Review
, Volume: 11( 6)A Review on Processing of Crude Oil and its Production of Hydrocarbon Intermediates
- *Correspondence:
- Dinesh KG , Department of Pharmaceutical Analysis and Quality Assurance, CMR College of Pharmacy, Medchal, Telangana, India, Tel: +919963330488; E-mail: dineshkumar.2431@gmail.com
Received: September 06, 2016; Accepted: September 24, 2016; Published: September 30, 2016
Citation: Dinesh KG. A Review on Processing of Crude Oil and its Production of Hydrocarbon Intermediates. Chem Technol Ind J.2016;11(6):105.
Abstract
The hydrocarbon intermediates alluded to in the past section are delivered by subjecting unrefined oils to different handling plans. The partition depends on contrasts of certain physical properties of the constituents, for example, the bubbling and liquefying focuses, adsorption affinities on a specific strong, and dispersion through specific layers. Air refining isolates the unrefined petroleum complex blend into various parts with moderately contract bubbling reaches. By and large, detachment of a blend into parts is construct fundamentally with respect to the distinction in the breaking points of the segments. The hot food enters the fractionator, which ordinarily contains 30-50 fractionation plate. The three vital warm splitting methods are coking, consistency breaking, and steam breaking. Butadiene, a conjugated diolefin, is regularly coproduced with C2-C4 olefins from various splitting procedures. Partition of these olefins from reactant and warm breaking gas streams could be accomplished utilizing physical and compound division strategies. The response is exceptionally endothermic, so it is favored at higher temperatures and lower weights. Superheated steam is utilized to lessen the halfway weight of the responding hydrocarbons (in this response, ethane).
Keywords
Thermal conversion; Crude oil; Vapours; Petroleum; Olefins; Hydrocarbons
Introduction
The hydrocarbon intermediates alluded to in the past section are delivered by subjecting unrefined oils to different handling plans. These incorporate an essential refining venture to isolate the unrefined petroleum complex blend into less difficult divisions. These parts are fundamentally utilized as powers. In any case, a little rate of these streams are utilized as optional crude materials or intermediates for acquiring olefins, diolefins, and aromatics for petrochemicals creation. Further preparing of these portions might be required to change their synthetic structure to the required items. These new items may likewise be utilized as fills of enhanced qualities or as substance feedstocks. For instance, changing a naphtha portion chemically delivers reformate rich in aromatics [1-6]. The real utilization of the reformate is to supplement the gas pool because of its high octane rating. Be that as it may, the reformate is additionally used to extricate the aromatics for petrochemicals use. Now, the creation of intermediates for petrochemicals is not distinct from the generation of powers. In this section, the creation of hydrocarbon intermediates is talked about in conjunction with various unrefined petroleum handling plans. These incorporate physical division systems and synthetic transformation forms. The creation of olefins is additionally examined in the last area [7-11].
Physical Separation Processes
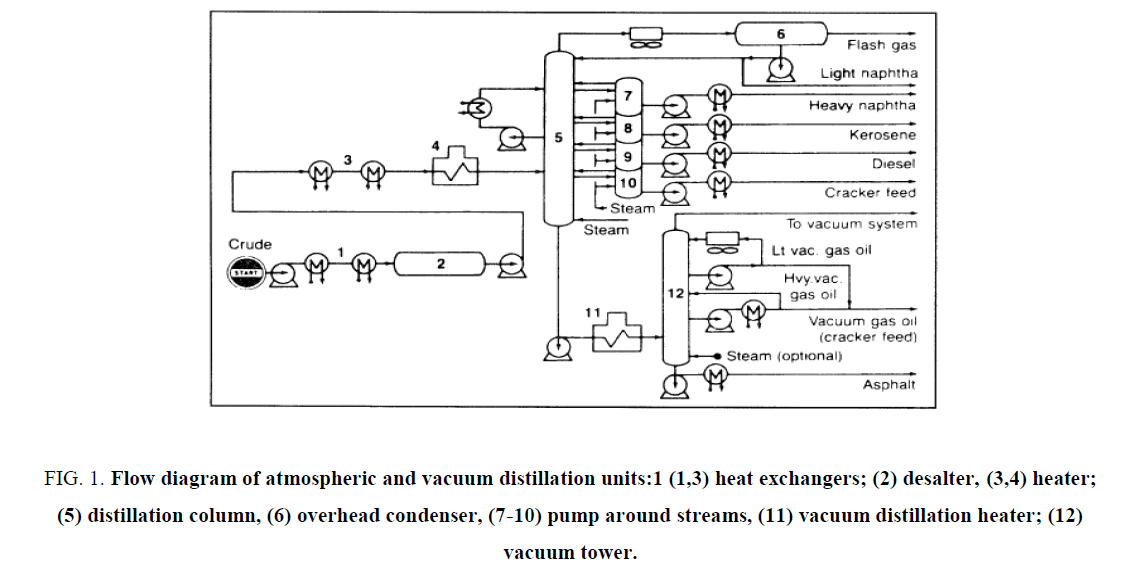
Physical division procedures isolate a blend, for example, a raw petroleum without changing the compound attributes of the segments. The partition depends on contrasts of certain physical properties of the constituents, for example, the bubbling and liquefying focuses, adsorption affinities on a specific strong, and dispersion through specific layers [12-16]. The imperative physical detachment forms, examined here, are refining, retention, adsorption, and dissolvable extraction ( Figure 1).
