Original Article
, Volume: 10( 6)Effect of Size and Surface Modification of Nano Particles on the Performance of Epoxy Coatings
- *Correspondence:
- Shalini D, Department of Metallurgical Engineering and Material Science, Indian Institute of Technology Bombay, Mumbai, 400076, Maharashtra, India, Tel: 912225764618; E-mail: 133110055@iitb.ac.in
Received: August 08, 2016; Accepted: August 31, 2016; Published: September 07, 2016
Citation: Shalini D, Anand SK. Effect of Size and Surface Modification of Nano ZnO Particles on the Performance of Epoxy Coatings. Nano Sci Nano Technol. 2016;10(6):107.
Abstract
The exterior durability of epoxy coatings is poor as they tend to chalking and yellowing due to weathering under sunlight. Also, after prolonged exposure to sunlight, cracking of the coating takes place. This leads to the exposure of metal substrate to corrosive environment and thereby leading to extensive corrosion. That is why all epoxy coated components are top coated with acrylics or polyurethanes which is a costly process. Another alternative is to use UV absorbers like nano zinc oxide (ZnO) which is a well-known Ultra-Violet (UV) absorber is used in the current study. In this work, the effect of nano ZnO in the epoxy coatings on its weathering properties has been investigated. In addition to this, nano ZnO can also improve the mechanical properties and corrosion resistance without affecting the optical clarity of clear epoxy coats. Nano ZnO powder was functionalized with 3-glycidyloxypropyl-trimethoxysilane (GPTMS) and incorporated into the clear epoxy resin its effect on performance, mechanical and optical properties of the coating. The results were compared with unmodified nano ZnO particles. Ultra-Violet weathering tests were done to study their weathering properties. Electro chemical impedance spectroscopy (EIS) was used for studying corrosion properties and mechanical tests like taber abrasion, pull-off adhesion and hardness tests were carried to evaluate the mechanical properties.
Keywords
Epoxy coating; Nano particles; Zinc oxide; Ultra-Violet; Electro chemical impedance spectroscopy
Introduction
All types of molecules selectively absorb the radiant energy of specific wave length across the electromagnetic spectrum. This can lead to photochemical reactions. These reactions include transformation of one compound to the other, volatilization and de-polymerization of organic compounds, breaking of chemical bonds or more complex reactions like formation of reactive free radicals from oxidizing compounds. Damage caused by these photo chemical reactions is called as photo degradation. Two main catalysts in these reactions are humidity and oxygen. Organic coatings are prone to such degradation [1,2]. Binders like epoxy with aromatic ring structures are absorptive and therefore susceptible to degradation [3]. The results are chalking and yellowing [4].
In order to control such damage HALS (hindered amine light stabilizers scavengers) and UV stabilizers are used [1]. UV stabilizers are the materials with high band gap energy and nano ZnO is one such material with high band gap energy of 3.3 eV to 3.4 eV. Nano ZnO is an inorganic compound, so it is non-migratory and more stable compared to organic UV stabilizers. Since ZnO is an inorganic compound, incompatibly arises between the matrix and nano ZnO. Hence, the interface between the nano ZnO particle and the epoxy matrix is vulnerable to stress and cracks initiation. Hence surface modification of nano particles with suitable coupling agent plays an important role in improving the properties of a coating. There are several examples of surface modification of nano particles with silanes to achieve the compatibility [5,6]. In the current study, effect of nano ZnO on epoxy coatings and the importance of its compatibility with the matrix have been studied.
