Original Article
, Volume: 12( 2)Experimental Study on the Pickling of Zircaloy Tubes and Appendages
- *Correspondence:
- Mandal D, Alkali Material & Metal Division, Bhabha Atomic Research Centre, Trombay, Mumbai-400085, India, Tel: +91-22-25505050; E-mail: dmandal10@gmail.com
Received: July 21, 2017; Accepted: August 18, 2017; Published: August 22, 2017
Citation: Bhattacharjee D, Mandal D. Experimental Study on the Pickling of Zircaloy Tubes and Appendages. Chem Technol Ind J. 2017;12(1):113
Abstract
The pressurized heavy water reactors use natural uranium oxide (UO2) as fuel and use cladding material made up of zircaloy, an alloy of zirconium. Pickling of zircaloy tubes and appendages viz., spacer and bearing pads is carried out to remove the oxide layer and surface contaminants. Experiments were conducted to optimize the composition of zircaloy pickling solution. The HF-HNO3 ratio in pickling solution and the effect of the optimized solution composition with both fresh and regenerated solution was studied. In this present paper, the authors present their experience about the recycling suitability of the used pickling solution and the rate of pickling reaction with the optimized solution.
Keywords
Zircaloy; Pickling rate; Nuclear regeneration; Composition; hexa-fluoro-zirconic acid
Introduction
Indian Pressurized Heavy Water Reactors (PHWRs) use natural uranium (0.7% U235) in the form of ceramic uranium di-oxide (UO2) as fuel and zircaloy, an alloy of zirconium (Zr), as cladding material. During the fabrication process of zircaloy as cladding material, different surface impurities and an oxide layer get deposited on the zircaloy tubes and appendages, viz. spacer pads and bearing pads. These surface contaminants, if not removed from the finished zircaloy product, may cause problems in the operation of the nuclear reactor [1,2]. These surface contaminants and oxide layer are normally removed by pickling process [3]. However, much information is not available in open literature, since the process has specific application and has very commercial importance. This paper discusses the process of pickling of zircaloy appendages, the ratio of the optimum concentration of the reagents and the rate of pickling reaction as studied at Nuclear Fuel Complex (NFC), Hyderabad, India.
Pickling Process
Pickling operation is a metal surface treatment process used to remove impurities like stains, contaminants, oxide film, scale etc. [3-7]. Pickling is usually carried out using an aqueous solution of hydrofluoric acid (HF) and nitric acid (HNO3) in a batch process. Since zirconium and its alloy, i.e. zircaloy, do not dissolve in all mineral acids readily, selection of a suitable pickling bath has to be done by studying the corrosion data [8,9]. Hlawka and Sutter [10] studied the solubility of Zr-F complexes in pickling solution with only HF and with a combination of HF-HNO3 zircaloy dissolves in hydrofluoric acid solutions, forming a black porous hydride film at the metal surface. The pickling reaction is even more aggressive when carried out in concentrated solutions. Along with hydrofluoric acid (HF), nitric acid is the main constituent of the zircaloy pickling solution as it a commonly used dissolving agent in metallurgical and nuclear industries [4,5]. Since zircaloy has a strong corrosion resistance for HNO3, the pickling process has been optimized for processing time using aqueous solution of HF and HNO3 [10]. Moreover, the choice of acids was made according to their potential in pickling by forming soluble salts with the main components with the compounds to be removed in agreement with published solubility data [6].
Interaction of zircaloy with HF is associated with formation of a strong Zr-F bonds and fluoro-zirconic complexes. Zirconium dissolves in aqueous HF with the evolution of NOx gases and the formation of hexa-fluoro-zirconic acid (H2ZrF6) in solution. The oxide layer too reacts to form hexa-fluoro-zirconic acid. Probable reactions, as shown in reactions (1-5) are take place during the pickling [3].
Zr+6HF+4HNO3 → H2ZrF6+4NO2+4H2O.………. (1)
3Zr+18HF+4HNO3 → 3H2ZrF6+4NO+8H2O… (2)
3Zr+4HNO3+12HF → 3ZrF4+4NO+8H2O……… .(3)
ZrF4+2HF → H2ZrF6………………………………(4)
ZrO2+2HF → H2ZrF6+8H2O……………………… (5)
Depending on the time of pickling operation, it is categorized into flash pickling or deep pickling. Based on the application and requirement for removal of surface layer, pickling time is decided. Deep pickling is carried out for about 5-10 min while flash pickling is carried out for about 3 min [10]. The pickling operation is usually carried out in a large bath, made of fibre reinforced plastic (FRP) and poly propylene.
