Original Article
, Volume: 10( 2)Synthesis of Cu-Chromite Catalyzer by Citrate Sol-gel
- *Correspondence:
- Ebrahimi Sobhan, Department of Material Science and Engineering, Sharif University of Technology, Iran, Tel: + 982166013126; E-mail: smpouresmaeily@hotmail.com
Received: April 29, 2017; Accepted: May 18, 2017; Published: May 22, 2017
Citation: Mohammad PS, Shirkavand BH, Mansour S, et al. Synthesis of Cu-Chromite Catalyzer by Citrate Sol-gel. 2017;10(2): 124.
Abstract
It has been introduced several ways for rising fuel burning rate. Using catalyzers is most common way to rising fuel burning rate. Cu-Chromite catalyzer used in solid fuels, as burning rate catalyzer in thermal decomposition of Ammonium Perchlorate and results were satisfying. This catalyzer is produced by several methods such as: ceramic, co precipitating, sol-gel, vacuum depositioning, but this paper explains producing catalyzer by Citrate sol-gel. Thermal analysis is used for studying process also SEM, XRD, TEM, FTIR tests used for determination of particle sizes.
Keywords
Nano Cu-Chromite; Citrate sol-gel; catalyzer; Burning rate; Ceramic method
Introduction
In recent years the Cu-Cr-O composites are found great promising in application as burning rate catalysts (ballistic modifier) for solid propellants used in defence (high explosives, ballistic missiles) and space vehicles (rocket propellants). Solid composite propellants are mixtures of pre polymer (binder), aluminium fuel, oxidizer salts (e.g. ammonium perchlorate), and other components, including curatives, plasticizers, bonding agents, stabilizers and catalysts. Even though added a few percent of the propellant binder, the catalysts used to control the burn rate are of high importance, since they allow improving the ballistics of rockets [1]. Combustion of this system involves the decomposition of AP (ammonium perchlorate) and the binder and mixing and oxidation-reduction of the decomposition products. Cu-Cr-O composite oxides via thermal decomposition of copper ammonium chromate and found that the propellant burning rate is enhanced by the addition of Cu-Cr-O composite oxides.
Cu-Cr-O nanocomposites as additives for the catalytic combustion of AP-based solid-state propellants, synthesized via a citric acid (CA) complexing approach. They showed that well crystallized Cu-Cr-O nanocomposites could be produced after the CA-Cu-Cr precursors are calcined at 500°C for 3 hrs. Addition of the as synthesized Cu-Cr-O nanocomposites as catalysts enhances the burning rate as well as lowers the pressure exponent of the AP-based solid-state propellants considerably. Noticeably, catalyst with a Cu/Cr molar ratio of 0.7 exhibited promising catalytic activity with high burning rate and low pressure exponent at all pressures, due to the effective phase interaction between the spinel CuCr2O4 and delafossite CuCrO2 contained in the as-synthesized Cu-Cr-O nanocomposites. synthesized p-type nano-CuO and CuCr2O4 by an electrochemical method and investigated the catalytic effect on the thermal decomposition behavior of ammonium perchlorate (AP) as a function of catalyst concentration using differential scanning calorimetry. The nano copper chromite (CuCr2O4) showed best catalytic effects as compared to nano-cupric oxide (CuO) in lowering the high temperature decomposition by 118°C at 2 wt.%. They observed high heat released of 5.430 and 3.921 kJ g-1 in the presence of nano-CuO and CuCr2O4, respectively. The decrease in the activation energy and the increase in the rate constant for both the oxides confirmed the enhancement in catalytic activity of AP. They proposed a mechanism based on an electron transfer process for AP in the presence of nanometal oxides.
Ceramic method
The vast majority of the powder synthesis of high temperature superconducting oxides to date has been carried out using the traditional “solid-state reaction route”. Copper chromite spinels are usually synthesized by the conventional high temperature method of solid state reaction (eqn. 1): [2]
6 hrs, 900°C
CuO(s) + Cr2O3 → CuCr2O4 (1)
Copper chromite by ceramic method, mixing copper (II) and chromium (III) oxides at 3 different ratios Cu/Cr = 0.61; Cu/Cr = 1.0; Cu/Cr = 1.5. The mixtures of oxides were homogenized with acetone followed by subsequent calcination at 900°C for 6 hours. This method results in spinel particles with low surface areas. In order to synthesize spinels with high surface area, they attempted different wet chemistry techniques.
