Original Article
, Volume: 15( 1)Synthesis and Characteristic Study of Co(II), Ni(II) and Cu(II) Complexes of New Schiff Base Derived from 4-Amino Antipyrine
- *Correspondence:
- Salih Hadi Kadhim , Department of Chemistry, College of Science, Babylon University, Iraq, Tel: 030249551; E-mail: hadi197019@yahoo.com
Received: February 21, 2017; Accepted: March 07, 2017; Published: March 15, 2017
Citation: Hadi Kadhim S, Qahtan Abd-Alla I, Jawad Hashim T. Synthesis and Characteristic Study of Co(II), Ni(II) And Cu(II) Complexes of New Schiff Base Derived from 4-Amino Antipyrine. Int J Chem Sci 2017;15(1):107
Abstract
The complexes of Co(II), Ni(II) and Cu(II) were prepared from the reaction between the new Schiff base derived from the reaction between Salicylaldehyde, p-amino acetophenone and 4-aminoantipyrineto to give the Schiff base: 4-(1-(4-(2-hydroxybenzylideneamino)phenyl) ethylidene amino)-1,5-dimethyl-2-phenyl-1H-pyrazol-3(2H)-one. The identification of the ligand and its complexes were carried out by the techniques: UV-visible, infrared and 1H-NMR spectroscopies, micro elemental analyses of metal ions and carbon, hydrogen, nitrogen, magnetic susceptibility, molar conductivity. The results of this studies show the coordination sites for the ligand with the metal ion were to be through the oxygen of the hydroxyl group and nitrogen of the azomethine (C=N)group. According to the observations, the octahedral geometric structure for the synthesized complexes were suggested.
Keywords
Schiff bases of salicylaldehyde; p-amino acetophenone; 4-aminoantipyrine
Introduction
The organic compounds which have azomethine group Called Schiff bases and that well known have a biologically activity [1], it is one of the most important ligands which have form a several coordination complexes through association with general elements and specialize with transition metal [2], the first imine compound which prepared by German scientist that called Hugo Schiff from the condensation reaction between aldehyde or ketones with a primary amines [3]. Schiff bases of 4-amino antipyrine and its complexes has a varied application in biological, clinical, pharmacological and analytical areas [4], the coordinated character of 4-amino antipyrine to binding with metal is varied by reaction with aldehydes and ketones. The biological behavior of 4-amino antipyrine has been compared with biological activity of transition metal complexes [5,6].
The aim of this work, is to synthesis and characterization of new Schiff base ligand derived from the reaction between Salicylaldehyde and p-amino acetophenone and treated the resulting product with 4-aminoantipyrine, and then prepared of its complexes with transition Co(II), Ni(II) and Cu(II) and suggested the geometrical structures of Schiff base complexes.
Experimental Part
Materials and instruments
Metal salts-CoCl2.6H2O (purity 99.9%), NiCl2.6H2O (purity 99.9), CuCl2 (purity 99%) (absolute ethanol (purity 99.99%), dimethyl formamide (purity 98%) and Dimethyl sulphoxide (purity 98%) were purchased from B.D.H company. 4-aminoantipyrine (purity 98%), p-aminoacetophenone (purity 98%) were purchased from Merck company. Salicylaldehyde (purity 99%) was purchased from Himedia company. The prepared ligand and its complexes were identified by UV-visible spectrophotometer type UV 6100PC double beam Spectrophotometer in the wave length range (200 nm to 800 nm). FT-IR spectroscopic analyses were recorded on Schimadzu FTIR 8400S spectrometer in the range (4000 cm-1 to 400 cm-1) using KBr pellet. The percentage of CHN were measured by using a EURO EA 3000 Single elemental analyzer. The metal percent in complexes were determination by Flame Atomic Absorption Spectrophotometer type (A.A-6300,Shimadzu). 1H-NMR spectra were recorded in 1H-NMR spectroscopy model 9300-UK ultra-shield at 300 MHz using TMS as an internal reference standard, Bruker’s NMR.
The molar conductivity of the metal complexes was measured by Digital Conductivity Meter type (Glassco-India) by using the solvents DMSO and DMF (1 × 10-3 molar) at room temperature. Magnetic moments measurements on Auto Magnetic Susceptibility Balance (Sherwood). The melting points were measured by Electro thermal melting point type (9300 U.K.).
