Original Article
, Volume: 15( 3)Heterogeneous Photo Catalytic Degradation of the Phenothiazine Dye, Azure A: A Kinetic Approach
- *Correspondence:
- David Swami, Department of Chemistry, SBN Govt. PG College, Barwani, India, Tel: 072902 22035; E-mail: david_swami@rediffmail.com
Received: May 02, 2017; Accepted: June 05, 2017; Published: June 14, 2017
Citation: David Swami, Brijesh Pare, Prakash More. Heterogeneous Photo Catalytic Degradation of the Phenothiazine Dye, Azure A: A Kinetic Approach. Int J Chem Sci. 2017;15(3):155
Abstract
The photo catalytic degradation of the phenothiazine day, Azure A was investigated in aqueous solutions containing TiO2 suspension as photo catalyst. The influence of parameters as initial dye concentration, amount of catalyst, solute, on pH addition of H2O2, K2S2O8, the presence of NaCl, Na2CO3, temperature, N2, O2 purging and COD, DO2 measurements were studied. The degradation rated was found to be strongly influenced by all the above parameters. Results showed that adsorption played an important role in the control of the photo catalytic degradation and an optimal TiO2 concentration of 300 mg/100 mg was determined. The reaction takes place much effectively at ph 9.00 and its play on important role in the characteristic of textile wastewater and generation of hydroxyl radicals. The results also indicated that the greater the formation rate of OH radical was, the higher photo catalytic activity was achieved. The degradation of dyes was also investigated under sunlight and the efficiency of degradation was compares with that of the artificial light source UV-Vis spectra, estimated COD, evaluation of CO2 confirmed the mineralization of dyes during photo catalytic degradation secondary pollution. So, in recent years, heterogeneous photo catalytic oxidation has received considerable attention of dyes and textile effluents, Heterogeneous photo catalysis is based on the irradiation of a photo catalyst, usually a semiconductor such as TiO2 with light energy equal to or greater than the band gap energy. This causes a valence band electron to be excited to the conduction band, causing charge separation. The conduction band electrons and valence band holes can then migrate to the surface and participate in interfacial.
Keywords
Phenothiazin; Azure A; TiO2; Photocatalytic degradation; Wastewater
Introduction
Textile dyes and other industrial dyestuffs constitute one of the largest group of organic compounds that represent an increasing environmental danger, About 1%-20% of the total world production of dyes is last during the dyeing process and is released in the textile effluents [1,2] for the treatment of dye containing wastewater, tradition methods such as flocculation carbon adsorption, reverse osmosis and activated sludge process has difficulties in the complete destruction of dye pollutants [3] and has the further disadvantage of potentially oxidation-reduction reactions, The oxidative degradation of an organic pollutant is attributed at the positive role where adsorbed water or hydroxyl groups are oxidized to hydroxyl radicals (aa•OH), which then read with the pollutant molecule [4]. Thus, finally it leads to their complete mineralization.
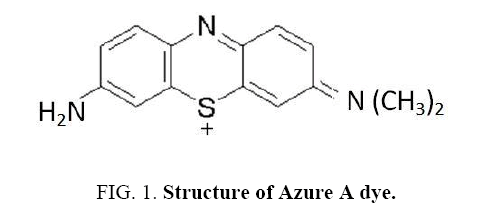
In the present work the photo catalytic degradation in aqueous solution of the phenothiazine aye, Azure A was investigated. Phenothiazine dyes represent the important class of commercial dyes and are mainly used for dyeing cotton silk, polyamide and leather. The structure of Azure A dye is shown in (Figure 1) To optimize the kinetic of the degradation process, several parameters were studied.
Experimental
Azure A was abstained from Aldrich Company. Photo catalyst TiO2 was obtained from the S.D. Fine Company. All Solutions were prepared in doubly distilled water. Photo catalytic experiments were carried out with 50 mL of dye solution (3.8 × 10-5 mol dm-3) using 300 mg of TiO2 photo catalytic under exposure to visible irradiation in specially designed double-walled slurry type batch reactor vessel made up of Pyrex glass (7.5 cm height, 6 cm diameter) surrounded by thermostatic water circulation arrangement to keep the temperature in the range of 30°C ± 0.3°C. Irradiation was carried out using 500 w halogen lamp surrounded by aluminum reflector to avoid irradiation loss.
During photocatalytic experiments after stirring for 10 min slurry composed of dye solution and catalyst was placed in dark for ½ h in order to establish equilibrium between adsorption and desorption phenomenon of dye molecule on photo catalyst surface. Them slurry containing aqueous dye solution and TiO2 was stirred magnetically to ensure complete suspension of catalyst particle while exposing to visible light. At specific time intervals aliquot (3 ml) was withdrawn and centrifuges for 2 min to remove TiO2 particle from aliquot to assess extent of decolorization photo metrically. Changes in absorption spectra were recorded at 480 nm on double beam UV-Vis, spectrophotometer (Systronic Model No. 166) Intensity of visible radiation was measured by a digital luxmeter (Lutron LX 101). pH of solution was measured using a digital PH meter. COD and CO2 estimation were performed. Performance efficiency was calculated as % Efficiency= 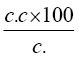 Where C and C. are initial and final dye lament ration.
