Research
, Volume: 17( 1) DOI: 10.37532/0974-7494.2023.17(1).181BIOSYNTHESIS AND CHARACTERIZATION OF COPPER OXIDE NANOPARTICLES USING JATROPHA TANJORENSIS AND VITELLARIA PARADOXA PLANTS
- *Correspondence:
- Aliyu AbdullahiChemistry Department, Modibbo Adama University of Technology, Yola; E-mail: aliyuabdullahi6064@gmail.com
Received date: 05-March-2023, Manuscript No. tsnsnt-23-92565; Editor assigned: 13-March -2023, Pre-QC No. tsnsnt-23-92565 (PQ); Reviewed: 18-March -2023, QC No. tsnsnt-23-92565 (Q); Revised: 25-March-2023, Manuscript No. tsnsnt-23-92565 (R); Published: 31-March-2023. DOI: 10.37532/0974-7494.2023.17(1).181
Citation: Abdullahi A., Shagal H M., Ali N M., Mohammed A.,Yelwa JM. Biosynthesis and Characterization of Copper Oxide Nanoparticles using Jatropha Tanjorensis and Vitellaria Paradoxa Plants. Nano Tech Nano Sci IndJ.2023;17(1):181
Abstract
The study aimed to synthesize copper oxide nano particles at room temperature using Jatropha Tanjorensis leaf extract and Vitellaria Paradoxa stem bark extract. This method is completely green, with no toxic or harmful solvents. Copper oxide nano particles were created by combining copper nitrate dehydrate (CuO(NO3)2.6H2O) with Jatropha Tanjorensis leaf extract and Vitellaria Paradoxa stem bark extract. The bio synthesized copper oxide nano-particles were characterized using Fourier Transform Infrared Spectroscopy (FTIR), UV-vis spectroscopy, and XRD, which revealed a single-phase monoclinic structure. The copper oxide nanoparticles were found to have a particle size of less than 100 nm. The surface morphology of these NPs was investigated using Scanning Electron Microscopy (SEM).
Keywords
Bio Synthesis, Nanoparticles, Bio-reduction, XRD Pattern
Introduction
Nanomaterials have the potential to solve many technological and environmental problems in the fields of solar energy conversion, medicine, and wastewater treatment [1]. Metal nanoparticles have proven to be extremely efficient and useful in the fields of electronics, photonics, and medicine [2]. Metal nanoparticles have different properties depending on their size, shape, and morphology. Copper oxide nanoparticles are significant due to their applications as antimicrobials and in gas sensors, batteries, high-temperature super conductors, solar energy conversion tools, and so on [3]. Copper (Cu) and copper complexes have been used for centuries as water purifiers, algaecides, fungicides, and antibacterial and antifouling agents. Copper-based compounds have effective biocidal properties and are commonly used in a variety of medical applications [3]. CuONPs can be synthesized using a variety of chemical methods, including chemical reduction, microwave radiation, and thermal decomposition [4]. However, the adsorption of toxic chemicals to the surface occurs during synthesis procedures using these methods. It has the potential to cause serious side effects, particularly in medical applications. The use of toxic chemicals in chemical methods, which endangers the environment and human health, has prompted researchers to develop new alternative methods [4].
Green chemistry, defined as the use of a dozen principles in the design, production, and use of chemical products that prevent or reduce the emergence and use of substances that are hazardous to the environment and human health, is an alternative approach that has received extensive research in recent years. Green synthesis is required to avoid the production of unwanted or harmful by-products by developing dependable, sustainable, and environmentally friendly synthesis procedures. To achieve this goal, ideal solvent systems and natural resources (such as organic systems) must be used. To accommodate various biological materials (e.g., bacteria, fungi, algae, and plant extracts), green synthesis of metallic nanoparticles has been used [5]. Plants are currently being used to produce green metal nanoparticles. Metal nanoparticle synthesis using inactivated plant tissue, plant extracts, exudates, and other living plant parts is a modern alternative for their production [3]. It is a very cost-effective method, making it a viable commercial option for large-scale production. Plant extracts such as Garcia papaya, Aloe vera, Centella Asiatic, Azadirachta indica (linden), Jatropha curcas (castor nuts), Acalypha indica (castor nettle), Cymbopogon sp (lemongrass) and Centella Asiatic were chosen by a few researchers for use in green synthesis of copper oxide nanoparticles because they are inexpensive and renewable natural resources.