Synthesis of Schiff base(I)
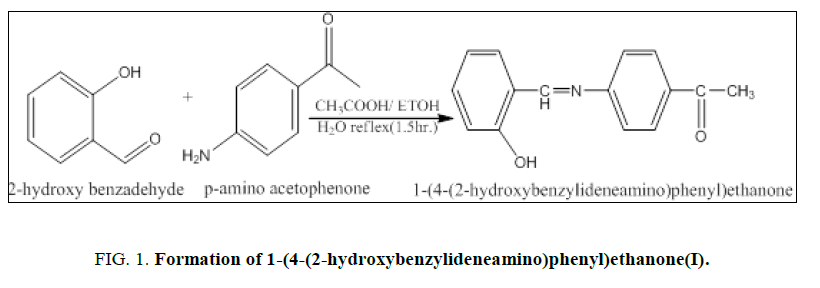
1.35 g of p-amino acetophenone (0.01 mol) was dissolved in 15 ml of absolute ethanol and mixed with a solution of 1.22 g of Salicylaldehyde (0.01 mol) in the equivalent volume and add 3 drops of glacial acetic acid as a catalyst and refluxed the mixture for (1.5 hours), then cooled the resultant solution to room temperature [7]. The resulting yellow solid 1-(4-(2-hydroxybenzylideneamino)phenyl)ethenone (I) was filtered washed and recrystallized in absolute ethanol (Figure. 1). The Yield percent is 81.7% and melting point equal to 104°C to 106°C.
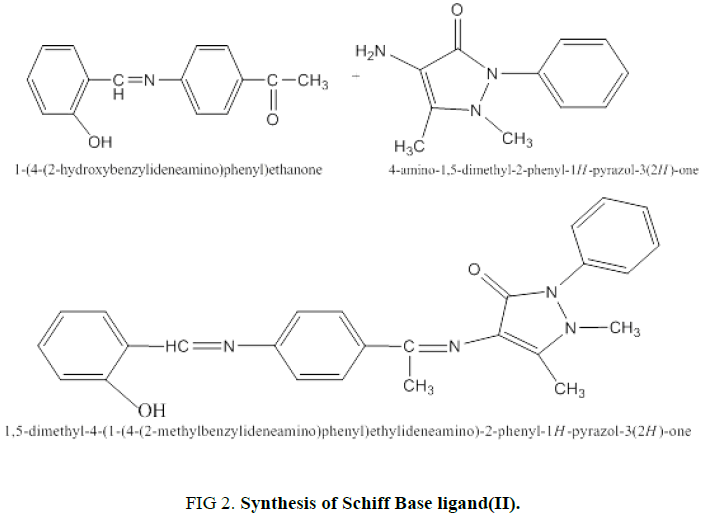
3-Synthesis of Schiff Base Ligand (L): 2.10 g of 20 ml of 1-(4-(2-hydroxybenzylideneamino)phenyl) ethenone (I) (0.0087 mol) was dissolved in 20 ml absolute ethanol and mixed with solution of 1-phenyl-2,3-dimethyl-4-amino pyrazol-5-one (4-amino antipyrine) (1.77 g/0.0087 mol) and added 5 drops of glacial acetic acid as a catalyst. The mixture was refluxed for (6 hours). The resulting solution was cooled to room temperature, filtered and washing the light yellow crystalline solid precipitate with absolute ethanol. The yield was 4-(1-(4-(2-hydroxy benzylidene amino) phenyl) ethylideneamino)-1,5-dimethyl-2-phenyl-1H-pyrazol-3(2H)-one(II) was recrystallized in absolute ethanol (Figure. 2). The Yield percent is 69.7%, and melting point equal to 178°C to 179°C.
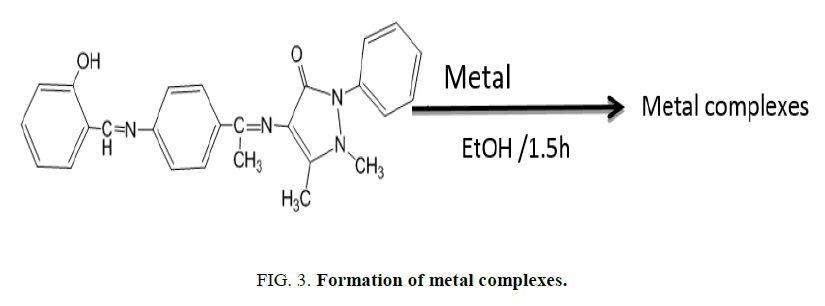
4-Synthesis of metal complexes: Complexes of the ions Co (II), Ni (II) and Cu (II) were prepared by reaction between the metal salt and the ligand in the molar ratio of 1:2 and refluxed for (1.5 hours), cooled the resultant mixture and allowed to crystallize. and then filtered, the precipitate was washed and recrystalized with ethanol absolute and dried in air (Figure. 3).
