Research
, Volume: 15( 2) DOI: 10.21767/0974-7532.1000155Preparation and Characterization of Xonotlite Whiskers Based on Fly Ash
- *Correspondence:
- Xu Peng
College of Textile and Light Industry
Inner Mongolia University of Technology
Huhhot 010050
The Inner Mongolia Autonomous Region
China
Tel: +86 471 657 6164
E-Mail: xupeng_hit@163.com
Received: May 19, 2020; Accepted: June 8, 2020; Published: June 15, 2020
Citation: Jiahui H, Peng X. Preparation and Characterization of Xonotlite Whiskers Based on Fly Ash. Res Rev Biosci. 2020;15(2):155.
Abstract
This paper discusses the preparation process of hydrothermal synthesis of xonotlite whisker with fly ash desiliconization solution as silicon source and high-quality lime milk as calcium source. The results show that the concentration of desiliconized filtrate SiO2 is 63.21 g/L, the concentration of refined lime milk CaO is 186.49 g/L, the molar ratio of calcium to silicon is 1.0, the water-solid ratio is controlled to 40:1, and the temperature is evenly raised to 240°C within 120 minutes, then kept at 240 min to ensure the reaction fully. Then it was cooled to room temperature, and after filtering, washing, and drying, xonotlite whisker product with an aspect ratio of 100 to 400 was obtained.
Keywords
Celosia; Drought stress; DUF538; Gene expression; Real-Time PCR
Abbreviations
XRD: X-Ray Diffraction; SEM: Scanning Electron Microscope
Introduction
Synthetic xonotlite whisker is a widely used reinforcing and toughening material. It not only has the high strength, high modulus, high elongation of common whisker, but also has high temperature resistance, biological activity, environmentally friendly and other excellent properties. It is a green environmental protection whisker material whose production and use process is not toxic to human body [1,2], and it is expected to be used in the fields of thermal insulation materials, construction materials, papermaking and bioactive materials [3-5]. This kind of material is made of calcium raw materials and siliceous raw materials through hydrothermal synthesis. Due to the harsh preparation conditions of whiskers, the selection of raw materials and the requirements of process conditions are relatively high. The theoretical chemical composition of xonotlite whiskers can be expressed as 6CaO•6SiO2•H20, and its crystal water is the least among all calcium silicate hydrates [6-11]. Compared with other inorganic whisker materials, xonotlite material has many excellent properties, mainly reflected in: (1) Excellent thermal stability; (2) Friendly to human body. Xonotlite is a kind of green material, which is non-toxic, harmless and safe to use; (3) High utilization rate. It can be used repeatedly.
Natural xonotlite minerals were first discovered by Rammelsherg in 1886, and research on artificial synthesis xonotlite was carried out in Nagai, Japan in 1932. In 1972, the dynamic hydrothermal synthesis method of xonotlite was discovered in Takahashi, Japan, which have been in the leading position in the world in the research and application of calcium silicate thermal insulation materials [12-14]. In the early 1970s, China began to develop calcium silicate thermal insulation materials. At present, it has been able to produce xonotlite thermal insulation products with a density of 170-260 kg/m3, and ultra-light products with a density below 130 kg/m3 have also been successfully tested [15,16].
At present, the xonotlite products used in industry are mainly obtained by dynamic hydrothermal synthesis reaction of industrial water glass as silicon raw material and calcium carbide slag and other calcium raw material, which Industrial water glass produced by alkali fusion of quartz sand and diatomaceous earth.
The use of diatomaceous earth or quartz sand and alkali to produce industrial water glass under molten conditions requires sodium hydroxide, and consumes a lot of energy. The residual alkali concentration in the sodium silicate solution is too high, which affects the synthesis of xonotlite. If the alkali concentration is controlled by dilution or water washing, it not only consumes a lot of water and energy, but also reduces production efficiency and increases reaction energy consumption. Large-scale mining of non-renewable mineral resources such as quartz and diatomaceous earth will destroy the ecological balance. So in this paper, the desilication solution after the alkali dissolution of fly ash is used as the silicon raw material instead of industrial water glass [17-19]. The calcareous raw material is refined lime milk obtained by digesting, sieving and blending of high-quality quicklime. This article focuses on the effect of different reaction temperature, time and other hydrothermal synthesis conditions on the morphology of synthetic xonotlite whiskers. In order to reduce the damage to the ecological environment and reduce the consumption of non-renewable resources, the use of fly ash as a raw material to prepare xonotlite greatly reduces the production cost of xonotlite whiskers and also provides an effective way for the reuse of fly ash.
