Original Article
, Volume: 15( 3)Studies of Solvent Effect of Aquo-Methanol Solvent System on Kinetics and Activation Parameters of Base Catalyzed Hydrolysis of Ethyl Cinnamate
- *Correspondence:
- Singh AK, Department of Chemistry, Teerthanker Mahaveer University, Moradabad, Uttar Pradesh, India, Tel: +91-8126721634; E-mail: anilkumar2_singh@yahoo.com
Received: July 19, 2017; Accepted: August 02, 2017; Published: August 05, 2017
Citation: Singh AK. Studies of Solvent Effect of Aqua-Methanol Solvent System on Kinetics and Activation Parameters of Base Catalyzed Hydrolysis of Ethyl Cinnamate. Int J Chem Sci. 2017;15(3):169
Abstract
The solvent effect of ethanol on the alkali catalyzed solvolysis reaction was studied by carring out of the hydrolysis of ester namely ethyl cinnamate in water-methanol media of varying composition consisting of 30 to 70% of methanol (v/v) at different Temperature ranging from 20°c to 70°c. The Specific rate constant values of the reaction were found to depleted with increasing concentration of methanol in reaction media. Enhancement in ï„G* with simultaneous depletion in ï„H* and ï„S* of the reaction, it has been concluded that reaction is enthalpy stimulating and entropy inhibiting and specific salvation take place in water methanol media. From the evaluated value of Iso kinetic temperature, which is less than 300 indicates that this reaction in water-methanol media obey Barclay-Butler rule and there is weak but considerable solvent-solute interaction taking place in reaction media.
Keywords
Solvent effect; Solvent-solute interaction; Iso-kinetic temperature; Specific salvation; Ethyl cinnamate; Base catalyzed; Activation parameter
Introduction
Study of solvent effect of protic and aprotic solvent on the reaction such as hydrolysis of ester and amide have been carried out by numbers of workers but explanation put forward are not quite satisfactory particularly solvolysis of ethyl cinnamate which is important for medicinal use as well as flavoring agent in cut tobacco [1-3]. There for kinetic study of the solvent effect on the base catalyzed hydrolysis ethyl cinnamate in water-methanol media of various compositions are needed to investigate which has not studies so far.
Experimental
The hydrolysis of ethyl cinnamate has been carried out volumetric in water-methanol having different concentration of solvent (methanol) varying from 30% to 70% (v/v) at five different temperatures ranging from 20°C to 40°C. The reaction was found to follow second order kinetics equation and evaluated value of specific rate constant has tabulated in Table 1 the evaluated thermodynamic activation parameter has been enlisted in Table 2.
| 30% | 40% | 50% | 60% | 70% |
|---|---|---|---|---|
| 15.66 | 11.22 | 10.00 | 8.12 | 6.45 |
| 20.89 | 16.59 | 13.48 | 10.59 | 8.49 |
| 27.54 | 21.87 | 16.98 | 13.64 | 10.83 |
| 38.01 | 30.19 | 23.17 | 18.62 | 14.62 |
| 45.70 | 36.30 | 27.86 | 22.38 | 17.74 |
Table 1: Hydrolysis of ethyl-cinnamate specific rate constant [k × 103 (dm)3/mole/mint] values of alkali catalyzed in water-methanol media.
Result and Discussion
Solvent effect on rate of reaction
The specific rate constant was calculated with help of second order kinetics and calculated values is inserted in Table 1 From Table 1 it is observed that the values of specific rate constant are decrease with increase of temperature which is quite in agreement with theory of Hughes and Ingold [4] and Singh AK [5].