Figure 1: Flow diagram of atmospheric and vacuum distillation units:1 (1,3) heat exchangers; (2) desalter, (3,4) heater; (5) distillation column, (6) overhead condenser, (7-10) pump around streams, (11) vacuum distillation heater; (12) vacuum tower.
Atmospheric Distillation
Air refining isolates the unrefined petroleum complex blend into various parts with moderately contract bubbling reaches. By and large, detachment of a blend into parts is construct fundamentally with respect to the distinction in the breaking points of the segments. In air refining units, one or additionally fractionating segments are utilized. Refining an unrefined petroleum begins by preheating the food by trade with the hot item streams. The food is further warmed to around 320°C as it goes through the warmer channel (pipe still radiator) [17-25].
The hot food enters the fractionator, which ordinarily contains 30-50 fractionation plates. Steam is presented at the base of the fractionator to take off light segments. The proficiency of partition is a component of the quantity of hypothetical plates of the fractionating tower and the reflux proportion. Reflux is given by gathering part of the tower overhead vapours. Reflux proportion is the proportion of vapours consolidating back to the still to vapours gathering out of the still (distillate). The higher the reflux proportion, the better the partition of the blend [26-35].
Conversion Processes
Change forms in the petroleum business are by and large used to:
• Upgrade lower-esteem materials, for example, substantial deposits to more important items, for example, naphtha and LPG. Naphtha is mostly used to supplement the gas pool, while LPG is utilized as a fuel or as a petrochemical feedstock.
• Improve the attributes of a fuel. For instance, a lower octane naphtha part is changed to a higher octane reformate item. The reformate is essentially mixed with naphtha for fuel detailing or extricated for acquiring aromatics required for petrochemicals creation.
• Reduce hurtful contaminations in petroleum divisions and deposits to control contamination and to abstain from harming certain handling impetuses. For instance, hydrotreatment of naphtha sustains to synergist reformers is key since sulfur and nitrogen polluting influences harm the impetus [36-42].
Transformation procedures are either warm, where just warmth is utilized to impact the required change, or synergist, where an impetus brings down the response actuation vitality. The impetus additionally coordinates the response toward a craved item or items (particular impetus).
Thermal Conversion Processes
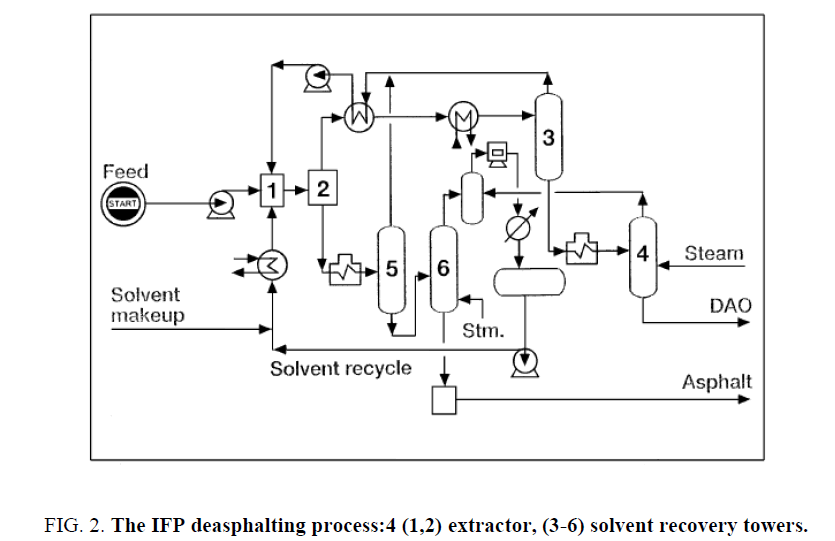
Thermal splitting was the primary procedure used to build gas generation. After the advancement of synergist splitting, which enhanced yields and item quality, Thermal breaking was given different parts in refinery operations [43-47]. The three vital warm splitting methods are coking, consistency breaking, and steam breaking ( Figure 2). Steam breaking is of unique significance as a noteworthy procedure planned particularly to produce light olefins. It is talked about independently later in this part.