Where C and C. are initial and final dye lament ration.
Results and Discussion
An aliquot was taken from the reaction mixture at regular time interval and the absorbance was measured spectra photo metrically at max value of 630 nm. The absorbance of the solution was found to decrease with increasing time, which indicates that the concentration of Azure a dye decreased with increasing time of exposure.
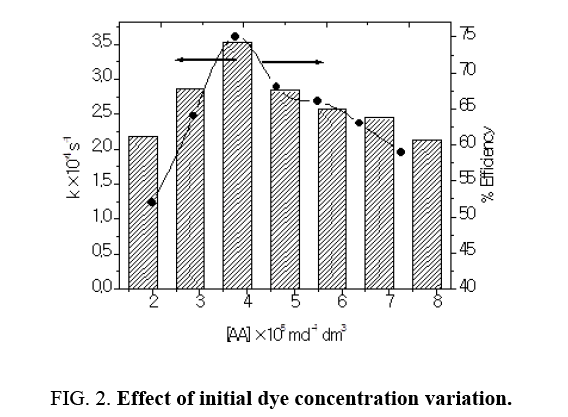
Effect of initial dye concentration variation
The influence of initial dye concentrations was investigated by varying the initial concentration from 1.8 × 10-5 mol dm-3 to 7.8 × 10-5 mol dm-3 at pH 9.0 and with catalyst loading 300 mg/100 ml. Rate constant increased with increase in dye concentration from 1.8 × 10-5 mol dm-3 to 3.8 × 10-5 mol dm-3. Rate constant has been found to be maximal at 3.8 × 10-5 mol dm-3 (Figure 2). The dye concentration influenced the extent of adsorption and rate of reaction at the surface of the photocatalyst. Increasing initial dye concentration would enhance the rate of the liquid phase reaction and surface reaction as well and the adsorption of dye molecules on the catalyst surface hinders competitive adsorption of OH‾ ions, thus it lowers the formation rate of hydroxyl radicals and consequently it affected the rate of reaction [5]. The increase in dye concentration also decreased the path length of photon entering into the dye solution and this might also reduce the catalytic efficiency (Table 1).
| [Azure A] ×105 mol-1dm3 | k × 10-4 s-1 | t1/2 × 103 s |
|---|---|---|
| 1.8 | 2.18 | 3.17 |
| 2.8 | 2.87 | 2.41 |
| 3.8 | 3.53 | 1.96 |
| 4.8 | 2.84 | 2.44 |
| 5.8 | 2.57 | 2.69 |
| 6.8 | 2.45 | 2.82 |
| 7.8 | 2.14 | 3.23 |
Table 1: Effect of initial dye concentration variation: TiO2=300 mg/100 mL pH=9.0, Light intensity=20 × 103 lux, Temperature=30 ± 0.3°C.
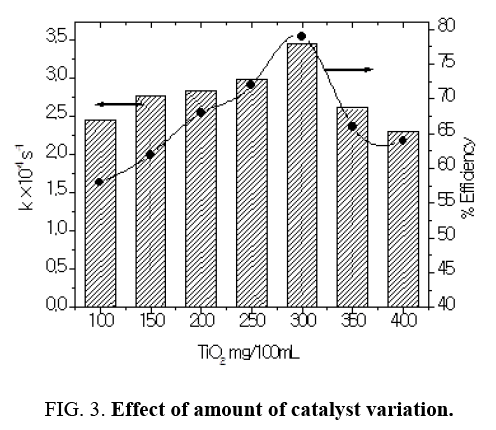
Effect of amount of catalyst variation
The catalyst dose is having strong influence on the degradation kinetics of dye. In order to determine the optimal amount of catalyst concentration, a series of experiments were carried out using different concentrations of TiO2 catalyst varying from 100 mg/100 mL to 400 mg/100 mL at optimized pH 9.0. It has been observed that as the concentration of catalyst increased from 100 mg to 300 mg/100 ml the degradation rate got increased rapidly. The increased degradation rate that followed the increase in the catalyst loading could be attributed to the fact that a larger number of photons were adsorbed, thus accelerating the process. The most effective decomposition of Azure A has been observed at catalyst amount 300 mg/100 ml. Further increase of the TiO2 concentration resulted in a decrease of rate of decoloration. On further increase in concentration all the molecules got adsorbed on TiO2 thus no improvement was achieved on further adding more catalyst. The decrease in efficiency, which is observed in the Figure 3, might be due to an increase in capacity of the suspension and to an enhancement of the light reflectance, because of the excess of TiO2 particles (Table 2). Additionally, in the case of high catalyst loading we observed agglomeration and sedimentation of TiO2 which made a significant fraction of catalyst to be inaccessible to either absorbing the dye or absorbing the radiation (Figure 3), with consequent decrease in active sites available to the catalytic reaction [6].
| TiO2 mg/100mL | k × 10-4 s-1 | t1/2 × 103 s |
|---|---|---|
| 100 | 2.45 | 2.82 |
| 150 | 2.76 | 2.51 |
| 200 | 2.84 | 2.44 |
| 250 | 2.99 | 2.31 |
| 300 | 3.53 | 1.96 |
| 350 | 2.61 | 2.66 |
| 400 | 2.3 | 3.01 |
Table 2: Effect of amount of catalyst variation: [Azure A] =3.8 × 10-5 moldm-3 pH=9.0, Light intensity=20 × 103 lux, Temperature=30 ± 0.3°C.