Results and Discussion
C.H.N.S. Analysis and physical properties of the prepared ligand and their complexes
The results of the C.H.N.S elemental analysis and the physical properties of the new Schiff base ligands with its complexes are summarized in Table 1. The molar conductivity values of the synthesis complexes show that there is no charge on the coordination sphere, this due to coordinated of anions to metal ions inside the coordination sphere [8,9].
| No. | Molecular formula |
Colour | m.p (C°) | Yield(%) | Found (Calc) %(M) |
Am Ohm-1 mol-1 cm2 |
Elemental Analyses |
|||
|---|---|---|---|---|---|---|---|---|---|---|
| DMF | DMSO | C% | H% | N% | ||||||
| 1 | C26H24N4O2 | Yellow | 178-179 | 69.7 | - | - | - | 74.22 (73.56) |
5.76 (5.69) |
13.36 (13.19) |
| 2 | [Co(C26H24N4O2)2 Cl2] | Dark yellow | 170-172 | 60 | 5.41 (5.58) |
3 | 4.3 | 64.24 (63.80) |
4.97 (4.94) |
11.52 (11.45) |
| 3 | [Ni(C26H24N4O2)2 Cl2] | Darkgreen | 190-191 | 57 | 4.85 (5.65) |
5.4 | 3.2 | 63.97 (63.82) |
4.87 (4.94) |
11.52 (11.45) |
| 4 | [Cu (C26H24N4O2)2 Cl2] | Black | 159-160 | 60 | 6.04 (6.46) |
4.7 | 5.3 | 63.76 (63.50) |
4.86 (4.92) |
11.47 (11.40) |
Table 1:The Physical properties and elemental values of the Schiff Base ligand its metal complexes.
There is an identical between the theoretical and experimental data for the C.H.N.S values, that gave an evidence for the formation of the desired ligands and its complexes.
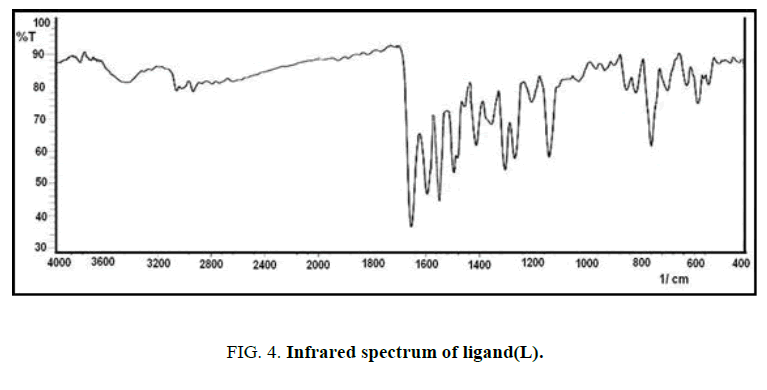
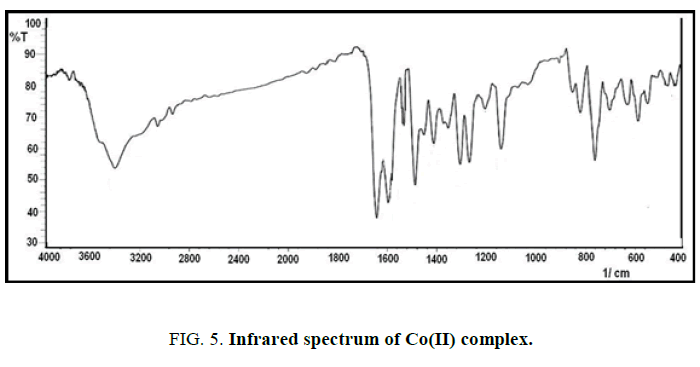
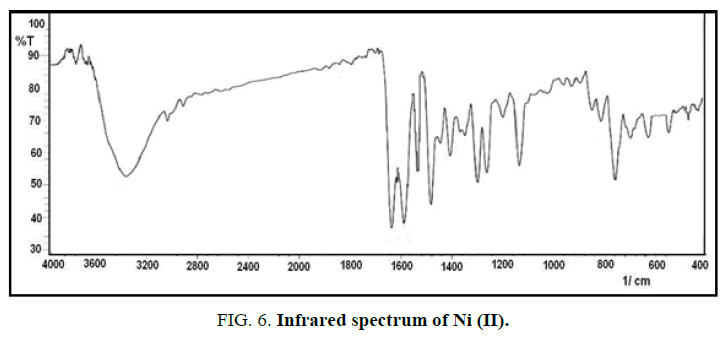
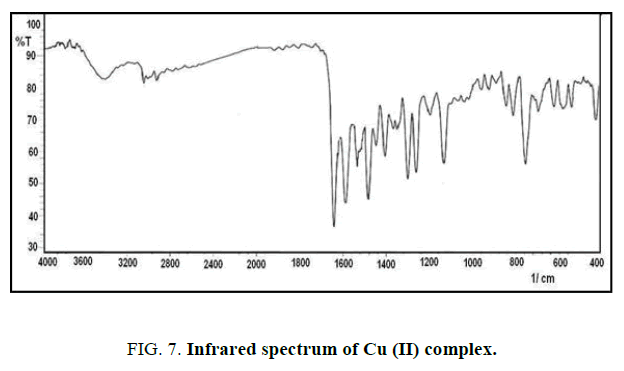