Experimental
Materials
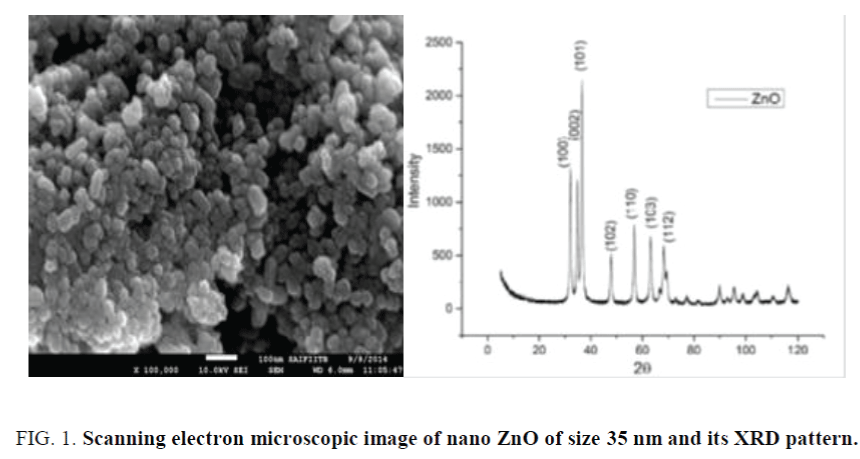
Nano ZnO with mean size 35 nm and 90 nm (synthesized in our lab) was used for experimental work. Nano ZnO was synthesized by using zinc acetate dehydrate as precursor, potassium hydroxide as reactant and methanol as solvent. Epoxy resin and amino adduct hardener was procured from Khamir industries. BYK-9076, a dispersing agent from BYK chemicals was used to disperse nano ZnO in epoxy matrix. Sodium lauryl sulphate (SLS) from Dow Chemicals and GPTMS from Sigma Aldrich were used to modify the surface of nano ZnO particles. Scanning electron microscope (SEM model no. 3400, Hitachi) was used for imaging. SEM image of 30 nm ZnO and its corresponding X-Ray diffraction pattern is shown in the Figure 1. The peaks appeared at values of 32°, 34°, 38°, 48°, 57°, 64° and 70° could be attributed to the diffraction peaks of (100), (002), (101), (102), (110), (103) and (112) planes of ZnO.
Preparation of coated samples
Control epoxy: Epoxy was mixed with the hardener and applied on the rust free, degreased and roughened (by 80 grit emery paper) mild steel samples using an applicator with 100 μ allowance. Substrate preparation was same in the below two cases.
Un-modified ZnO/epoxy coating (ZnO/epoxy): Nano ZnO of 30 nm size was dispersed in the solvent using dispersing agent by ultra-sonication at 70% amplitude for half an hour. This dispersion was added to the epoxy resin and stirred for 1 h. Hardener was added into the resin and stirred for 10 min. The coating was applied on steel panels with an applicator. The coating was designated as 30-ZNO/epoxy.
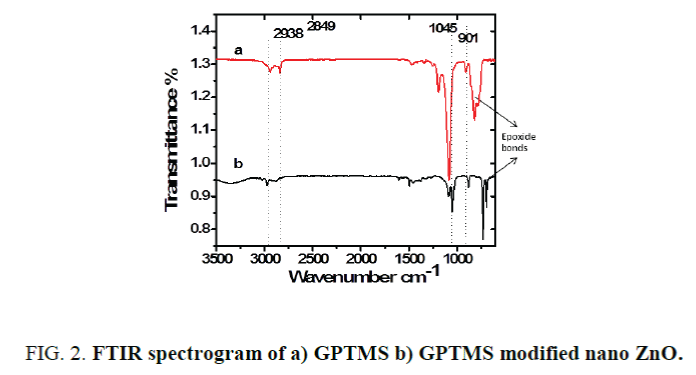
Modified ZnO/epoxy coating (M-ZnO/epoxy): Nano ZnO (30 nm and 90 m) was soaked in 30% Butyl alcohol and 70% xylene mixture to form OH bonds on the surface of the particles. 10 W% GPTMS with respect to the weight of nano ZnO was added to this solvent along with 0.1 W% SLS (W% with respect to the weight of ZnO). This mixture was kept under continuous stirring for 24 h at 65°C. Surface of the nano ZnO gets modified with GPTMS and it was confirmed by the FTIR spectroscopy as shown in the Figure 2. The mixture along with butyl alcohol/xylene mixture was used as solvent in the epoxy resin. A few drops of BYK-9076 were added. This entire mixture was added into the epoxy resin and stirred for 1 h. In the end, hardener was added and stirred for 10 min. This coating was applied on the steel panels using an applicator.