Materials and Methods
By this method, Cu and Potassium dichromate with Ammoniac and deionized water mixed with specific ratio (determines the particle size of final Chromite) then dried the outcome precipitate in 110°C and 500°C for twice finally, Cu-Chromite produces
2CuSO4(aq) + K2cr2O7(aq) + 4NH3 + 3H2O → 2Cu(OH)NH4CrO4(s) + K2SO4(aq) + (NH4)
100°C to 500°C
2Cu(OH)NH4CrO4(s) → CuO(s) +Cucr2O4(s) + 5H2O (2)
Vacuum precipitating is a laboratory method, that isn’t economical because of problems of performing such as: low temperature, high vacuum for producing catalyzer. By studying different factors: ease of perform, low costs, uniform particle size, Citrate sol-gel was choice for producing catalyzer.
Catalyzer fabrication
Cu-Cr-O nanocomposites by citric acid (CA) complexing approach in which 0.01 mol Cu(NO3)2 and 0.02 mol Cr(NO3)3 are dissolved in 100 mL deionized water to obtain a mixed metal nitrate solution. Then citric acid is added to this solution and the molar ratio of citric acid to the total metal ions is fixed to be 2:1. After stirring for 30 min, the solution is heated at 95°C for several hours to evaporate the water solvent to produce dark brown transparent viscous gels. The gels are then dried at 160°C for 2 hrs to obtain the foamy dark powders, which are denoted as precursors of Cu-Cr-O Nano composites (CA-Cu- Cr). After grinding, the precursors are successively heated at 600°C for 3 h to obtain the final black Cu-Cr- O Nano composites [3].
Characterization methods
Thermal decomposition process acts with thermal analysis of DTA/TG6 model SAT1649 device with heating rate 10°C/min in air. Solid phases detects with XRD7 device, model Xpert-Philips with CuKα radiations. Outcome Chromite nano particles calcinated sizes measures with SEM8 images of Philips-Xl30 model device.
Results and Discussion
Citrate self-burning behavior
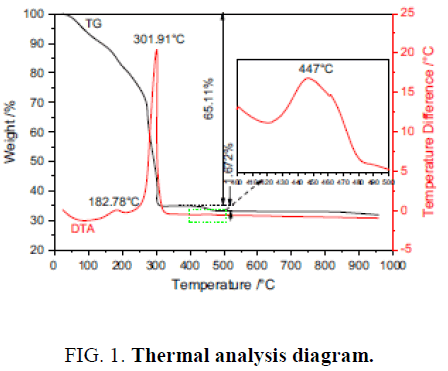
Results shows that produced Citrate gel from metal Nitrates and Citric acid goes through self-burning. By heating, bright points appear. This process studied by thermal analysis. (Figure. 1) shows that in DTA diagram, exothermic reaction acts Scanning Electron Microscope system and Nitrate [4]. Diagram TG also shows that decomposition of gel occurs suddenly and randomly. In this temperature (237°C) gel begins to burn . Weight loss of gel is about 80% that occurs with self-burning. This weight loss is because of removing water vapor, carbon dioxide and nitrogen oxide.
Phase analysis and particle size determination
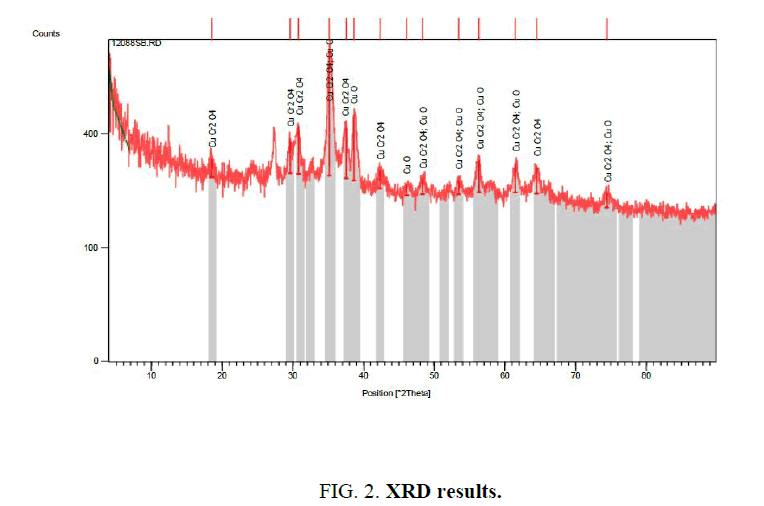
Figure. 2 shows that ash produces as result of drying in 160°C then calcination in 600°C, includes different oxides of Cu and Cr compounds in Figure. 2, also Figure. 3 shows nano particle sizes which measured with SEM.