FT-IR spectra: The infrared spectrum of IR for the new ligand with its complexes are shown in Figures. 4-7 and Table 2. The main functional groups frequencies (C=N, C=O, H-C=N) in IR spectrum of ligand undergo a significant shifting in their frequencies positions in the IR spectrum of metal ions complexes, that refer to the coordinated to the metal ions and formation the complexes. The phenolic (O-H) seems at the same position in IR spectrum of ligand and its complexes (3439 cm-1), this due to not bonding to the metal ions in the complexes [10]. The appearance of new absorption bands at 550 cm-1 and 440 cm-1 refer to the coordinated bond between the metal ions and base sites in the ligand, the azomethine (C=N) and (C=O) carbonyl groups to form five member [11,12].
| Compound | νC=O | νC=N | νH-C=N | νM-N | νM-O |
|---|---|---|---|---|---|
| L | 1654(s) | 1552(s) | 1595(s) | - | - |
| Ni+L | 1644(s) | 1537(m) | 1593(s) | 549(w) | 462(w) |
| Co+L | 1642(s) | 1531(m) | 1595(s) | 545(w) | 454(w) |
| Cu+L | 1643(s) | 1543(m) | 1593(s) | 551(w) | 430(w) |
Table 2: The infrared absorption frequencies data of ligand and its complexes. m=medium, w=weak, s=strong.
The absorption band at 3385 cm-1 refer to υ(O-H) of water that is present in the spectrum of complexes and absent in the spectrum of the ligand [13,14].
Electronic spectra and magnetic moment measurements
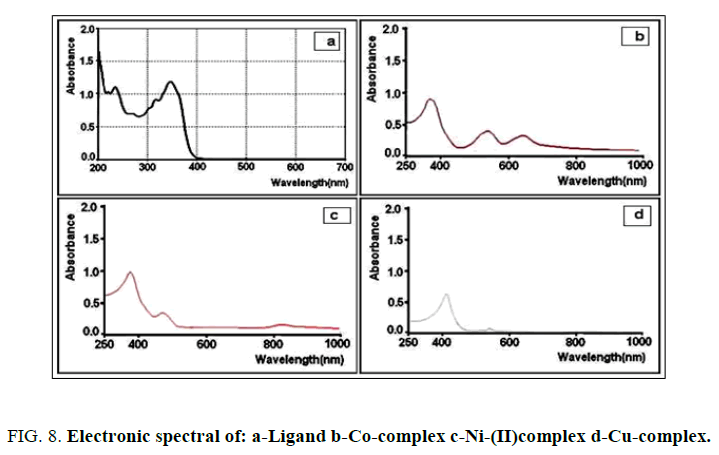
Figure. 8a show there are three band of the UV-visible spectrum of the ligand at 202, 236 and 348 nm due to π→π* and n→π* transition within the ligand [15]. These three band in the metal ions complexes are slightly shifted to blue or red regions (Figure. 8c-8d). The appearance of new bands in the visible region due to d→d transitions [12].