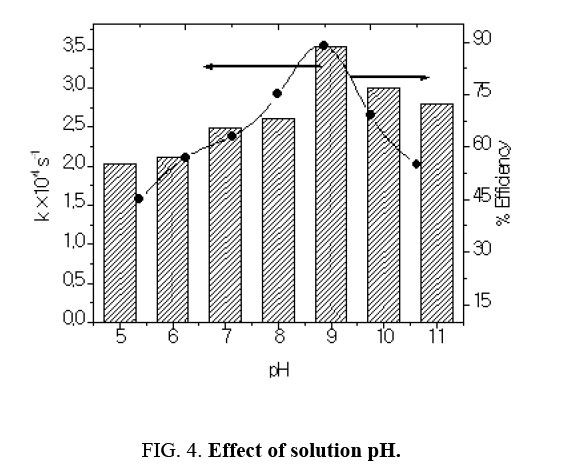
Effect of solution pH
The waste water from textile industries usually has wide range of pH values. pH plays an important role both in the characteristic of textile wastewater and generation of hydroxyl radicals. The effect of pH on the rate of degradation has been investigated in the pH range 5.0 to 11.0. Upon changing the pH, the surface hydroxyl group could undergo protonation and deprotonation according to the following reaction [7].
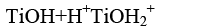 (1)
(1)
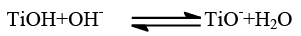 (2)
(2)
Bahnemann et al have reviewed that acid-base properties of the metal oxide surfaces could have considerable implications upon their photocatalytic activity. The point of zero charge of the TiO2 is at pH 6.8. Thus, the TiO2 surface becomes positively charged in acidic media, whereas it is negatively charged under alkaline conditions [8]. Since Azure A dye is cationic dye, thus there was a poor adsorption in the acidic medium. Therefore, decrease in pH caused decrease in degradation rate. Higher pH favors electrostatic interactions between the negative TiO2 surface and cationic dye led to strong adsorption and enhancing degradation rate (Table 3). The optimal pH was observed at 9.0. The results obtained in (Figure 4) indicated that, at pH 9.0 a strong adsorption of the Azure A dye on the TiO2 particles was observed as a result of the electrostatic attraction of the negatively charged TiO2 with the dye. The inhibitory effect observed to be more pronounced in the higher alkaline range. At high pH values the hydroxyl radicals are rapidly scavenged and they do not have the opportunity to react with dye [9].
| pH | k × 10-4 s-1 | t1/2 × 103 s |
|---|---|---|
| 5 | 1.5 | 4.62 |
| 6 | 2.11 | 3.28 |
| 7 | 2.49 | 2.78 |
| 8 | 2.61 | 2.65 |
| 9 | 3.53 | 1.96 |
| 10 | 3.1 | 2.23 |
| 11 | 2.99 | 2.31 |
Table 3: Effect of solution pH: [Azure A]=3.8 × 10-5 moldm-3 TiO2=300 mg/100 mL, Light intensity=20 × 103 lux Temperature=30 ± 0.3ºC.
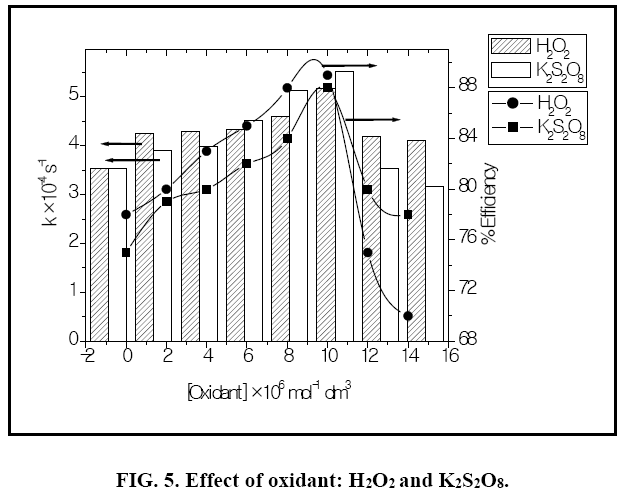
Effect of H2O2 and K2S2O8
The addition of other power oxidizing species such as H2O2 and K2S2O8 to TiO2 suspension is a well-known procedure and lead to an increase in the rate of photo-oxidation. The photocatalytic degradation has been studied at different H2O2 concentrations. H2O2 is well-known to have two functions in the process of photocatalytic degradation. It accepts a photo generated electron from the conduction band and it also forms •OH radicals according to following equation.
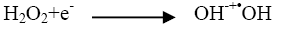 (3)
(3)
 (4)
(4)
The rate of reaction has been found to be increased as the concentration of H2O2 increased and it reached to the optimum value at 10 × 10-6 mol dm-3. (Figure 5) On further addition of H2O2 it decreased as the concentration of the H2O2 increased beyond the optimum because excess H2O2 might act as a ?OH scavenger and form peroxo compounds, which are detrimental to the photocatalytic action [10].