Production of Olefins
The most vital olefins and diolefins used to fabricate petrochemicals are ethylene, propylene, butylenes, and butadiene. Butadiene, a conjugated diolefin, is regularly coproduced with C2-C4 olefins from various splitting procedures. Partition of these olefins from reactant and warm breaking gas streams could be accomplished utilizing physical and compound division strategies. Be that as it may, the petrochemical interest for olefins is much more prominent than the sums these operations produce. Most olefins and butadienes are created by steam breaking hydrocarbons. Butadiene can be on the other hand delivered by other engineered courses examined with the union of isoprene, the second major diolefin for elastic generation.
Steam Cracking of Hydrocarbons
The fundamental course to produce light olefins, particularly ethylene, is the steam breaking of hydrocarbons. The feedstocks for steam splitting units range from light paraffinic hydrocarbon gasses to different petroleum portions and buildups. The splitting responses are chiefly bond breaking, and a considerable measure of vitality is expected to drive the response toward olefin generation. The least difficult paraffin (alkane) and the most broadly utilized feedstock for delivering ethylene is ethane. As said before, ethane is gotten from characteristic gas fluids. Breaking ethane can be imagined as a free radical dehydrogenation response, where hydrogen is a coproduct. The response is exceptionally endothermic, so it is favored at higher temperatures and lower weights. Superheated steam is utilized to lessen the halfway weight of the responding hydrocarbons' (in this response, ethane) [48-50].
Conclusion
The processing of crude oil is a fundamental step in the oil production. However, the hydrocarbon intermediate production is a crucial step and needs attention for improved production of quality product. Generally, the simplest refineries consist of crude, vacuum, reforming and some hydro treating capacity. Refining splits crude oil into constituents used for a variety of purposes, from high-performance fuels to plastics. A wide spectrum of research is done in the refinery domain and many of the aspects still needs to be focused.
References
- Okoh A, Ajisebutu S, Babalola G, et al. Potential of Burkholderiacepacia RQ1 in the biodegradation of heavy crude oil. IntMicrobiol. 2001;4:83-7.
- UNEP. Environmental setting in Ogoniland and the Niger Delta: Environmental Assessment of Ogoniland. United Nations Environment Programme, Nairobi, Kenya, 2011;30-3.
- Hatch LF. Crude Oil Processing and Production of Hydrocarbon Intermediates. In: Chemistry of Petrochemical Processes. 2nd ed. London: Butterworth-Heinemann, UK; 2001.
- Mishra S, Jyot J, Kuhad RC, et al. Evaluation of inoculum addition to stimulate in situ bioremediation of oily-sludge-contaminated soil. Appl Environ Microbiol. 2001;67:1675-81.
- O?Reilly KT, Magaw RI, Rixey WG. Predicting the Effect of Hydrocarbon and Hydrocarbon-Impacted Soil on Groundwater. American Petroleum Institute, USA; 2001.
- Leahy JG, Colwell RR. Microbial degradation of hydrocarbons in the environment. Microbiol Rev. 1990;54:305-15.
- Atlas RM. Petroleum biodegradation and oil spill bioremediation. Marine PollutBull. 1995;31:178-82.
- Margesin R, Schinner F. Efficiency of indigenous and inoculated cold-adapted soil microorganisms for biodegradation of diesel oil in alpine soils. Appl Environ Microbiol. 1997;63:2660-4.
- Desai A, Vyas P. Hydrocarbon degradation. Applied Microbiology: Petroleum and Hydrocarbon Microbiology. Vadodara: M.S. University of Baroda, India; 2006.
- Foght JM, Westlake DWS. Biodegradation of hydrocarbons in freshwater. In: Vandermeulen JH, Hrudey SB, editors. Oil in Freshwater: Chemistry, Biology, Countermeasure Technology. New York: Pergamon Press, USA;1987. p. 217-30.