Gundelia tournefortii (kenger) plant, Psidium guajava plant, lemon juice, and Gloriosa superba plant are used specifically in CuONP synthesis. Because of the phenolic species present in plants, no other agents are required in the production of metal nanoparticles [4]. As a result, studies on plants and plant extracts, as well as nanoparticle synthesis, have accelerated in recent years. CuONP synthesis was performed in this study using the root extracts of Jatropha Tanjorensis and Vitellaria paradoxa.
Materials
Reagents
Jatropha Tanjorensis and Vitellaria Paradoxa leave and stem bark, Zinc nitrate, Hanna instrument 2211 PH meter, Jenway 6400 UV-visible spectrophotometer, Perkin Elmer Frontier Fourier Transform Infrared Spectroscopy, Ultrasonic bath, measuring cylinder, analytical balance, beakers, conical flasks, magnetic stirrer, heating mantle, spatula, funnel, reagent bottles, burette, magnetic stirrer, thermometer, oven, desiccator, retort stand, test tubes, test tube rack, Whatman filter paper, centrifuged.
Reagents: Ethanol, distilled water, Potassium Bromide (KBr), Copper Oxide, diethyl ether, copper nitrate, ferric chloride, ammonia, Hydrochloric Acid (HCl), dragen dorff’s, H2SO4, acetic acid, ammonium hydroxide, sodium chloride, n-butanol, methanol, ferrocyanide, FeCl3.
Methods
The method used by Elumalai et al. will be adopted for the synthesis of the Copper oxide nanoparticles [6].
Sample collection and pre-treatment
Jatropha Tanjorensis and Vitellaria Paradoxa leaves and stem bark will be collected from Sangere Numan Road Yola South and Federal Housing Girei Local government area of Adamawa State. The sample will be washed thoroughly with distilled water to remove dust allowed to dry at room temperature on a clean polythene plastic bag for at least two weeks. The dried leaves will be grounded mechanically using sterile pestle and mortar. The sample will be sieved, parked in polyethylene bags labelled and stored for phytochemical screening, extract preparation for synthesis of copper oxide nanoparticles and sox hlet extraction for anti-microbial activity analysis.
Preparation of leave and stem bark extract
10 g of the dried leaves and stem bark will be placed in a 250 mL Erlenmeyer flask and boiled with distilled water for 20 minutes and then the mixture will be allowed to cool under room temperature. The solution will then be filtered with Whatmann filter paper No. 1 to obtain the extract of Jatropha Tanjorensis leave and Vitellaria Paradoxa stem bark which will be later used as reducing agent for the bioreduction of hydrate Copper nitrate in the synthesis of the Copper oxide nanoparticles.
Preparation of copper oxide nanoparticles
Biogenic synthesis of CuO. NPs were carried out according to the method with modifications [6]. The crude plant extract (about 25 mL) was heated (60°C–80°C) on a magnetic stirrer. When the temperature of the extract reached 60°C, 2.5 g of Copper nitrate hexahydrate (CuO(NO3)2.6H2O) was added and left for about 1h till a white precipitate appeared. This mixture then was left overnight in a hot air oven at 60°C or till a creamy paste formed. This paste was collected and washed several times with a solution of distilled water: Ethanol (3:1). Afterwards, the collected paste was transferred to a ceramic crucible cup and heated in a furnace at 400°C for 2 hour. The resultant white powder was stored in an airtight container for characterization.