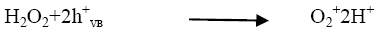 (5)
(5)
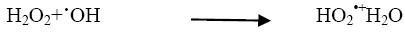 (6)
(6)
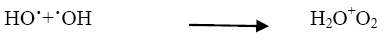 (7)
(7)
The effect of persulphate ion on the photocatalytic degradation of the dye was investigated. The concentration of K2S2O8 was 2 × 10-6 mol dm-3 to 14 × 10-6 mol dm3. The data have been presented in Figure 2. The percentage degradation of the dye increased with increasing amount of persulphate ion concentration. K2S2O8 proved to be a beneficial oxidizing agent in photocatolytic detoxification since it generates SO4?‾ oxidant as per reactions (12) and (13) utilizing the semiconductor generated electrons (e-cb).
 (8)
(8)
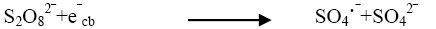 (9)
(9)
The strong oxidant sulphate radical anion 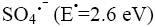 reacted with dye molecules in the following three possible modes of reactions: By abstracting a hydrogen atom from saturated carbon, by adding it to unsaturated or aromatic carbon and by removing one electron from carboxylate anion or from certain neutral molecules [11].
reacted with dye molecules in the following three possible modes of reactions: By abstracting a hydrogen atom from saturated carbon, by adding it to unsaturated or aromatic carbon and by removing one electron from carboxylate anion or from certain neutral molecules [11].
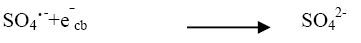 (10)
(10)
 (11)
(11)
Thus, formation of hydroxyl radical and sulphate radical anion enhanced the degradation of dye molecules at faster rate [12]. The optimum concentration (Table 4) has been found to be 10 × 10-6 moldm-3. Above thus optimum concentration the rate constant values decreased due to the absorption of sulphate ions on the surface of TiO2 deactivating a section of photocatalyst (Figure 5).
| [Oxidant] × 106mol-1dm3 | H2O2 | K2S2O8 | ||
|---|---|---|---|---|
| k × 10-4 s-1 | t1/2 × 103s-1 | k × 10-4s-1 | t1/2 × 103s-1 | |
| 0.0 | 3.53 | 1.96 | 3.53 | 1.96 |
| 2.0 | 4.26 | 1.62 | 3.91 | 1.77 |
| 4.0 | 4.29 | 1.61 | 3.99 | 1.73 |
| 6.0 | 4.33 | 1.60 | 4.52 | 1.53 |
| 8.0 | 4.60 | 1.50 | 5.14 | 1.34 |
| 10.0 | 5.18 | 1.33 | 5.52 | 1.25 |
| 12.0 | 4.18 | 1.66 | 3.53 | 1.96 |
| 14.0 | 4.10 | 1.69 | 3.17 | 2.18 |
Table 4: Effect of H2O2 and K2S2O8: [Azure A]=3.8 × 10-5 mol dm-3 TiO2=300 mg/ 100 mL, pH=9.0, Light intensity=20 × 103 lux Temperature=30 ± 0.3°C.
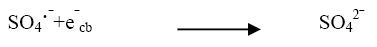 (12)
(12)
 (13)
(13)
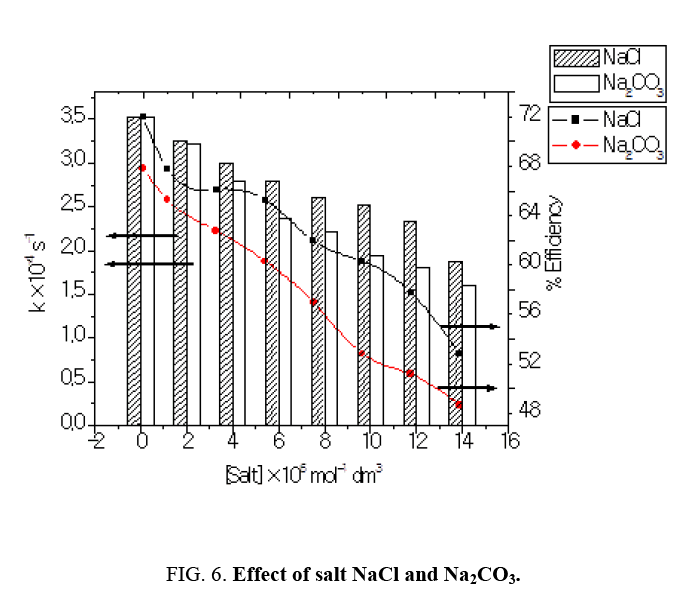
Effect of NaCl and Na2CO3
Industrial effluents contain, apart from the pollutants, different salts at different concentrations. The inorganic anion may have an effect on the rate of photocatalytic degradation. The presence of anions such as chloride, carbonate, sulphate and bicarbonate is common in industrial effluents. These ions affect the adsorption of the degrading species, acts as hydroxyl ion scavengers and may absorb light as well [13]. The presences of sodium chloride decreased the photocatalytic degradation rate considerably due its effect on adsorption. The degradation rate of Azure A dye decreased with increase in the amount of chloride ions from 2 × 10-6 mol dm-3 to 14 × 10-6 mol dm-3. The inhibiting effect of Cl‾ ion on the degradation has been due to its ability to act as hydroxyl radical (•OH) scavengers.