FT-IR spectrum
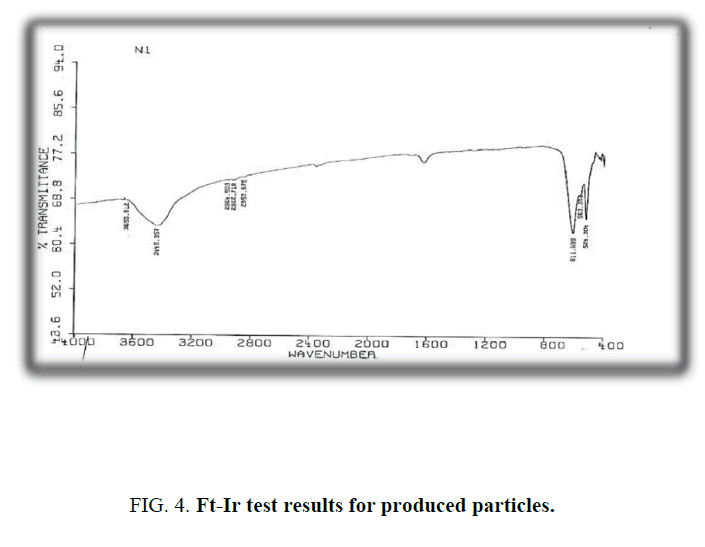
FT-IR spectrum of produced particles shows some peaks in 524 cm-1 563 cm-1 and 611 cm-1,, represents bending vibrations of Chromite anion (Cr2O4) in solid phase (CuO.CuCr2O4) (Figure. 4). The peak of 1637 cm-1 is for CuO vibrations. Existing solid phase (CuO.CuCr2O4) can proved by spectrum data [5]. Reported studies compatibility the empirical data with FT-IR spectrums by Miller and Willkins for mineral on ions.
Measuring produced particles size
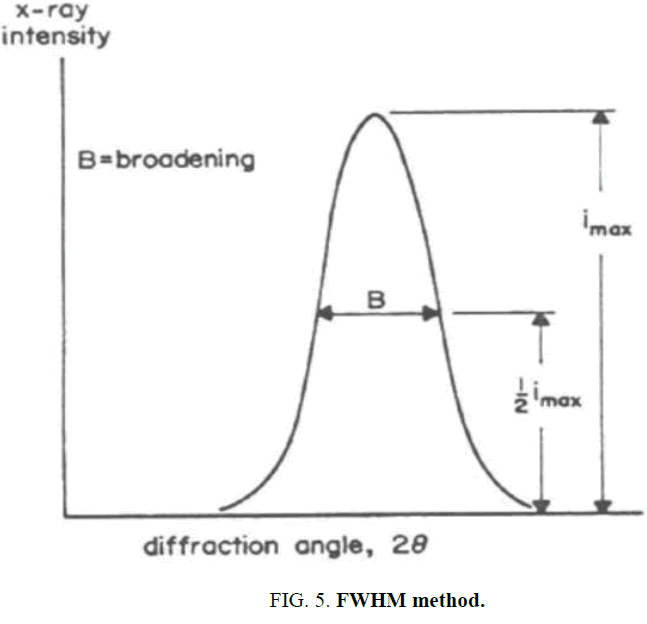
In polycrystalline materials, width of peak of XRD increases with decreasing crystalline plane spacing. The most useful pattern for measuring grain size is using the FWHM 9 formula that shown in Figure. 5. FWHM depends on the number of reflecting crystalline plane. Scherer formula relates the crystalline grain size to width of maximum peak in half height and other conditions [6].