Effect of salvation power of reaction media on thermodynamic activation parameters of reaction
It is a great significance to study of solvent effect on thermodynamic activation parameters, such as enthalpy of activation (ΔH*), free energy of activation (ΔG*), and entropy of activation (ΔS*). These parameters are calculated using Wynne-Jones [6] and Eyring equation have been recorded in Table 2.
| % of MEOH |
Mole% | ΔH* in KJ/Mole | 20°C | 25°C | 30°C | 35°C | 40°C | |||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| ΔG* | -ΔS* | ΔG* | -ΔS* | ΔG* | -ΔS* | ΔG* | -ΔS* | ΔG* | -ΔS* | |||
| 30% | 16.03 | 53.31 | 93.64 | 137.64 | 94.27 | 137.44 | 94.98 | 137.52 | 95.60 | 137.30 | 96.31 | 137.38 |
| 40% | 22.90 | 52.95 | 94.20 | 140.78 | 94.91 | 138.42 | 95.65 | 140.89 | 96.31 | 140.77 | 97.03 | 140.83 |
| 50% | 30.82 | 52.87 | 94.68 | 142.6 | 95.44 | 140.30 | 96.23 | 143.10 | 96.96 | 143.14 | 97.75 | 143.38 |
| 60% | 40.06 | 51.28 | 95.16 | 149.76 | 95.88 | 147.24 | 96.72 | 149.96 | 97.43 | 149.83 | 98.23 | 150.00 |
| 70% | 50.97 | 50.51 | 95.64 | 154.02 | 96.39 | 151.44 | 97.19 | 154.05 | 97.96 | 152.56 | 98.77 | 154.18 |
Table 2: Thermodynamics activation parameters of the reaction in water-acetone media ΔH* and ΔG* in KJ/Mole, ΔS* in J/K/Mole.
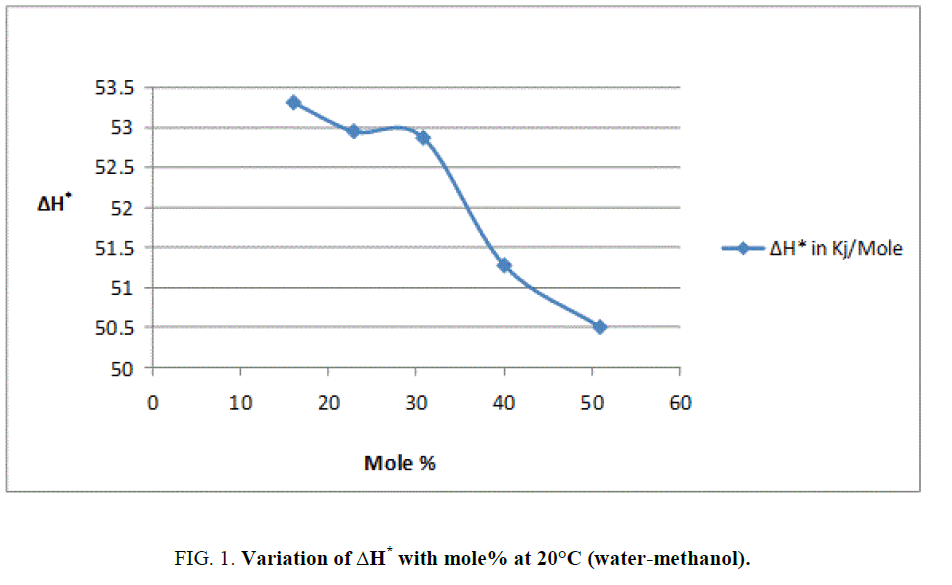
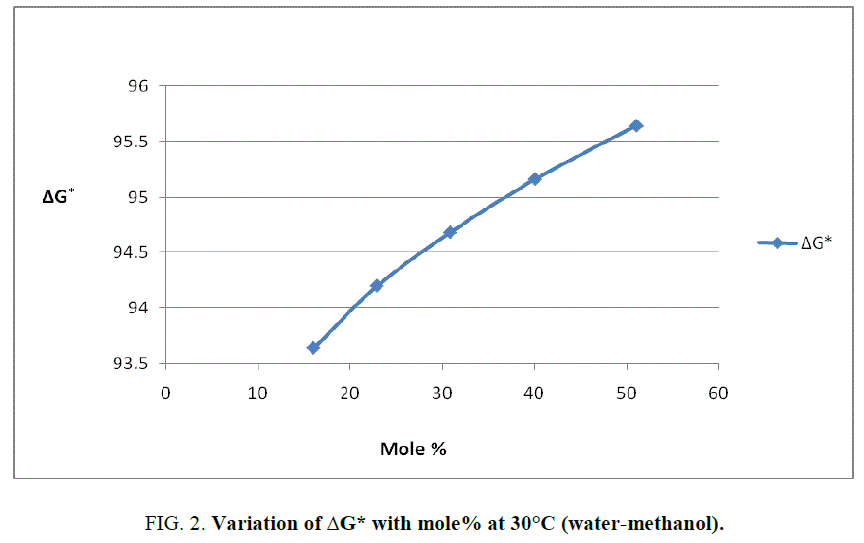
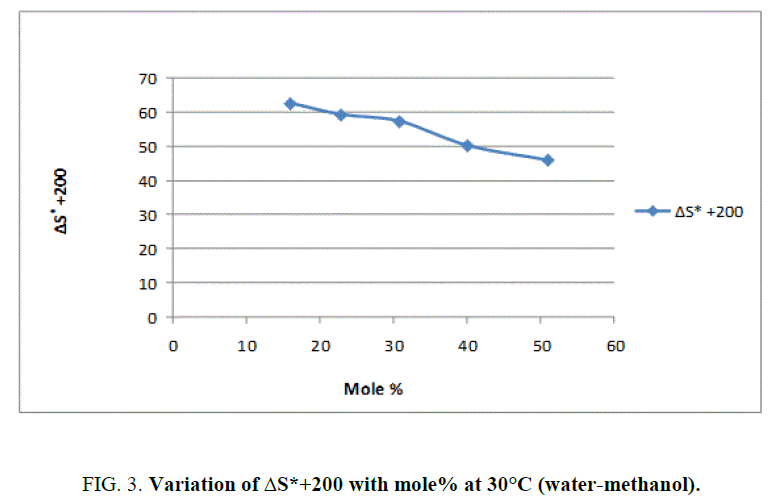