- Atlas RM, Bartha R. Hydrocarbon biodegradation and oil spill bioremediation. AdvMicrobEcol. 1992;12:287-338.
- Cheesbrough M. District Laboratory Practice in Tropical Countries 1 & 2. Cambridge: Cambridge University Press, UK; 2002.
- Abed RMM. Interaction between cyanobacteria and aerobic heterotrophic bacteriain the degradation of hydrocarbons. IntBiodeterBiodegr. 2010;64:58-64.
- Moore DM, Dowhan D. Current Protocols in molecular biology. New Jersey: John Wiley and Sons Inc, USA; 2002.
- King CR, Scott-Horton T. Pyrosequencing: a simple method for accurate genotyping. Methods Mol Biol. 2007;373:39-56.
- Pruesse E, Quast C, Knittel K, et al. SILVA: a comprehensive online resource for quality checked and aligned ribosomal RNA sequence data compatible with ARB. Nucleic Acids Res. 2007;35:7188-96.
- Abed RMM, Koster J. The direct role of aerobic heterotrophic bacteria associated with cyanobacteria in the degradation of oil compounds. IntBiodeterBiodegr. 2005;55:29-37.
- Jones DM, DouglasAG, ParkesRJ, et al. The recognition of biodegraded petroleum-derived aromatic hydrocarbons in recent marine sediments. Marine PollutBull. 1983;14:103-8.
- Adebusoye SA, Ilori MO, Amund OO, et al. Microbial degradation of petroleum hydrocarbons in a polluted tropical stream. World JMicrobiolBiotechnol. 2007;23:1149-59.
- Bartha R, Bossert I. The treatment and disposal of petroleum wastes. In: Atlas RM,editors. Petroleum Microbiology. New York: Macmillian, USA; 1984. p. 553-78.
- Rahman KS, Rahman TJ, Kourkoutas Y, et al. Enhanced bioremediation of n-alkane in petroleum sludge using bacterial consortium amended with rhamnolipid and micronutrients. Bioresour Technol. 2003;90:159-68.
- Jadoon S, Amin AA, Malik A, et al. Effects of Crude Oil Contamination under the Controlled Conditions on the Physicochemical Properties of Water in Khurmala and Guwayar, Kurdistan Region, Iraq. J Pollut Eff Cont. 2016;4:165.
- Ebonwu BI, Ugwu LLC. Effect of Crude Oil Water Soluble Fraction Toxicity on Tilapia Guineensis Fingerlings Using Histology of the Kidney as a Bioassay Indicator. J Pet Environ Biotechnol. 2016;7:287.
- Saini D. Modeling of Pressure Dependence of Interfacial Tension Behaviors of a Complex Supercritical CO2+ Live Crude Oil System Using a Basic Parachor Expression. J Pet Environ Biotechnol. 2016;7:277.
- Ogbulie TE, Nwaokorie FO. Molecular Diversity of Microbes with Probable Degradative Genes in Agricultural Soil Contaminated with Bonny Light Crude Oil. J Ecosys Ecograph. 2016;S5:002.
- Ahmed DF, Khalaf AH. Artificial Neural Networks Controller for Crude Oil Distillation Column of Baiji Refinery. J Chem Eng Process Technol. 2016;7:272.
- Saborimanesh N, Mulligan CN. Effect of Sophorolipid Biosurfactant on Oil Biodegradation by the Natural Oil-Degrading Bacteria on the Weathered Biodiesel, Diesel and Light Crude Oil. J Bioremed Biodeg. 2015;6:314.
- Ogbulie TE, Duru C, Nwanebu FC. Interaction Effects of Plants and Indigenous Micro-organisms on Degradation of N-Alkanes in Crude Oil Contaminated Agricultural Soil. J Ecosys Ecograph. 2015;5:166.
- Chikere CB, Obieze CC, Okerentugba P. Molecular Assessment of Microbial Species Involved in the Biodegradation of Crude Oil in Saline Niger Delta Sediments Using Bioreactors. J Bioremed Biodeg 2015;6:307.
- El-Hussein, Marzouk A. Characterization of Petroleum Crude Oils using Laser Induced Fluorescence. J Pet Environ Biotechnol. 2015;6:240.
- Ipeaiyeda AR, Nwauzor GO, Akporido SO. Biodegradation of Polycyclic Aromatic Hydrocarbons in Agricultural Soil Contaminated with Crude Oil from Nigeria Refinery using Pleurotus sajor-caju. J Bioremed Biodeg. 2015;6:301.