| Pos. [°2Th.] |
Height [cts] |
FWHM [°2Th.] |
d-spacing | Rel. Int. [%] |
Tip width [°2Th.] |
Matched by |
|---|---|---|---|---|---|---|
| 18.4897 | 55.39 | 0.4723 | 4.79874 | 11.48 | 0.4800 | 01-072-1212 |
| 29.5914 | 111.12 | 0.3936 | 3.01887 | 23.04 | 0.4000 | 01-072-1212 |
| 30.7835 | 155.31 | 0.4723 | 2.90462 | 32.20 | 0.4800 | 01-072-1212 |
| 35.1639 | 482.28 | 0.2755 | 2.55218 | 100.00 | 0.2800 | 01-072-1212 |
| 37.5089 | 162.56 | 0.3936 | 2.39784 | 33.71 | 0.4000 | 01-072-1212 |
| 38.6402 | 215.94 | 0.2362 | 2.33020 | 44.78 | 0.2400 | 01-080-1917 |
| 42.3909 | 50.25 | 0.6298 | 2.13231 | 10.42 | 0.6400 | 01-072-1212 |
| 46.0739 | 26.27 | 0.9446 | 1.97007 | 5.45 | 0.9600 | 01-080-1917 |
| 48.3479 | 39.30 | 0.7872 | 1.88259 | 8.15 | 0.8000 | 01-072-1212 |
| 53.4776 | 35.78 | 0.6298 | 1.71348 | 7.42 | 0.6400 | 01-072-1212 |
| 56.3144 | 90.43 | 0.3936 | 1.63371 | 18.75 | 0.4000 | 01-072-1212 |
| 61.4957 | 82.90 | 0.4723 | 1.50791 | 17.19 | 0.4800 | 01-072-1212 |
| 64.4867 | 72.49 | 0.6298 | 1.44501 | 15.03 | 0.6400 | 01-072-1212 |
| 74.4494 | 33.89 | 1.1520 | 1.27334 | 7.03 | 0.9600 | 01-072-1212 |
TABLE 1: Shows the FWHM details, by substituting values in formula of FWHM, the mean particle size is 47nm.
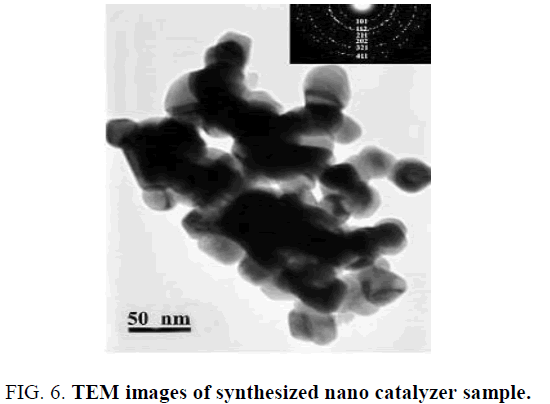
Measuring and determining synthesized productions crystalline type by TEM images
Figure. 6 shows that synthesized particles size is 50 nm that confirms diffraction data that synthesized production by Citrate sol-gel method in PH of 9, has spinel crystalline that is In accordance with the detected structure in XRD. Synthesized Cu-Chromite in PH of 9 is solid phase of CuO, CuCr2O4 that has spinel crystalline structure.
Synthesis of Cu-Chromite nano catalyzer in different PHs
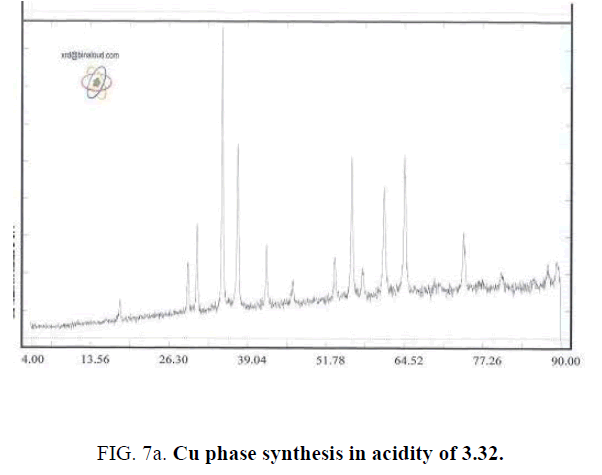
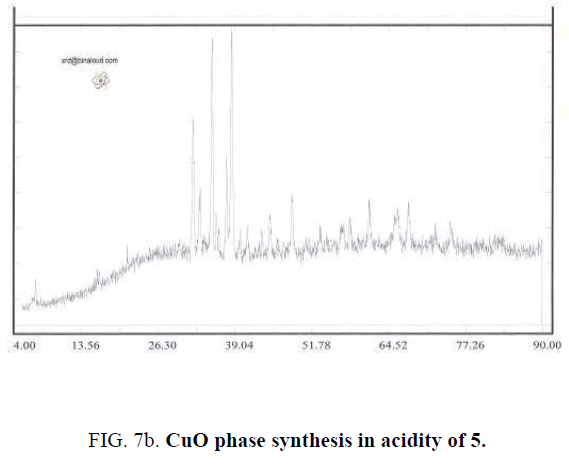
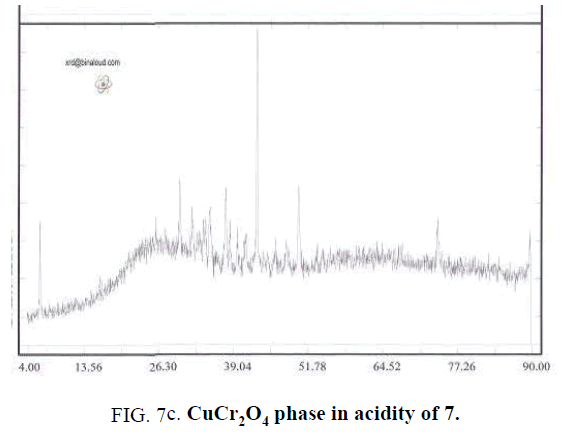