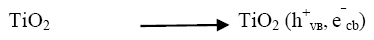 (14)
(14)
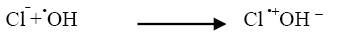 (15)
(15)
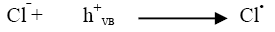 (16)
(16)
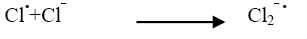 (17)
(17)
We have also studied the effect of carbonate anions on photodegradation, an increment in concentration of CO32- from 2 × 10-6 mol dm-3 to 14 × 10-6 mol dm-3, resulted into decrease of rate of reaction (Table 5). When carbonate ions react with •OH radicals (Figure 6) they generate carbonate radical anions that is a weak oxidizing agent and hardly reacts with other molecules and thus the rate of reaction got decreased due to the hydroxyl scavenger property of carbonate ions [14].
| [Salt] × 106mol-1 dm3 | NaCl | Na2CO3 | ||
|---|---|---|---|---|
| k × 10-4 s-1 | t1/2 × 103 s-1 | k × 10-4 s-1 | t1/2 × 103 s-1 | |
| 0 | 3.53 | 1.96 | 3.53 | 1.96 |
| 2 | 3.26 | 2.12 | 3.22 | 2.15 |
| 4 | 3 | 2.31 | 2.8 | 2.47 |
| 6 | 2.8 | 2.47 | 2.37 | 2.92 |
| 8 | 2.61 | 2.65 | 2.22 | 3.12 |
| 10 | 2.53 | 2.73 | 1.95 | 3.55 |
| 12 | 2.34 | 2.96 | 1.8 | 3.85 |
| 14 | 1.88 | 3.68 | 1.6 | 4.33 |
Table 5:Effect of NaCl and Na2CO3: [Azure A]=3.8 × 10-5 mol dm-3 TiO2=300 mg/100 mL, pH=9.0, Light intensity=20 × 103 lux Temperature=30 ± 0.3°C.
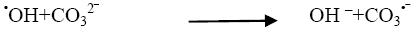 (18)
(18)
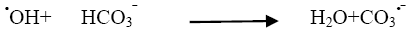 (19)
(19)
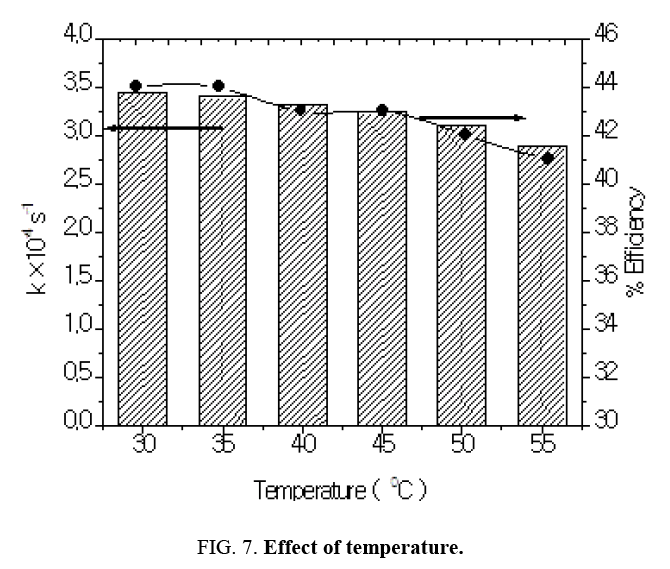
Effect of temperature
One of the advantages of photocatalytic reactions is that it is not affected or slightly affected by temperature change. The influence of temperature was studied in the range 30°C-55°C. The results are shown in Table 6 and Figure 7. Increase in temperature led to decrease the rate of degradation. This gradual decrease in the reaction rate values could be attributed to the following reasons: the adsorption rate decreased with increasing temperature because the adsorption is a heat releasing process, increase in reaction temperature tend to increase electron-hole recombination and with increase in temperature the solubility of oxygen in water decreased.
| Temperature (°C) | k × 10-4 s-1 | t1/2 × 103 s |
|---|---|---|
| 30 | 3.45 | 2 |
| 35 | 3.4 | 2.38 |
| 40 | 3.32 | 2.08 |
| 45 | 3.24 | 2.13 |
| 50 | 3.1 | 2.23 |
| 55 | 2.9 | 2.38 |
Table 6: Effect of temperature: [Azure A]=3.8 × 10-5 mol dm-3, pH=9.0 TiO2=300 mg/100 mL, Light intensity=20 × 103 lux, Temperature=30 ± 0.3°C.