- El Mahdi AM, Aziz HA, Abu Am SS, et al. Performance of Isolated Kocuria sp. SAR1 in Light Crude Oil Biodegradation. J Bioremed Biodeg. 2015;6:303.
- Okpashi VE, Wallace I, Akpo DM. Crude Oil Contaminant and Bio-remediation using Brewery Mash and Earthworm (Nsukkadrilus mbae.) a Consortium to Cleaning-up and Restoring Soil Fertility Potentials. J Pet Environ Biotechnol. 2015;6:223.
- As?ad AM, Yeneneh AM, Obanijesu EO. Solvent Dewaxing of Heavy Crude Oil with Methyl Ethyl Ketone. J Pet Environ Biotechnol. 2015;6:213.
- Obinna AO, Chibuike SU, Onwurah INE. Variation in the Carbon (C), Phosphorus (P) and Nitrogen (N) Utilization during the Biodegradation of Crude Oil in Soil. J Pet Environ Biotechnol. 2015;6:206.
- Dawoodi V, Madani M, Tahmourespour A, et al. The Study of Heterotrophic and Crude Oil-utilizing Soil Fungi in Crude Oil Contaminated Regions. J Bioremed Biodeg. 2015;6:270.
- Burugupalli S. Multifractal Detrended Crosscorrelation Analysis of Gold and WTI Crude Oil Price Time Series. J Bus Fin Aff. 2015;3:130.
- Ofoegbu RU, Momoh YOL, Nwaogazie IL. Bioremediation of Crude Oil Contaminated Soil Using Organic and Inorganic Fertilizers. J Pet Environ Biotechnol. 2015;6:198.
- Onyeike EN, Ohiri RC, Uwakw AA. Toxicological Parameters of Albino Rats Fed with Amarantus hybridus Grown on Crude Oil Post-remediated Agricultural Soil. J Environ Anal Toxicol. 2014;5:251.
- Odokuma LO, Ubogu M. Phragmitis australies Growth and Tolerance to Crude Oil Contamination in Mangrove Swamp Soil. J Bioremed Biodeg. 2014;5:256.
- Elmahdi AM, Aziz HA, El-Gendy NS, et al. Optimization of Libyan Crude Oil Biodegradation by Using Solid Waste Date as a Natural Low-Cost Material. J Bioremed Biodeg. 2014;5:252.
- Abdeen Z, El-Sheshtawy HK, Moustafa YMM. Enhancement of Crude Oil Biodegradation by Immobilizing of different Bacterial Strains on Porous PVA Hydrogels or Combining of them with their produced Biosurfactants. J Pet Environ Biotechnol. 2014;5:192.
- Ichor T, Okerentugba PO, Okpokwasili GC. Biodegradation of Total Petroleum Hydrocarbon by Aerobic Heterotrophic Bacteria Isolated from Crude Oil Contaminated Brackish Waters of Bodo Creek. J Bioremed Biodeg. 2014;5:236.
- Ismail W, Alhamad NA, El-Sayed WS, et al. Bacterial Degradation of the Saturate Fraction of Arabian Light Crude oil: Biosurfactant Production and the Effect of ZnO Nanoparticles. J Pet Environ Biotechnol. 2013;4:163.
- George AK, Singh RN, Arafin S. Equation of State of Crude Oil Samples. J Phylogenetics Evol Biol. 2013;4:162.
- Jones JC. Energy-Return-On-Energy-Invested (EROEI) For Crude Oil and Other Sources of Energy. J Phylogenetics Evol Biol. 2013;4:150.
- Shohaimi NAM, Abu Bakar WAW, Jaafar J, et al. Treatment of Acidic Petroleum Crude Oil Utilizing Catalytic Neutralization Technique of Magnesium Oxide Catalyst. Mod Chem Appl. 2013;1:103.
- Belhaj H, Khalifeh HA. Asphaltene Stability in Crude Oil during Production Process. J Phylogenetics Evol Biol. 2013;4:142.
- Kuburi LS. Effect of Flow Velocity on the Inhibition Efficiency of Low Carbon Steel Corrosion in Crude Oil. J Appl Mech Eng. 2013;2:121.
- Agarry SE, Aremu MO, Aworanti OA. Kinetic Modelling and Half- Life Study on Enhanced Soil Bioremediation of Bonny Light Crude Oil Amended with Crop and Animal-Derived Organic Wastes. J Phylogenetics Evol Biol. 2013;4:137.