In both the cases above, the concentration of ZnO was maintained constant i.e., 1 W% with respect to the weight of epoxy resin. Both 30 nm ZnO and 90 nm ZnO were functionalized separately. The coating with 30 nm ZnO was designated as M-30-ZnO/epoxy and one with 90 nm ZnO particles was named as M-90-ZnO/epoxy.
Results and Discussions
Characterization of modified ZnO
The IR spectra of GPTMS and M-ZnO/epoxy in the range of 500 cm-1 to 4,000 cm-1 were recorded using Perkin Elmer ATR spectrum 100 FTIR spectrometer. Figure 2 shows the FTIR analysis of GPTMD and GPTMS modified nano ZnO. FTIR confirms silane modification of ZnO surface. A peak at 901 cm-1 is attributed to epoxide in the silane. Peaks at 2,938 cm-1 and 2,849 cm-1 are due to C–H stretching [7]. The peak at 1,045 cm-1 is due to Si–O bonds [8].
Visual observation of dispersion stability of nano ZnO
The stability of the particles in xylene was visually observed for 30-days. The particles with only BYK-9076 dispersant got settled down as shown in the Figure 3a. Whereas GPTMS modified nano ZnO in xylene with a few drops of BYK-9076 was well dispersed and no sedimentation was observed as can be seen in Figure 3b. This shows that GPTMS acts as a stabilizing agent for maintaining good suspension of nano ZnO in xylene.
FIG. 3. 30-days visual observation of a) sedimentation of un-modified ZnO in solvent with BYK-9076 b) modified ZnO dispersion in solvent.
Weathering tests
All the coatings including control epoxy coating were subjected to accelerated weathering in QUV chamber (QUV weathering test chamber, QUV Co. lab) for 220 h. The chamber simulates extreme weathering conditions as per ASTM G53 standards. That means the samples are exposed to UV-B radiations of wave length of 315 nm for 8 h at 100% humidity followed by condensation for 4 h. The yellowing index and loss in gloss were measured by BYK gardener spectrometer. The readings were taken after every 32 h. The increase in the yellowness of the coating is measured by yellowing index.
Gloss loss and yellowness index reports
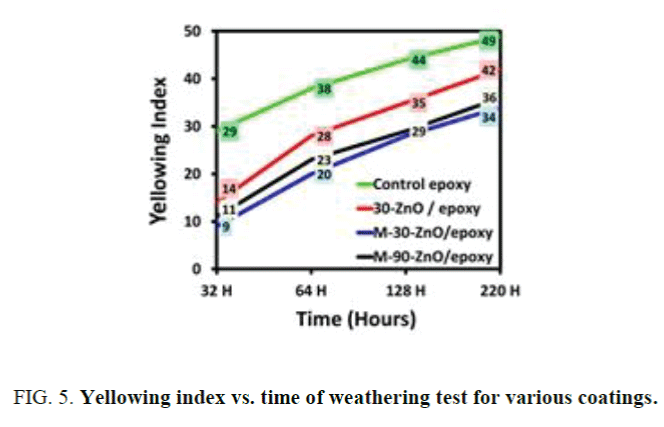
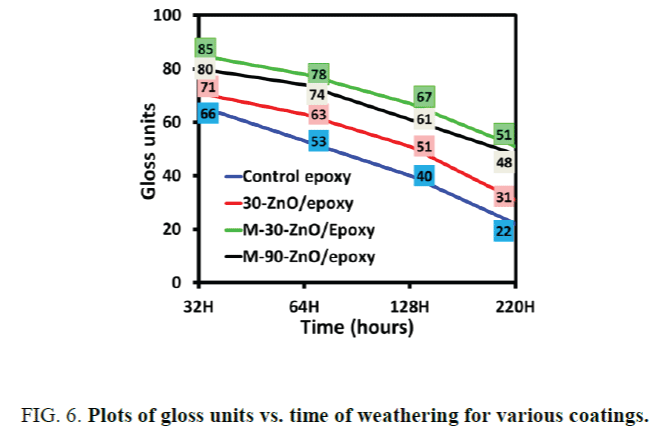
Effect of modification of ZnO particles with GPTMS: Figure 4 shows the samples of control epoxy and ZnO stabilized epoxies subjected to accelerate weathering for 220 h. Chalking was started in the case of control epoxy sample as can be seen in the Figure 4d, whereas ZnO stabilized epoxies were still glossy and reflective as can be seen in Figure 4a-4c. The yellowness index of unmodified ZnO and modified ZnO of various sizes are compared in the Figure 5. The initial yellowing index for all the coatings was 5. The results of loss in gloss are expressed in terms of gloss units and have been represented in Figure 6. The initial gloss unit of all the coatings before testing was 90. This shows that ZnO doesn’t have any negative effect on gloss of the coating. Also, the optical clarity wasn’t affected by nano ZnO. In all the above cases, the results were better in the case of GPTMS modified nano ZnO because of good compatibility with the matrix and stability of the particles in epoxy resin.