Pickling operation of zircaloy appendages
During the fuel bundle manufacturing process, the zircaloy appendages viz. spacer pads and bearing pads are subjected to pickling operation to provide a smooth surface finish. Pickling also increases the weld strength of the appendages, as there are lesser surface defects. The appendages are manufactured by punching zircaloy sheets, which gives a higher thickness of the appendages than that specified for their application. For appendage welding operations on the tubes, the acceptable thickness should be about 0.03 mm to 0.05 mm less than the initial product manufactured by punching. This thickness reduction of the appendages is performed by pickling operation. Study of the present practice at the plant indicates that the time required for pickling is quite high and the productivity can be increased by obtaining a suitable composition of pickling solution.
As the pickling reaction proceeds, the concentration of HF decreases while the concentration of Zr increases in the pickling bath. Once the bath gets saturated with Zr it cannot be further used for pickling operation after which the bath is replaced. Previous studies carried out by the authors [1,2] indicate the possibility of regenerating and reusing the pickling solution. The same experimental procedure was carried out to regenerate the pickling solution and the solution was made up of the required concentration.
Experimental
Pickling operation was done with varying the amount of HF-HNO3 to estimate their ratio required for optimal pickling composition to increase the productivity. One of the quantitative ways to measure the pickling rate is by determination of mass reduction achieved at different time. Experiments were performed in which mass reduction of zircaloy samples due to pickling, by using fresh and regenerated pickling solutions, were