To explain the effect of solvent on these activation parameters, the value of these parameters was plotted against mole% at 30°C of methanol which is shown in Figure 1, Figure 2 and Figure 3.
From Figure 2 and the value s of ΔG* recorded in Table 2, obviously indicate that the variation in ΔG* is small and it increases from 94.98 KJ/Mole to 97.19 KJ/Mole at 30°C with change of proportion of methanol from 30% to 70% (v/v). The small but considerable increase in ΔG* and nonlinear variation in ΔH* and ΔS* curves with the increasing mole% as shown in Figure 1 and Figure 3 are indication of specific salvation taking place in process of activation as already proposed by Tommila [7], Elsemogy [8], Singh [9], Barclay and Butler [10] have also observe the similar increase in ΔG* values. Increase in ΔG* with simultaneous decrease in ΔH* and ΔS* values are only possible when extent (degree) of depletion in ΔS* value is greater than ΔH* values and from this, it may be inferred that alkali catalyzed hydrolysis of ethyl cinnamate in water-methanol media act as Entropy inhibitor and Enthalpy stimulator solvent. Such inference has also recently been supported by recent view of Leffler [11].
Barclay and Butler relationship and solvent-solute interaction
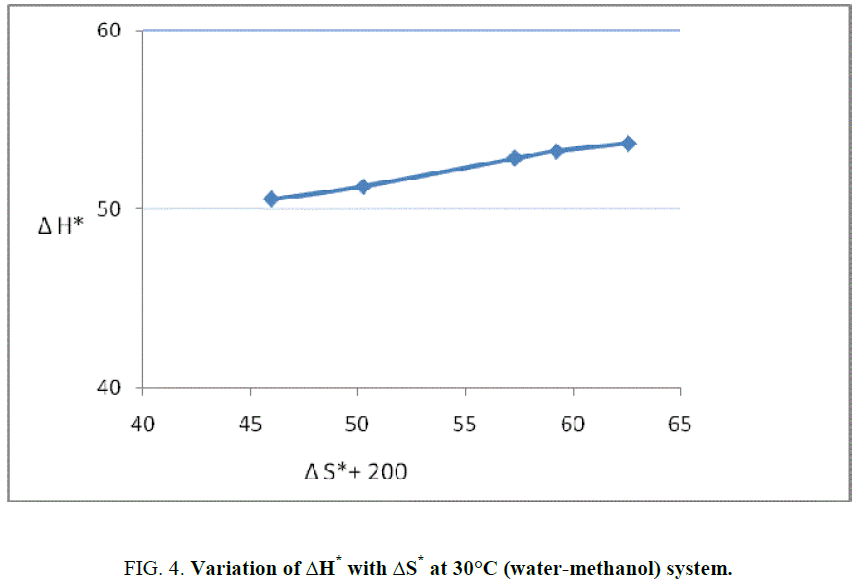
Barclay and Butler relationship between enthalpy and entropy of activation, which is as follows [12]:
δm (ΔH*)=β δm (ΔS*).