Effect of N2 and O2 purging
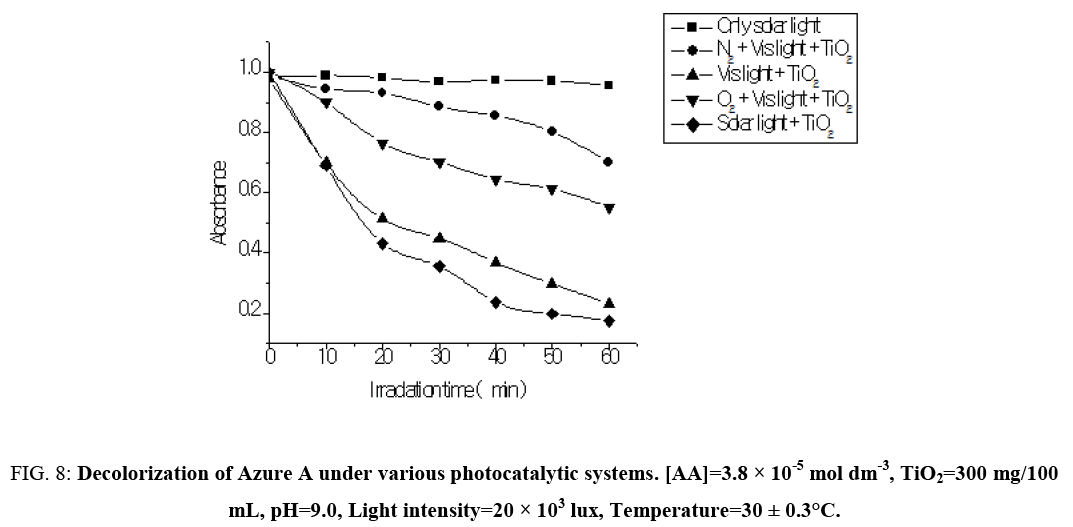
The effect of nitrogen and oxygen has been widely studied in photocatalytic oxidation systems. Studies have shown that the bubbling of pure N2 in the system retarded the degradation of the dye but bubbling of O2 in the system enhanced the degradation of the dye. The degradation of Azure A has been increased from 3.53 × 10-4 s-1 to 5.60 × 10-4 s-1 on bubbling of oxygen through the dye solution but decreased from 3.53 × 10-4 s-1 to 2.45 × 10-4 s-1 on purging of pure N2 through the dye solution. Oxygen as an electron acceptor and strongly electrophillic, forming the superoxide radicals (O2‾•) upon scavenging of the electrons (Figure 8). This helped to prevent the recombination of electrons and holes and enables the system to maintain a favorable charge balance necessary for the photocatalytic redox process. Thus, oxygen often used as an electron acceptor in photocatalytic oxidation processes since it is readily available, soluble under most conditions and non-toxic. The surface chemical reactions of conduction band (CB) electrons are blocked by N2 purging, consequently the formation of superoxide radical anion and hydroxyl radicals are prevented. As a result, the photocatalytic degradation process would be significantly suppressed.
Figure 8: Decolorization of Azure A under various photocatalytic systems. [AA]=3.8 × 10-5 mol dm-3, TiO2=300 mg/100 mL, pH=9.0, Light intensity=20 × 103 lux, Temperature=30 ± 0.3°C.
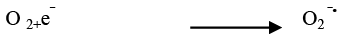 (20)
(20)
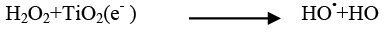 (21)
(21)
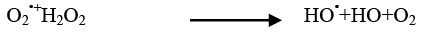 (22)
(22)
 (23)
(23)
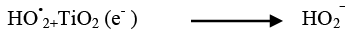 (24)
(24)
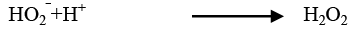 (25)
(25)
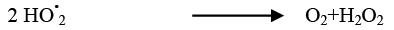 (26)
(26)
COD and CO2 measurements during mineralization process
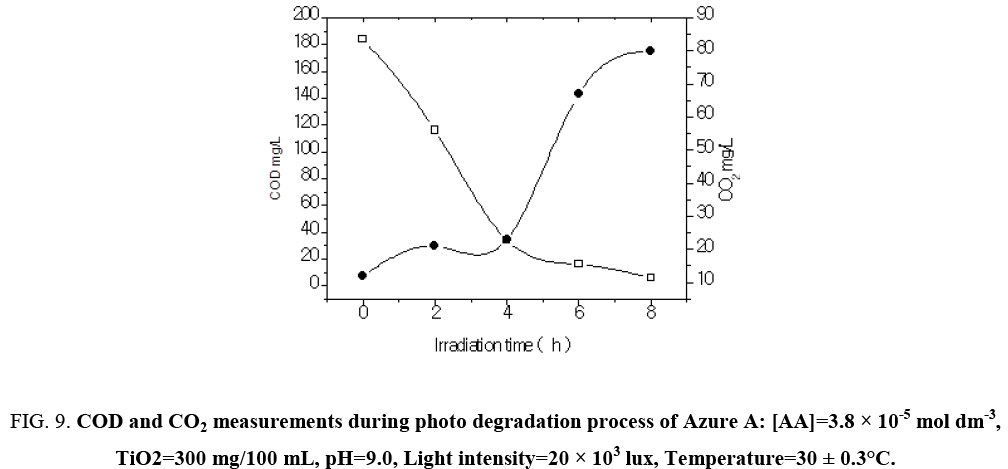
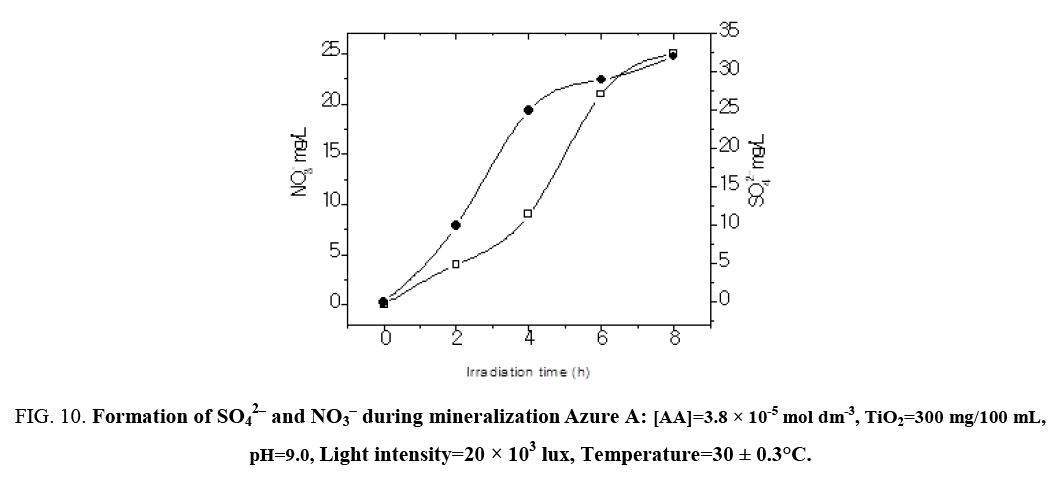