Characterization of copper oxide nanoparticles
Copper oxide nanoparticles synthesized by this green method were characterized by XRD, SEM, UV-Visible spectrophotometer (Shimadzu) in the range 200 nm–800 nm with a resolution of 1nm at room temperature and Fourier-Transform Infrared (FTIR-Shimadzu) spectrum in the range 4000 cm-1-400 cm-1.
Results and Discussion
Fourier Transform Infrared Spectroscopy (FT-IR)
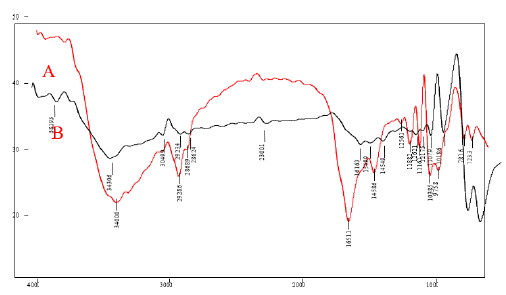
The FTIR characterization is used to find the molecules and their functional group present in the samples. FIG. 1 shows the overlay FTIR spectra of A) (CuO nanoparticles synthesized using Jatropha Tanjorensis) and B) (CuO nanoparticles synthesized using Vitellaria Paradoxa). In the spectrum of the nanoparticles (A), a broad band at 3400.0 cm-1 was assigned to O–H stretching which may be due to the binding of (CuO(NO3)2.6H2O) to –OH groups. The sharp bands at 2868.9 cm-1-2928.6 cm-1 are assigned to the asymmetric stretching vibration of C-H bond. Another band at 1188.2 cm−1 was attributed to NO2 absorption and 1038.5 cm−1 was assigned as absorption peaks of -C-O-C- or -C-O-. The band at 781.6 cm−1 for C–H bend of alkynes, while the band at 1256.1 cm-1 indicated the presence of amine groups, as expected due to the plant origin of the samples. The band at 1651.1 cm−1 was assigned to carbonyl and carboxylic (CO) stretching bands of peptide linkages (stretching of amides). The band at 1458.6 cm−1 was assigned to –O– H bend of carboxylates. In the FTIR spectrum of (B), the most significant absorption peaks were those observed at 751.3 and 841.0 cm−1 which corresponds to the stretching vibration of the Cu-O bond in monoclinic CuO which is in close agreement with the work of [7]. The strong peaks at 1018.6 cm−1 and 1107.9 cm−1 were due to C-O stretching vibration in the carboxylic group and flavanones. The characterized peaks observed at 1262.1 cm−1 were due to C-N stretching vibration in the amine group. The strong band at 1616.3 cm−1 was due to aromatic CC bending vibration, and absorption peaks at 2862.4 cm−1 and 2923.4 cm−1 were attributed to the asymmetric and symmetric C-H stretching mode caused by the phenolic compounds. The broad band at 3430.6 cm−1 was due to O-H stretching vibration in alcohols [8]. The presence of these biologically active plant compounds might have been responsible for the reduction and capping of CuO NPs. These results were similar to the previous report on the synthesis of CuO NPs using different plant extracts [7].