FIG. 4. Epoxy coated mild steel samples subjected to accelerated weathering tests for 220 h a) M-30-ZnO/epoxy b) M-90-ZnO/epoxy c) 30-ZnO/epoxy d) control epoxy.
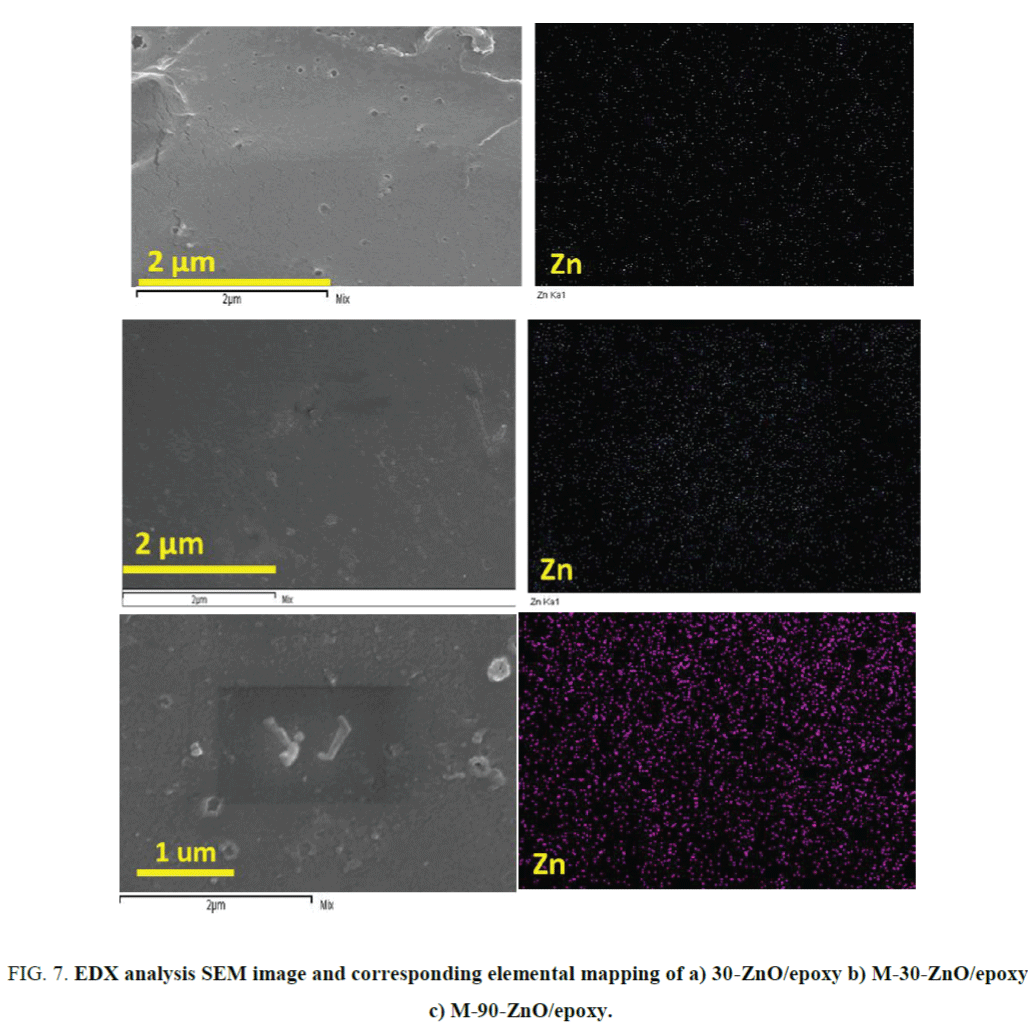
Observation of Zn distribution in epoxy matrix: The distribution of nano ZnO in the above three cases can be seen in elemental mapping as shown in the Figure 7. The distribution of Zn is mapped using elemental mapping technique in the scanning electron microscope. The distribution was found to be more effective in the case of GPTMS modified ZnO/epoxy coatings as it is evident from the Figure 7b and 7c. In the Figure 8a, the scanning electron microscopic picture of a ZnO agglomerate embedded in the ZnO/epoxy coating matrix shows cracks around its surface at 16,000X magnification whereas no such cracks can be seen in the case of M-ZnO/epoxy coating even at 45,000X magnification as can be seen in the Figure 8b. The unmodified ZnO particles tend to settle down in the epoxy matrix due to the high density whereas GPTMS stabilizes ZnO particles which prevent them from forming sedimentation. Hence, a uniform distribution can be seen even after curing in the case of GPTMS modified ZnO.
