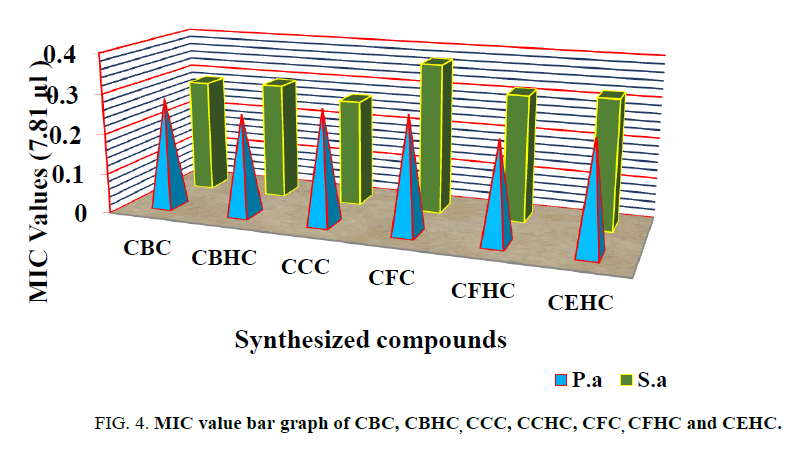
MICs results revealed that the OD value was higher in the control because the bacteria caused turbidity. There was a gradual decrease in the optical density at higher dilution, whereas, the compound CCC showed more inhibitory activity at the lowest dilutions (MIC 7.8 μg/ml) than other compounds (MIC 7.8 μg/ml) against Gram-negative pathogens such as Pseudomonas aeruginosa; and Gram-positive bacteria such as Staphylococcus aureus (Figure 4). 7.8 μg/ml was the effective (MIC) concentration for the inhibitory effect of all compounds tested against bacteria. The anti-microbial activity of compound P-30 was weaker against both microorganisms.
Solubility of the synthesized compounds
The solubility data of the synthesized compounds are presented in the Table 3. The CBC, CBHC, CCC, CFC, CFHC and CEHC are almost soluble in all the solvents used for solubility test except water, benzene and n-hexane but freely soluble in methanol, ethanol, dimethyl sulfoxide, dimethylformamide and tetrahydrofuran.
| Polymer | H2O | MeOH | EtOH | CHCl3 | DMSO | DMF | Acetone | C6H6 | THF | n-Hexane |
|---|---|---|---|---|---|---|---|---|---|---|
| CBC | _ | + | + | + | + | + | + | + | _ | |
| CBHC | _ | + | + | + | + | + | + | _ | + | _ |
| CCC | _ | + | + | + | + | + | + | _ | + | _ |
| CFC | - | ± | ± | ± | + | ± | ± | - | ± | _ |
| CFHC | _ | + | ± | ± | + | + | + | _ | + | _ |
| CEHC | _ | + | ± | ± | + | + | + | _ | + | _ |
+ = Soluble, - = Insoluble and ± = partially soluble
Table 3: Solubility Data of the novel chalcones at 30°C.
Conclusion
There are six novel compounds were synthesized by substituting various molecules in to the cyanuric chloride. All the synthesized compounds were characterized by FT-IR, NMR and UV spectroscopy techniques. All the synthesized chalcones were completely soluble in polar protic solvents like acetone, alcohol, DMSO, THF, ether but insoluble in water. Melting points of all the synthesized compounds were determined in an open capillary and found to be dependent on the molecular weight of the compounds. Antimicrobial activity of CBC, CBHC, CCC, CFC, CFHC and CEHC were tested on Staphylococcus aureus and Pseudomonas aeruginosa. CFHC showed more inhibitory activity at the lowest dilutions (MIC 7.8 μg/ml) than other compounds (MIC 7.8 μg/ml) against Gram-negative pathogens such as Pseudomonas aeruginosa (0.247). Whereas CCC showed excellent activity on tested gram positive Staphylococcus aureus bacteria’s with the MIC value of 0.265.
References
- Sonja F, Carola S Ulrich BF, et al. In vitro anti-plasmodial activity of prenylated chalcone derivatives of hops (Humulus lupulus) and their interaction with haemin.J Antimicrob Chemoth.2005;55: 883-7.
- SivaPM, PrabhawathiV, RamalingamN, et al. Chalcone coating on cotton cloth: An approach to reduce attachment of live microbes.BiomaterSci.2014;2: 990-5.
- AmritaD, Gopi KA, Krishna J, et al. An efficient synthesis of highly substituted indanones and chalcones promoted by superacid. RSC Adv. 2014;4: 26662-6.
- Dawane BS, GaikwadMV,Rahul D, et al. Bleaching earth clay (pH 12.5): a green catalyst for rapid synthesis of pyranopyrazole derivatives via a tandem three-component reaction. Res ChemIntermed. 2013;39: 3859-66.
- Brantner A, Grein E. Antibacterial activity of plant extract used externally in traditional medicine. J Ethnopharmacol. 1994;44: 35-40.