For studying PH effect in synthesis process of Cu-Chromite nano catalyzer, all of last studied method include metal percentage, amount of used Citric acid and drying and calcinating method (environmental conditions) are used and Ammonium solution of 0.1 molar used for controlling PH values. In this step, values of PH set to 3.32 (that is equal to PH of sample without adding Ammonium solution), 5, 7 and 9. TABLE 2 shows the synthesized samples.
| Sample No. | Cu-Cr ratio | Citric acid to total metals molar ratio | Drying | Calcination | PH value |
|---|---|---|---|---|---|
| 1 | 0.05 | 2:1 | 2 hrs in 160°C | 3 hrs in 700°C | 3.32 |
| 2 | 0.05 | 2:1 | 2 hrs in 160°C | 3 hrs in 700°C | 5 |
| 3 | 0.05 | 2:1 | 2 hrs in 160°C | 3 hrs in 700°C | 7 |
| 4 | 0.05 | 2:1 | 2 hrs in 160°C | 3 hrs in 700°C | 9 |
TABLE 2: Cu-Chromite synthesized samples in different PHs.
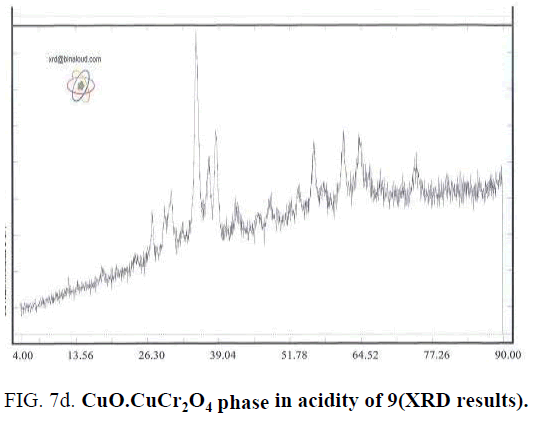
Effect of PH is because of formation of complex of metals and Citrate ion. Actually Citrate ion produces metal citrate complex in solution with free hydrolyzed metals. Synthesizing complex cause of sol to gel transferring. Synthesis this complex is strongly dependent of PH value that can observe by changing the color of synthesized complex in different PH values. For example, the color of complex in PH of 5 is black and is brown for PH of 7. Changing color is because of difference of synthesized Citrate complex [7]. Type of synthesized complex, stable ability amount and thermal decomposition method are effective in phase of solid produced. In different PHs, a synthesis different metal complex that leads to different solid phases by later steps of drying and calcination that by reducing water and other materials. The mean of crystalline dimensions of Cu-Chromite powder calculated by Debby- Scherer relation in PH of 9 is about 85 nm. Results shows that by determining a specific PH could specify the desired type of solid-phase catalysts (FIG. 7a – FIG. 7d).
Cu-Chromite that used in increasing fuels burning rate has solid phase of CuO.CuCr2O4 that synthesis in PH of 9 according to XRD results.