FIG. 7. EDX analysis SEM image and corresponding elemental mapping of a) 30-ZnO/epoxy b) M-30-ZnO/epoxy c) M-90-ZnO/epoxy.
FIG. 8. Scanning electron microscopic image of a) agglomerate of ZnO in ZnO/epoxy showing cracks b) compatible small agglomerate in M-ZnO epoxy.
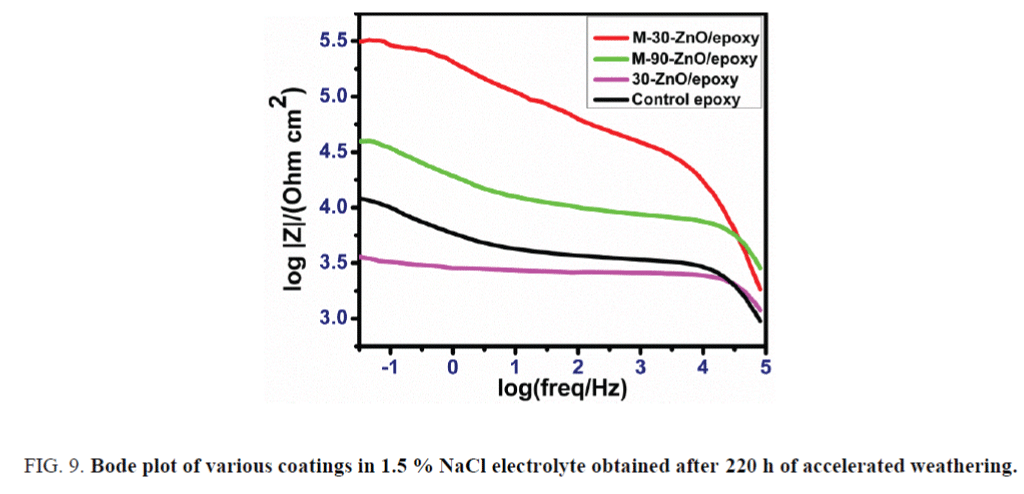
Electro chemical impedance spectroscopy after 220 h of UV exposure: The EIS analysis was carried using software controlled CH instrument (model 600C series). Bode plots of all the coatings from electrochemical impedance spectroscopy after 220 h of UV exposure are represented in Figure 9. There was two orders difference in the magnitude of impedance between the control epoxy and M-30-ZnO/epoxy. This shows the better performance of M-30-ZnO/epoxy coating due to less damage in the accelerated weathering test. This is due to less number of defects at the interface of nano particles and epoxy matrix. Another factor is presence of water repellant silane i.e., GPTMS in the coating. The Figure 9 also shows that the performance of M-30-ZnO/epoxy coating is better than M-90-ZnO/epoxy.