Figure 1: FTIR spectra of CuO Nanoparticles synthesized using (A) Jatropha Tanjorensis and (B) Vitalaria Paradoxa
UV-Visible Spectroscopy
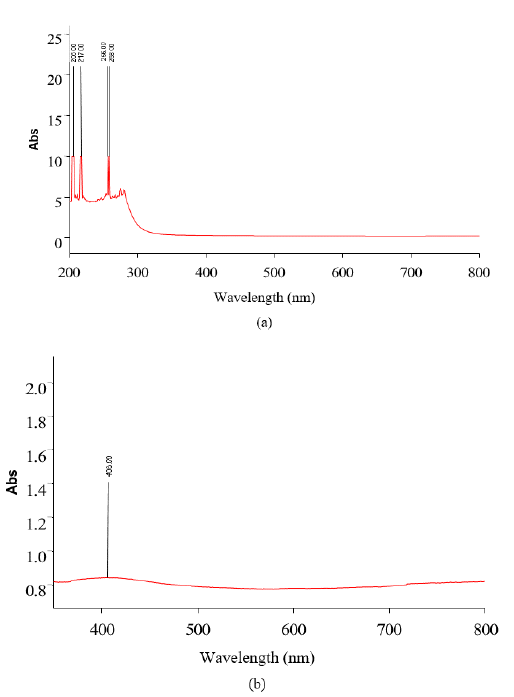
Figures 2(a) and 2(b) show the UV-Vis spectrum of green synthesized CuO NPs synthesized using Jatropha Tanjorensis and Vitalaria Paradoxa respectively. The biosynthesized CuO NPs showed high absorbance over the visible wavelength range which is one of the indications that the narrow-band gap CuO NPs were successfully synthesized. In FIG. 2(a) it was observed that the solutions of CuONPs synthesized by Jatropha Tanjorensis gave four characteristic plasmon bands at 206, 217 256, and 258 nm at room temperature corresponding to the characteristic band of copper oxide nanoparticles. However, in FIG. 2(b), a single band observed at 408 nm was assigned to the absorption of copper oxide nanoparticles.
Figure 2: UV-visible spectrum of copper oxide nanoparticles synthesized using (a) Jatropha Tanjorensis and (b) Vitalaria Paradoxa
X-Ray Diffraction (XRD) studies
XRD pattern of CuONPs synthesized from Jatropha Tanjorensis and Vitellaria Paradoza
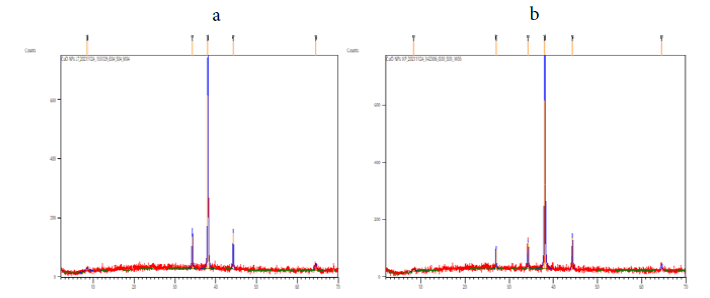
Powder XRD was a rapid analytical technique primarily used for phase identification of a crystallite material and can provide information on unit cell dimensions. The XRD pattern of the CuO nanoparticles was in monoclinic phase was shown in the FIG. 3. According to JCPDS data (80-0076), the exhibited diffraction peaks at 2θ = 8.4804°, 34.2290°, 38.0412°, 44.2433° and 64.5471° is indexed as (004), (111), (111) (202), (020) and it is found that Monoclinic crystal system. The information obtained was in agreement with the literature. The calculated crystalline sizes for all the samples are presented in TABLE 1. As it is well-known, any change in the interlayer of d-spacing of a lattice by organic modification results in the shifting of peak position, its broadness, and intensity in the XRD spectra.