determined at different time. Both fresh and regenerated pickling solutions of 500 ml were prepared for the experiments. From the mass loss, the reduction in thickness was determined by assuming uniform mass loss. The mass reduction values were converted to thickness reduction values for different time periods. Experiments were carried out at room temperature. The appendages can be visualized as a trapezoid, having thickness very small than the other sides. Since one area (measured along the length and breadth) is quite larger than the other area (measured along the thickness side), the mass loss can be assumed to be occurring on the thickness side only. The cross-sectional area (measured along the length and breadth) of the component was thus assumed constant (e.g. for bearing pads, the area is around 5 cm2) while the density of the zircaloy component was determined experimentally to be 6 g/cc. The rate of pickling for both fresh pickling solution and regenerated pickling solution was studied and average pickling rate was determined for both. The pickling rate [cm.s-1] was calculated based on the eqn. (6).
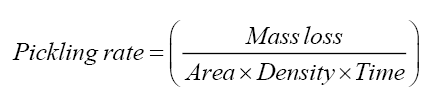 (6)
(6)
Results and Discussions
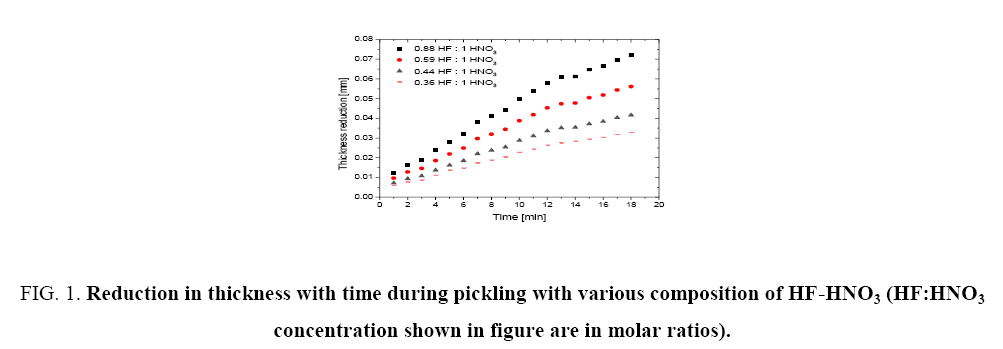
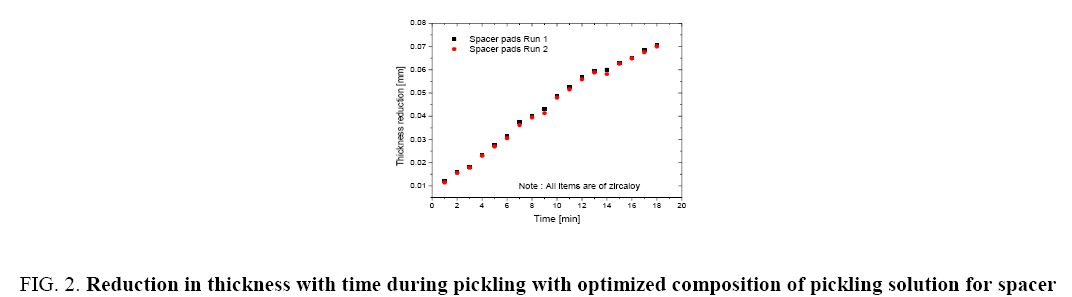
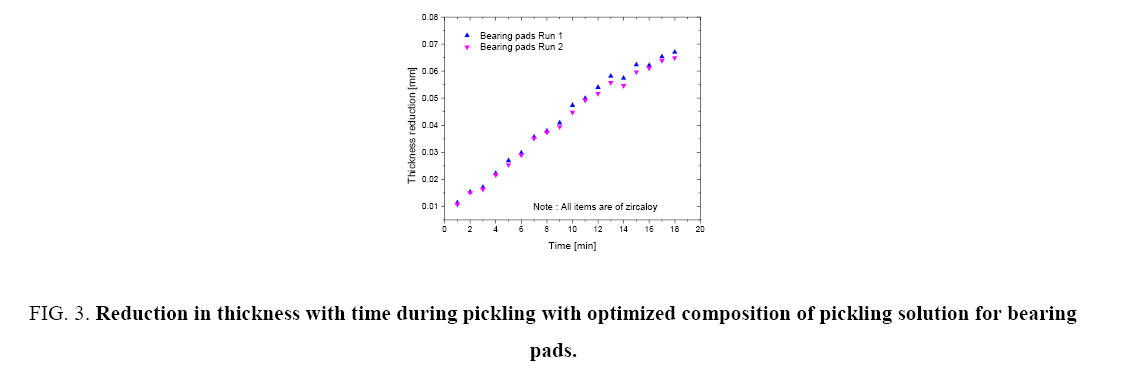
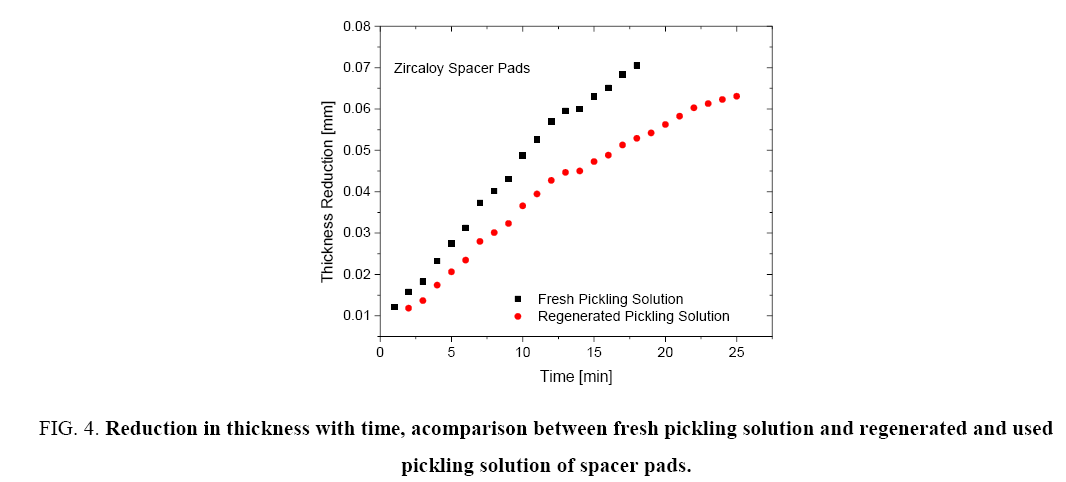
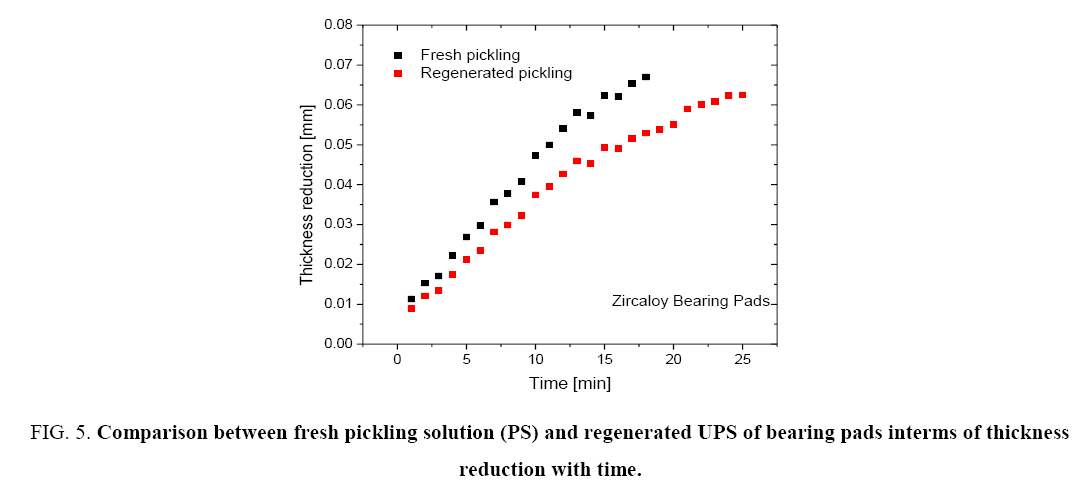
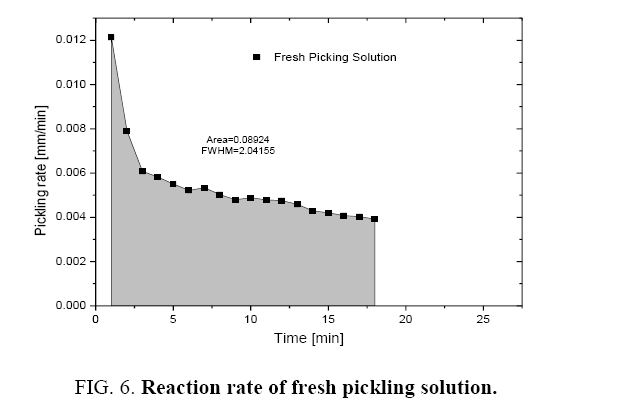
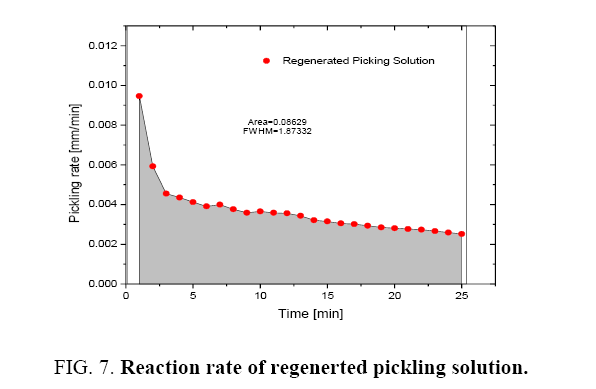
Figure 1 shows the variation of molar ratios of HF-HNO3 used for determining pickling solution composition for spacer and bearing pads. Figure 2 and 3 show the effect of pickling on spacer and bearing pads respectively with the optimum the amount ratio of HF-HNO3. Figure 4 and 5 compare the effects of fresh pickling solution and regenerated pickling solution of spacer and bearing pads respectively, with HF composition of regenerated pickling solution matching the fresh pickling solution composition. Figure 6 and 7 show the pickling rate of fresh and regenerated pickling solution respectively.