According to diagram for the d7 system configuration show that the presence of three transitions in their electronic spectra: V14T1g(F) → 4T2g(F), V24T1g(F)→4A2g(F), V3(4T1g(F) → 4T1g(P) found at 649 nm (15408 cm-1), 566 nm (17677 cm-1) and 370 nm (27027 cm-1) respectively for the Co(II) complex Figure. 9. This indicate the presence of an octahedral geometric structure of cobalt(II) complex. The magnetic moments effected, μ eff (4.36 B.M.) (Table 3) of Co(II) complex are in the range of the octahedral geometries.
| Complex | Bands (nm) |
Bands v cm-1 |
Assignment | μeff (B.M) | Geometry Suggested |
|---|---|---|---|---|---|
| Co+L | 649 | 15408 | 4T1g (F) →4T2g (F) | 4.36 | Octahedral |
| 566 | 17667 | 4T1g (F) →4A2g (F) | &a|||
| 370 | 27027 | 4T1g (F) →4T1g (P) | |||
| Ni+L | 830 | 12048 | 3A2g (F) →3T1g (F) | 3.09 | Octahedral |
| 442 | 22624 | 3A2g (F) →3T1g (P) | |||
| 363 | 27584 | C.T | |||
| Cu+L | 534 | 18726 | 2Eg →2T2g | 1.94 | Octahedral |
| 406 | 24630 | C.T |
Table 3: Electronic absorption spectral and magnetic moment data for the ligand and their metal complexes.
Nickel (II) ion has a (d8) system configuration having three peaks in their electronic spectra, at 830 nm (12048 cm-1), V1(3A2g(F)→3T1g(F), 442 nm (22624 cm-1), V2(3A2g(F)→3T1g(P)), 363 nm (27584 cm-1) V3(C.T) transitions respectively for the Ni(II) ion complex Figure. 10. The first transition did not appear or is very weak V1(3A2g(F)→3T2g(F). The effected magnetic moment, μ eff (3.09 B.M.) (Table 3), of the Ni(II) complex are in the environment of the octahedral geometries.
The (d9) system configuration of Copper (II) has the following absorption found at 534 nm (18726 cm-1), 406 nm (24630 cm-1) which may be assigned to V1(2Eg→2T2g), V2(C.T) transitions respectively, corresponding to a distorted of octahedral geometry around the Cu(II) ion. The effected magnetic moments, μ eff (1.94 B.M.) (Table 3), of Cu(II) complex is in the environment of the octahedral geometries [16].
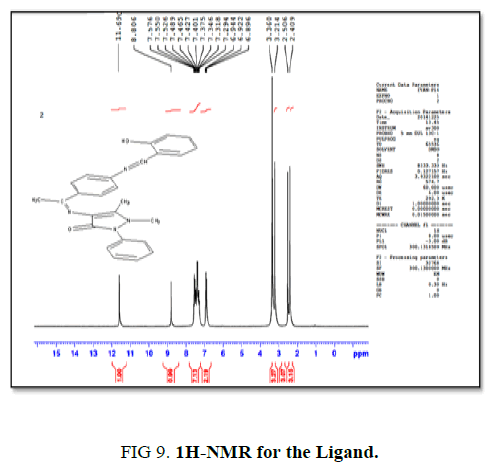
H-NMR for the ligand
The single band at (11.69 ppm) belong to one proton of hydroxyl group (OH), the single band at (8.80 ppm) belong to one proton of benzylidenimin (-CH=N-). The multiple bands at (6.89 ppm to 7.57 ppm) belongs to nine protons of aromatic rings, the two single bands at (3.21 ppm to 3.36 ppm) due to the protons of methyl group (CH3), while the band at (2.40 ppm to 2.50 ppm) related to H2O in deuterated DMSO as in Figure. 9.
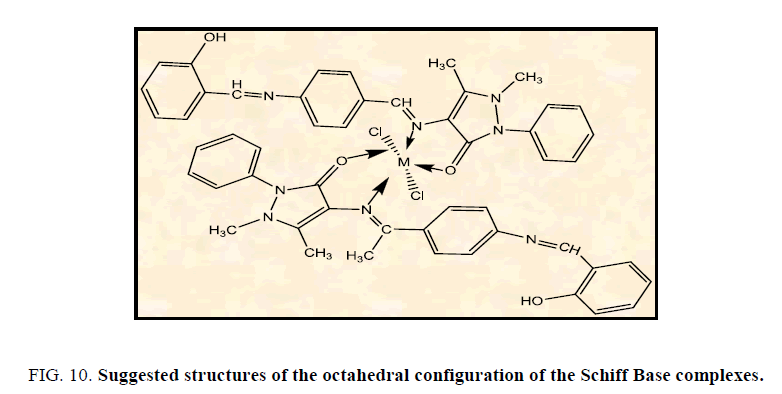
The suggested structures of metal complexes
According to the analytical and Spectroscopic studies of the prepared complexes and magnetic properties we can show the structure of the complexes as in Figure. 10.