The result presented in Table 7 show that the photocatalyic process led, apart from decolorization, to a substantial decrease of the chemical oxygen demand (COD) of the solution. As the irradiation time increased, dye molecules are degraded to components of lower and lower molecular weight fractions and consequently led to complete mineralization that can be is ascertained by COD and CO2 measurements. The COD is widely used as an effective technique to measure the organic strength of wastewater. The test allowed the measurement of waste in terms of the total quantity of oxygen required for the oxidation of organic matter to CO2 and H2O. The COD of the dye solution was estimated before and after the treatment. The reduction in COD values of the treated dye solution indicated the mineralization of dye molecules along with color removal (Figure 9). The aqueous solution of a mixture of Azure A (3.8 × 10-5 mol dm-3) was exposed separately to visible light in the presence of TiO2 at a dose of 300 mg/100 mL. Aliquots were taken at regular intervals and COD was measured using closed reflux titrimatric method. As the irradiation time, increased dye molecule got degraded to compounds in the estimated COD value from 184 mg/L to 0 mg/L. CO2 value increased from 55 mg/L to 242 mg/L in 6 h of illumination indicated photo degradation of treated dye solution. The reduction in pH of solution has also been observed with increase in the extent of mineralization (Figure 10).
Figure 9: COD and CO2 measurements during photo degradation process of Azure A: [AA]=3.8 × 10-5 mol dm-3, TiO2=300 mg/100 mL, pH=9.0, Light intensity=20 × 103 lux, Temperature=30 ± 0.3°C.
Figure 10: Formation of SO42‾ and NO3‾ during mineralization Azure A: [AA]=3.8 × 10-5 mol dm-3, TiO2=300 mg/100 mL, pH=9.0, Light intensity=20 × 103 lux, Temperature=30 ± 0.3°C.
| Irradiation time (h) | COD (mg/L) | CO2 (mg/L) | Efficiency (%) | NO3- (mg/L) | SO42‾ (mg/L) | pH |
|---|---|---|---|---|---|---|
| 0 | 184 | 12 | 0 | 0 | 0 | 8.7 |
| 2 | 116 | 21 | 36 | 4 | 10 | 6.3 |
| 4 | 34 | 23 | 81 | 9 | 25 | 4.7 |
| 6 | 16 | 67 | 91 | 21 | 29 | 4 |
| 8 | 6 | 80 | 96 | 25 | 32 | 3.2 |
Table 7: COD and CO2 measurements during photodegradation process: [Azure A]=3.8 × 10-5 mol dm-3, TiO2=300 mg/100 mL, pH=9.0, Light intensity=20 × 103 lux, Temperature=30 ± 0.3°C.