Figure 1: Reduction in thickness with time during pickling with various composition of HF-HNO3 (HF:HNO3 concentration shown in figure are in molar ratios).
Figure 2: Reduction in thickness with time during pickling with optimized composition of pickling solution for spacer pads.
Figure 3: Reduction in thickness with time during pickling with optimized composition of pickling solution for bearing pads.
Figure 4: Reduction in thickness with time, acomparison between fresh pickling solution and regenerated and used pickling solution of spacer pads.
Figure 5: Comparison between fresh pickling solution (PS) and regenerated UPS of bearing pads interms of thickness reduction with time.
It was found that the thickness reduction of 0.03 mm to 0.05 mm within the time period of deep pickling regime could be achieved with a molar ratio of HF-HNO3 as 0.88:1 as shown in Figure 1. It was also observed that increasing the HF content (keeping other parameters fixed) further increased the pickling rate considerably but the reaction was difficult to control and white patches developed on the surface of the appendages which could lead to rejection of the appendages for welding. It was also observed that these compositions of pickling solution demonstrate an excellent repeatability with the above pickling solution composition as shown in Figure 2 and 3. The values of each run shown in Figure 2 and 3 are the mode values of experimental results of 4 sets of experiments. Therefore, keeping in view the acceptable quality of appendages and smooth pickling operating conditions, the above HF-HNO3 molar ratio of 0.88:1 was fixed for operation.