FIG. 9. Bode plot of various coatings in 1.5 % NaCl electrolyte obtained after 220 h of accelerated weathering.
Effect of nano ZnO on mechanical properties of epoxy coating
All the epoxy samples were subjected to taber abrasion testing by ASTM D4060 with 1 kg load on CS 17 wheels. The weight loss of the coating after taber abrasion test is called taber abrasion index and these values are represented in Table 1. Abrasion resistance was increased by three times after adding ZnO. Nano particles embedded in organic coating matrix will increase the rigidity of the coating. Hence improves the resistance to abrasion [9]. Silane modification of ZnO has no additional effect on abrasion resistance. Impact test was conducted on all the above samples according to ASTM D2794. The results are measured in kg-meter. All the samples showed the same values. Scratch resistance was carried out according to ASTM D 5178 and the results were same in all the cases.
| Sample name | Taber abrasion index(grams) | Impact strength(kg-meter) | Scratch hardness (kg) |
|---|---|---|---|
| Control epoxy | 150 | 10 | 8 |
| 30-ZnO/epoxy | 55 | 10 | 8 |
| M-30-ZnO/epoxy | 66 | 10 | 8 |
| M-90-ZnO/epoxy | 69 | 10 | 8 |
Table 1: Taber abrasion index, impact resistance and scratch hardness of various coatings.
Conclusion
In this work, effect of nano ZnO and impact of its surface modification on the weathering properties was studied. Nano ZnO can act as an effective UV blocker without affecting the optical clarity of clear epoxies and their gloss. However, its surface modification with silane can further improve the resistance to weathering. Even after 220 h of exposure to weathering, silane modified ZnO showed good corrosion performance. Size of nano particles is also an important factor. As the size reduces, the surface area increases. Thus the resistance to weathering and corrosion increases. Coating with modified 30 nm ZnO particles showed better weathering resistance and corrosion resistance compared to that of 90 nm ZnO particles. Nano ZnO improves the abrasion resistance also.
References
- Step E, Turro NJ. Gande ME. et al. Mechanism of polymer stabilization by hindered-amine light stabilizers (HALS). Model investigations of the interaction of peroxy radicals with HALS amines and amino ethers. Macromolecules.1994;27(9):2529-39.
- Dabrowska D, Kot WA,Namie?nik J. The importance of degradation in the fate of selected organic compounds in the environment. Part II. Photo degradation and biodegradation. Pol J Environ Stud. 2004;13(6):617-26.
- Rajagopalan N. Khanna A. Effect of size and morphology on UV-blocking property of nano ZnO in epoxy coating. Int J Sci Res Publ. 2013;3(4):01-14.
- Malshe V, Waghoo G. Chalk resistant epoxy resins. ProgOrg Coat. 2004;51(3):172-80.
- Eslami FR, Khosravi H, Fayazzadeh S. Using 3-Glycidoxypropyltrimethoxysilane Functionalized SiO2 Nanoparticles to Improve flexural properties of glass fibers/epoxy grid-stiffened composite panels. Int J Chem Mol Nucl Mater Metallu Eng. 2015;9(12):1377-80.
- https://www.shinetsusilicone-global.com/catalog/pdf/ResinModification_e.pdf
- Chen S, Bo Y, Shuxue Z, et al. Preparation and characterization of scratch and mar resistant waterborne epoxy/silica nanocomposite clearcoat.J Appl Polym Sci.2009;112(6):3634-9.
- Nazir T, Adeel A, Humaira MS, et al. The influence of temperature and interface strength on the microstructure and performance of sol-gel silica-epoxy nanocomposites. Polym Bull. 2011;67(8):1539-51.
- Dittanet P. Fracture behavior of silica nanoparticle filled epoxy resin. Pennsylvania: Lehigh University; 2011.