Figure 3: XRD pattern of synthesized copper oxide nanoparticles synthesized using (a) Jatropha Tanjorensis and (b) Vitalaria Paradoxa
TABLE. 1 XRD Data of CuO Nanoparticles synthesized using Jatropha Tanjorensis and Vitellaria Paradoza
| CuO nanoparticles from Jatropha Tanjorensis | ||||
|---|---|---|---|---|
| 2θ of the intense peak | (h k l) | FWHM of intense peak (β) in radian | Size of the particle (D) nm | d-spacing (Å) |
| 8.4804 | (004) | 0.9446 | 8.81 | 10.42687 |
| 34.2290 | (111) | 0.1574 | 55.18 | 2.61972 |
| 38.0412 | (111) | 0.1771 | 49.58 | 2.36551 |
| 44.2433 | (202) | 0.1378 | 65.03 | 2.04724 |
| 64.5471 | (020) | 0.4723 | 20.79 | 1.44380 |
| CuO nanoparticles from Vitellaria Paradoza | 54.15 | |||
| 2θ of the intense peak | (h k l) | FWHM of intense peak (β) in radian | Size of the particle (D) nm | d-spacing (Å) |
| 8.2870 | (110) | 0.6298 | 13.21 | 10.66973 |
| 26.9792 | (112) | 0.1181 | 72.28 | 3.30493 |
| 34.2208 | (202) | 0.1181 | 73.54 | 2.62033 |
| 38.0268 | (111) | 0.1968 | 44.61 | 2.36637 |
| 44.2918 | (311) | 0.1574 | 56.94 | 2.04511 |
| 64.5189 | (220) | 0.2362 | 41.56 | 1.44437 |
The average particle size of silver nanoparticles synthesized by the present green method were calculated using Debye-Scherrer equation:
D = Kλ / β cos θ (1)
Where D = the crystallite size of Cu Nanoparticles
λ = the wavelength of x-ray source (0.1541 nm) used in XRD
β = the full width at half maximum of the diffraction peak.
K = the Scherrer constant with value from 0.9 to 1. θ = the Bragg angle.
Scanning Electron Microscopy Analysis
SEM analysis of Jatropha Tanjorensis and Vitellaria Paradoza
The morphology of the prepared nanoparticles was examined using scanning electron microscopy. FIG. 4 shows the surface morphology of the copper oxide nanoparticles. SEM image showed individual copper oxide particles as well as a number of aggregates. The SEM image showed most of the nanoparticles are spherical in shape.
Conclusion
The green chemistry approach used in the present work for the synthesis of copper oxide nanoparticles is simple, cost effective and the resultant nanoparticles are highly Table and reproducible.
The CuO nano particles were characterized by UV-Vis spectroscopy, FTIR, and XRD and SEM. The reduction of copper nitrate to copper oxide nanoparticles was confirmed by UV-Visible spectro photometer. The bending vibrations and stretching bonds present in the sample was confirmed by the Fourier Transform Infrared spectroscopy (FTIR). XRD study showed that the CuO nanoparticles had monoclinic phase and the average particle size were in the range of 39 nm-50 nm.
REFERENCES
- Tiwari DK, Behari J, Sen P. Application of nanoparticles in waste water treatment. World Appl Sci J. 2008;3(3):417-33.
- Cohen-Karni T, Langer R, Kohane DS. The smartest materials: the future of nanoelectronics in medicine. ACS nano. 2012;6(8):6541-5.
- Begum SN, Esakkiraja A, Asan SM, et al. Green Synthesis of Copper Oxide Nanoparticles Using Catharanthus Roseus Leaf Extract and Their Antibacterial Activity. Int. J. Sci. Res. in Multidisciplinary Studies. 2019;5:8.
- Calhan SD, Gundogan M. Copper oxide nanoparticles: synthesis, characterization, antimicrobial activities and catalytic reduction of methylene blue. 2020;7(2):561-70..
- Singh J, Dutta T, Kim KH, et al. Green’synthesis of metals and their oxide nanoparticles: applications for environmental remediation. Journal of nanobiotechnology. 2018;16(1):1-24.
- Elumalai K, Velmurugan S, Ravi S, et al. Green synthesis of zinc oxide nanoparticles using Moringa oleifera leaf extract and evaluation of its antimicrobial activity. 2015
- Andualem WW, Sabir FK, Mohammed ET, et al. Synthesis of copper oxide nanoparticles using plant leaf extract of Catha edulis and its antibacterial activity. Journal of Nanotechnology. 2020;2020:1-0.
- Yelwa JM, Osemeahon SA, Nkafamiya II, et al. Synthesis And Characterization Of Hydroxylated Sunflower Seed Oil/Poly Vinyl Acetate Copolymer As A Binder For Possible Application In The Coating Industry. International Journal of Innovative Research and Advanced Studies (IJIRAS). 2017;4:417-8.