This reaction is found to follow the above Barclay and Butler relation where β represent iso kinetic temperature. It is also known as Leffler-Grunwald solvent stabilizer operator. From the value of slope of plot (Figure 3), the value of iso kinetic temperature was evaluated which come to less than 300 (200 aprox) (Figure 4). It shows that change in structure of reactant or solvent is due to weak interaction between solvent and solute in similar way as reported by Leffler [12] and recently supported by Singh [13].
Conclusion
In this research work, namely hydrolysis of cinnamate ester the specific rate constant show decreasing trend at all specify temperature indicate that transition state is more desolveted than initial state. The increase in the value of and ΔG* with simultaneous decrease in ΔH* and ΔS* for the hydrolysis ethyl cinnamate in water-methanol is enthalpy dominating and enthalpy control. The values of Iso-kinetic which is less than 300, clearly indicates that there is no appreciable interaction between solvent and solute present in the reaction media, i.e., reaction is not ion-dipole but ion-molecule type.
Acknowledgement
My special gratitude to my supervisor Prof. R T Singh Veer Kunwar Singh (VKS) university ARA, Bihar, India for his proper guidance and thanks to my friend Dr. Ajit, Dr. Parag and Mr. LK Tiwari for his cooperation during preparation of this content.
References
- Nada A, Jallal A, Ismail AM. Solvent effect of kinetics of amide bond cleavage in p. chloro and p. bromo oxazolinanes in acetone nitrile-water mixture. J Solution Chem. 2012;41:2154-63.
- Fathalla MF. Kinetics of reaction of 2-chloro-quinosalin with hydroxide ion in CAN-H2O and DMSO-H2O binary solvent mixture. J Solution Chem. 2011;40:1258-70.
- Singh AK. Solvent effect on the enthalpy and entropy of activation for the hydrolysis of ethyl cinnamate in mixed solvent system. J Phys Chem Biophys. 2017;7:238.
- Hughes ED, Ingold CK. Mechanism of substitution at saturated carbon atom part IV. A discussion of constitution and solvent effect on mechanism, kinetics, velocity, and orientation of substitution. J Chem Soc. 1935;244-55.
- Singh AK. The influence of solvent on the solvolysis of ethylcinnamate in water acetone mixed solvent system. Int J Engg Appl Comp Sci. 2017;2(2):79-82.
- Wynne-Jones WFK, Eyring H. The absolute rate of reaction in condense phase. J Chem Phys. 1935;3:492-502.
- Tommila E. Influnce of solvent on reaction velocity. Acta Chem Scand. 1955;pp:957-88.
- Elsemogy MM, Abu Elamayem MS, Mussa MNH. Solvent effect on the enthalpy and entropy of activation for the hydrolysis of ethyl cinnamate in mixed solvent system. Z Physik Chem (Neuetold). 1975;94:69.
- Singh AK. Solvent effect on the enthalpy and entropy of activation for the hydrolysis of ethyl cinnamate in mixed solvent system. J Phys Chem Biophys. 2017;2(4):123-6.
- Barclay L, Butler JAV. The entropy of solution. J Am Chem Soc. 1938;pp:1445.
- Leffler JE, Grunwald E. Rate and equlibria of organic reaction. J Chem Edu. 1964;pp:407.
- Leffler JE. Entropy relationship and implication for organic chemistry. J Org Chem. 1955;pp:1201.
- Singh AK. Solvolysis rate and activation parameter of ethyl acetate in mixed dipolar organic solvent systems-A solvent effect. Int J Res Appl Sci Engg Tech. 2016;4(16):704-10.