UV- Vis spectral analysis during mineralization
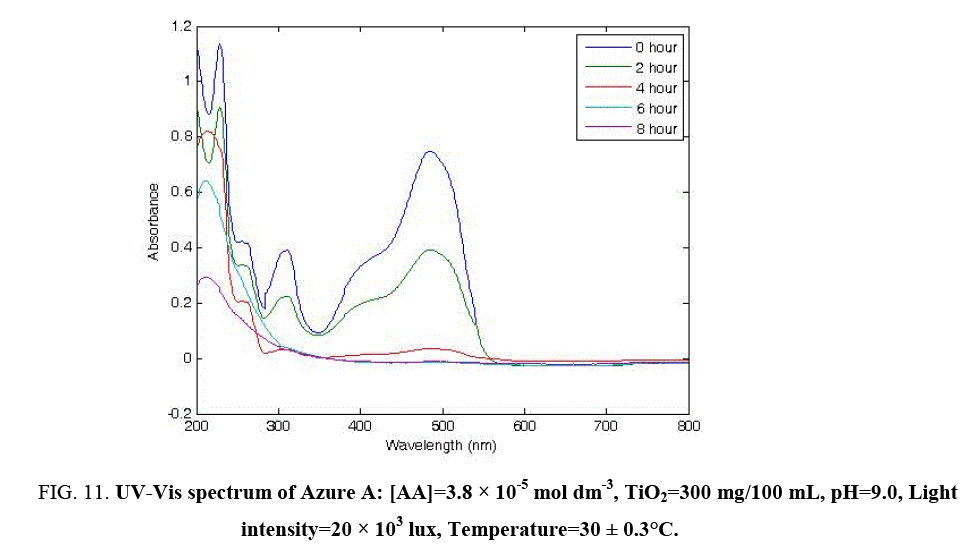
The UV-Vis absorption spectra of AA were studied at different times of irradiation. AA is phenothiazine dye in which the chromophore part of molecular structure contains phenothiazine group. The changes in the absorption spectra of AA solution during the photocatalytic degradation at different irradiation times are presented in Figure 2. both in the UV and visible region. Its λmax is 480 nm (Figure 11). The decrease of absorption peaks actually indicated a rapid decoloration and degradation of AA dye. The nearly perfect disappearance of peaks in UV region reveals that AA dye is eliminated in presence of TiO2 after 8 h of irradiation.
Figure 11: UV-Vis spectrum of Azure A: [AA]=3.8 × 10-5 mol dm-3, TiO2=300 mg/100 mL, pH=9.0, Light intensity=20 × 103 lux, Temperature=30 ± 0.3°C.
Conclusion
Photo assisted mineralization of Azure A can be effectively carried out utilizing TiO2 with visible light. Amount of catalyst, solution pH has been found to be Vitol parameter. The presence of inorganic salts such as sodium carbonate and sodium chloride hinders the photo catalytic degradation of textile dyes. Synergetic of H2O2 and K2S2O8 has ols-ban investigated. The reduction in COD of the effluent suggested that the dye molecules were completely mineralized. UV-Vis spectra confirmed the degradation of Azure A dye.
Acknowledgement
Authors acknowledgment the support and Laboratory facilities provides by Chemistry Department Govt. Madhav Science P.G. College, Ujjain, Madhya Pradesh, India.
References
- H. Zollinger.Color chemistry, synthesis, properties and application of organic dyes and pigments, second revised ed., VCH. 1991.
- Herrera F, Lopez A, Mascola G, et al. Catalytic decomposition of the reactive dye uniblueA on hematite. Modeling of the reactive surface. Water Res.2001;35:750-60.
- PouliosI, Tsachpinis I.Photodegradation of the textile dye Reactive Black 5 in the presence of semiconducting oxides. J ChemTechnolBiotechnol.1999;71:349-57.
- Davis AP, Huang CP. The removal of substituted phenols by a photocatalytic oxidation process with cadmium sulfide. Wat Res. 1990;24:543-50.
- Graetzel C, Jirousek M,Graetzel M.Decomposition of organophosphorus compounds on photoactivated TiO2 surfaces. J MolCatal. 1990;60:375-87.
- Nasr C, Vinodgopal K, Kotchandani S, et al.Photocatalytic reduction of azo dyes naphthol blue black and disperse blue 79. ChemIntermed.1997;23:219-31.
- Minero C, Pelizzetti E, Malato S, et al. Large solar plant photocatalytic water decontamination: Degradation of pentachlorophenol. Chemosphere.1993;26:2103.
- Abdullah M, Gary KCL,Matthews RW. Effects of common inorganic anions on rates of photocatalytic oxidation of organic carbon over illuminated titanium dioxide.JPhys Chem.1990;94:6620-5.
- Haarstrick A, Kut OM,Heinzle E. TiO2-assisted degradation of environmentally relevant organic compounds in wastewater using a novel fluidized bed photoreactor. Environ Sci Technol.1996;30:817-24.
- Wang S.A comparative study of Fenton and Fenton-like reaction kinetics in decolourisation of wastewater. Dyes and Pigments.2008;76:714-20.
- Lea J,Adesina AA. The photo-oxidative degradation of sodium dodecyl sulphate in aerated aqueous TiO2 suspension.J PhotochemPhotobiol A: Chem.1998;118:111-22.
- Chen F, Xie Y, Zhao J, et al.Photocatalytic degradation of dyes on a magnetically separated photocatalyst under visible and UV irradiation.Chemosphere.2001;44:1159-68.
- Qamar M,Muneer M.A comparative photocatalytic activity of titanium dioxide and zinc oxide by investigating the degradation of vanillin. Desalination. 2009;249:535-40.
- Kavitha SK,Palaisamy PN. World Academy of Science, Engineering and Technology C: Civil and Environmental Engineering. 2010;3:1.