Figure 4 and 5 show that the regenerated pickling solution can also be used for pickling operations and the time required for achieving the given thickness reduction is higher than that required by the fresh pickling solution. The amount of HF made up was the same as the fresh pickling solution. However due to the presence of higher nitrates in the regenerated pickling solution, the corresponding molar ratio of HF-HNO3 was observed to be 0.44:1 (based on the objective that volume of regenerated pickling solution should not exceed that of the fresh pickling solution). This resulted in the lower rate of pickling for the regenerated solution.
It was observed that the initial rate of pickling operation is high for both the fresh and regenerated pickling solution as shown in Figure 6 and 7. As the time increases, the pickling rate decreases. The pickling bath could be replaced with a new one after the reaction rate becomes low. It was also found that the rate of reaction can be determined from the Figure 6 and 7 and these are listed in Table 1.
| Sr. No. | Parameter | Fresh pickling solution (mm/min) |
Regenerated pickling solution (mm/min) |
|---|---|---|---|
| 1. | Initial picking rate | 5.74 × 10-3 | 4.31 × 10-3 |
| 2. | Average pickling rate | 4.796 × 10-3 | 3.45 × 10-3 |
Table 1: Reaction rate of pickling solutions.
Conclusion
It was found from the experiments that HF-HNO3 in the molar ratio of 0.88:1 could be used as pickling solution composition to increase the production. The average pickling rate of fresh and regenerated solution as calculated from the experiment corresponds to 4.96 μm/min and 3.45 μm/min respectively. The used pickling solution is an effluent from the process and was regenerated and reused for pickling. It was observed from the experiments that the regenerated pickling solution could be used for pickling and had considerable reaction rate compared to the fresh pickling solution.
However, reactor core studies need to be performed with the appendages pickled with regenerated pickling solution to establish their suitability in the manufacturing process. Also, studies with no constraint on the volume of regenerated pickling solution could be performed and the reaction rate of both fresh and regenerated pickling solution having the same molar ratios of HF-HNO3 i.e. 0.88:1 could be observed.
Acknowledgements
The authors would like to thank the technical staff of the concerned Pickling Plant and HPU, at NFC, Hyderabad for their kind help in providing the chemicals, plant data and other equipment for the experiments.
References
- Bhattacharjee D, Mandal D, VisweswaraRao RVRL, et al. Regeneration and reuse of zircaloy pickling solution. Indian Chemical Engineering Congress (CHEMCON 15); 2015 December 27-30; IIT Guwahati, India:Nuclear and Alternative Energy (NA) and others (OT).
- Bhattacharjee D, Mandal D, VisweswaraRao RVRL, et al. Recovery of zirconium from pickling solution, regeneration and its reuse. Nuclear Engineering and Design. 2017;316:89-92.
- Joseph, Megy A, Richard LP. Process for regenerating a pickle acid bath. US Patent 4105469 A. 1978.
- Mandal D, Ghosh SK. Reduction of nitrates present in aqueous waste. Environ Geochem. 2010;8:244-9.
- Jaiswal SK, Mandal D, VisweswaraRao RVRL. Recovery and reuse of nitric acid from effluents containing free nitric acid in absence and presence of metal nitrates. ChemEng J. 2015;266:271-8.
- Handbook of Chemistry and Physics. 45th ed (1964-1965). The Chemical Rubber Co. Cleveland, Ohio.
- D Mandal, D Sen, S Mazumder, et al. Sintering behaviour of lithium-titanate pebbles: Modifications of microstructure and pore morphology. Ceramic Engineering and Science Proceedings. 2011;32(2):165-70.
- Walker. Process of regenerating spent HF-HNO3 pickle acid containing (ZrF6)-2. US Patent 5082523 A. 1992.
- Ravi Theja DS. Reuse of zircaloy pickling solution. M.Tech Thesis, HBNI, 2015.
- Hlawka F, SutterEMM. Reactivity of the surface of zirconium during pickling in nitric-hydrofluoric acid. Materials and Corrosion. 1991;42(8):428-36.