Innovation in catalyzer synthesis
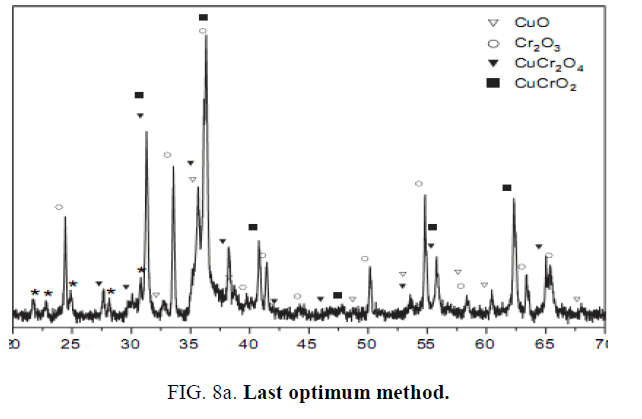
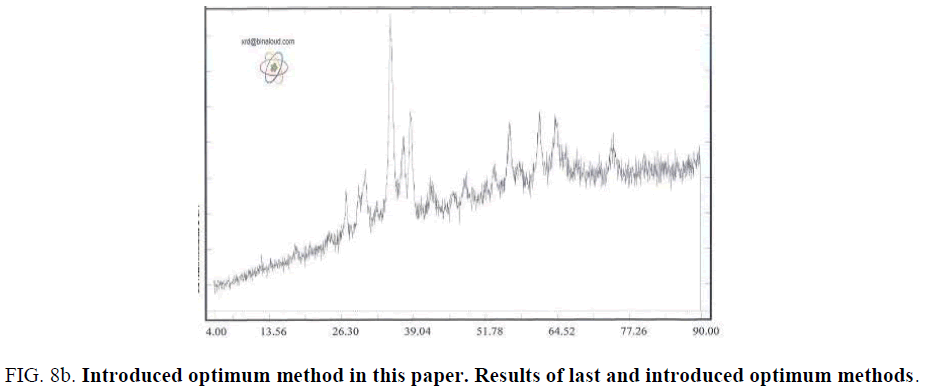
Synthesis Cu-Chromite catalyzer with desired solid phase CuO.CuCr2O4 leads the researchers attention to synthesis of selected phases of this catalyzer. This phase could synthesize In all of discussed methods. Mentioned phases in TABLE 2 represent the main consisting phase of particles. Last researches in sol-gel Citrate were based on studying calcination temperature and metals ratio for phase controlling. We informed in this project that changing PH is effective in type of solid phase. It has done for different calcination temperature and metal ratio, leads to finding the optimum calcination temperature and metal ratio [8]. By this method, the best values for calcination temperature is 700oc for 3 hrs and Cu to Cr ratio of 0.5 FIG. 8a and FIG. 8b shows the XRD results analysis of synthesized catalyzer by last optimum method and introduced method of this paper. Also shows that introduced optimum method (calcination temperature of 700oc for 3 hrs and Cu to Cr ratio of 0.5 in PH of 9) synthesis the single phase catalyzer and there isn’t other phases like Cr2O3 or CuCrO2.
Conclusion
Producing Cu-Chromite catalyzer according to applications in solid fuels is important for burning rate catalyzer. Thermal analysis shows that Citrate gel have self-burning behavior and sol-gel method can used because of low costs, ease of action and uniform particle size for producing nano Cu-Chromite.
References
- Liu T, Wang L, Yang P, et al. Preparation of nanometer CuFe 2 O 4 by auto-combustion and its catalytic activity on the thermal decomposition of ammonium perchlorate. Mater Lett. 2008;62(24):4056-8.
- Patron L, Pocol V, Carp O, et al. New synthetic route in obtaining copper chromite I. Hydrolysis of some soluble salts. Mater Res Bull. 2001;36(7):1269-76.
- Patil PR, Krishnamurthy VE, Joshi SS. Effect of Nano?Copper Oxide and Copper Chromite on the Thermal Decomposition of Ammonium Perchlorate. PropellExplosPyrot. 2008;33(4):266-70.
- Tagliaferro FS, Fernandes EA, Bacchi MA, et al. INAA for the validation of chromium and copper determination in copper chromite by infrared spectrometry. J RadioanalNucl Ch. 2006;269(2):403-6.
- Sitthisa S, Sooknoi T, Ma Y, et al. Kinetics and mechanism of hydrogenation of furfural on Cu/SiO 2 catalysts. J Catal. 2011;277(1):1-3.
- Prasad R, Singh P. Applications and preparation methods of copper chromite catalysts: a review. Bulletin of Chemical Reaction Engineering and Catalysis. 2011;6(2):63-113.
- Roy S, Ghose J. Syntheses and studies on some copper chromite spinel oxide composites. Mater Res Bull. 1999;34(7):1179-86.
- Li W, Cheng H. Synthesis and characterization of Cu-Cr-O nanocomposites. Journal of Central South University of Technology. 2007;14(3):291-5.