Conclusions
1-Schiff base ligand: 4-(1-(4-(2-hydroxybenzylideneamino)phenyl)ethylideneamino)-1,5-dimethyl-2-phenyl-1Hpyrazol- 3(2H)-one was synthesis from salicylaldehyde, p-amino acetophenone and 4-aminoantipyrine with metals such as Co(II), Ni(II) and Cu(II) in tow steps.
2-The ions Co(II), Ni(II) and Cu(II) were coordinated to the Schiff base through the nitrogen of group(C=N) and the oxygen of the carbonyl group of the five-member ring and the Schiff base behaves as a bidentate ligand. 3-from the molar conductivity data suggest that uncharged the coordination sphere, and presence of chloride ion binding with Co(II), Ni(II) and Cu(II) ions inside the coordination sphere. 4-The molar ratio of M:L is 1:2.
Recommendations
Study the thermal behavior of the ligands and their complexes to illustrate how much benefit in the liquid crystal field. Study the biological activity for this compounds because it has active azo-methane group. Employment this ligands in the quantitative determination for study of other metals ions as long as have ability to form a chelate complexes with synthesized ligands.
References
- Cotton F, Wilkinson G.Advancedinorganic chemistry".4thedn, Interscincience publishers, New York. 1980;567-71.
- Saeed AAH, Titinch SJ, Abbo HS, et al. J Edu and Sci. 1989;8:30.
- Wilkionson G, Gillordandj R. Comprehensive coordination chemistry".1st edn, pregamon press Oxford, England. 1987;197-99.
- Li Y, Liu Y, Wang H, et al. Synthesis, crystal structure, vibration spectral, and DFT studies of 4-aminoantipyrine and its derivatives. Molecules. 2013;18:877-93.
- YamaguchiY,Hayashl C.Determination of urinary total phenolic compounds with use of 4-aminoantipyrine: Suggested screening test for hyperthyroidism and for catecholamine-producing tumor. Clin Chem. 1977;23:2151-4.
- Ismail K. Transition metal chemistry. 2000;25:522-8.
- Diehl H, Hach CC. Inorganic synthesis. 1950;3:196-201.
- Shabani F, Saghatforoush LA, Ghammamy S. Synthesis, characterization and anti-tumour activity of iron(Iii) Schiff base complexes with unsymmetric tetradentate ligands.Bull Chem Soc Ethio. 2010;24:193-9.
- Sharma R, Singh PR, Pawar S, et al. Studies of transition metal complexes and their antibacterial activities.JAmSci. 2010;6:559-64.
- Suresh MS, Prakash V. Preparation and characterization of Cr(III), Mn(II), Co(III), Ni(II), Cu(II), Zn(II) and Cd(II) chelates of Schiffs base derived from vanillin and 4-amino antipyrine.J Phys Sci. 2010;5:2203-11.
- Mosoarca EM, Tudose R, Linert W, et al. Mononuclear complex of Fe(III) with N, N?-tetra(4-antipyryl-methyl)-1,2 diaminoethane. Synthesis and Spectral Properties Chem Bull. "POLITEHNICA" Univ (Timisoara). 2006;51:1-2.
- Abdel-Wahab ZH, Mashaly MM, Faheim AA. Synthesis and characterization of Cobalt(II), Cerium(III) and dioxouranium(VI) complexes of 2,3-dimethyl-1-phenyl-4-salicylidene-3-pyrazoline-5-one mixed ligand complexes, pyrolytic products and biological activities. Chem Pap.2005;59:25-36.
- Khedr AM, Marwani HM. Synthesis, spectral, thermal analyses and molecular modeling of bioactive Cu (II)-complexes with 1,3,4-thiadiazole Schiff base derivatives. Their catalytic effect on the cathodic reduction of oxygen. Int J Electrochem Sci. 2012;7:10074-93.
- Mohamed GG, Omar MM. Metal complexes of Schiff Bases: Preparation, characterization, and biological activity. Turk J Chem. 2006;30:361-82.
- Redayan MA. J Baghdad for Sci. 2012;9:536-8.
- Nichols D. The chemistry of Iron, Cobalt and Nickel. 1stedn, pergamon, oxford. 1973.