The Experiment
Experimental material
The xonotlite crystal formation reaction equation can be expressed as:
6Ca(OH)2+6SiO2=6CaO•6SiO2•H2O+5H2O
The reaction is just a simplified final reaction equation. In fact, the whole reaction is a very complex chemical kinetics process. It is generally believed that the reaction goes through the gel and hydrated calcium silicate formation stage, the tobermorite formation stage, and the xonotlite formation stage. In the reaction process, a certain amount of impurities such as Fe2+, Al3+, Mg2+ can replace Si4+ or Ca2+ in the intermediate product tobermorite, which can change the tilt angle of the bridging structure of the tetrahedral framework with a high degree of freedom. The lattice C axis is elongated, and the lattice volume increases. Thus, the stability of tobermorite with lamellar structure is improved, and the lattice of xonotlite shrinks, which inhibits the growth of xonotlite whiskers [16]. Therefore, the effective removal of Fe2+, Al3+, Mg2+ impurities in the reaction raw materials is particularly important for the synthesis of high-quality xonotlite whiskers. The raw materials should be effectively purified and removed impurity before the reaction.
Preparation of desilication solution of silicon raw material fly ash: The desilication of fly ash is to mix fly ash with a sodium hydroxide solution with a concentration of 14% to 20% at a certain ratio, and then pump it to a heating sleeve. Then it is heated to about 130°C with steam and left in the holding tank for 20 to 40 minutes, so that part of the SiO2 in the coal ash reacts with NaOH to form a Na2SiO3 solution to achieve the purpose of desilication from the coal ash. Then use a disc filter to separate the desilication solution from the coal ash residue, and wash the coal ash. The resulting liquid is filtered again through the leaf filter to remove the small particles of impurities containing Fe2+ and Al3+ components in the solution. The solution obtained after filtration is the refined desilication filtrate. The reaction chemistry formula is expressed as:
SiO2+2NaOH=Na2SiO3+H2O
After analysis, the concentration of refined desilication filtrate SiO2 was 63.21 g/L.
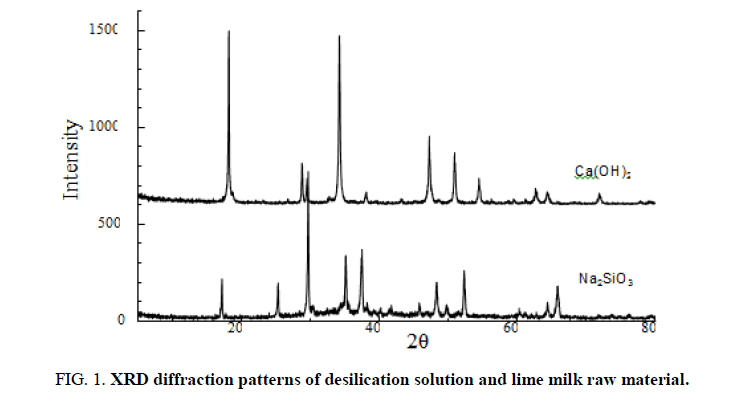
Preparation of calcium raw material refined lime milk: The high-quality limestone obtained from the quarry is crushed, washed, and sorted to obtain limestone raw materials with a size of 75°C~150 mm, which are calcined in a vertical kiln using 20 mm~40 mm anthracite. The obtained lime is sieved to remove the cinder (ash), small lime and lime dust, crushed to 10 mm~30 mm and mixed with warm water at 55°C~65°C according to lime: water=1:4.8 for digestion. Dilute the digested coarse lime milk with hot water and stir it evenly after the lime and water react completely. Then, after filtering through 35 mesh, 80 mesh and 120 mesh three-layer vibrating screens and purifying by hydrocyclone, and the impurities containing Mg2+ in the crude lime milk which can’t react with water can be removed, and the refined lime emulsion can be obtained. The concentration of CaO in the refined lime milk prepared in this experiment was 186.49 g/L. The XRD diffraction peaks spectrum of desilication solution and lime milk which are the main raw material in this reaction is shown in ( FIG. 1).
Figure 1. XRD diffraction patterns of desilication solution and lime milk raw material.
Preparation method and equipment
Experimental method: Orthogonal experiments were carried out according to four factors and three levels. The four factors are: the molar ratio of calcium and silicon of the reaction material, the water-solid ratio, the reaction temperature and the holding time. The three levels condition was the calcium-silicon molar ratio of raw materials: CaO/SiO2=0.95, 1.00, 1.05; water-solid ratio=30.0, 40.0, 50.0; reaction temperature: 200°C, 220°C, 240°C; holding time: 2 h, 4 h, 6 h, as shown in TABLE 1 below.
| Factor | Reaction temperature | Ca/Si | Water-solid ratio | Holding time |
|---|---|---|---|---|
| Experiment 1 | 200 | 0.95 | 30 | 2 |
| Experiment 2 | 200 | 1 | 40 | 4 |
| Experiment 3 | 200 | 1.05 | 50 | 6 |
| Experiment 4 | 220 | 0.95 | 40 | 6 |
| Experiment 5 | 220 | 1 | 50 | 2 |
| Experiment 6 | 220 | 1.05 | 30 | 4 |
| Experiment 7 | 240 | 0.95 | 50 | 4 |
| Experiment 8 | 240 | 1 | 30 | 6 |
| Experiment 9 | 240 | 1.05 | 40 | 2 |
Table 1: Orthogonal experimental schedule for the synthesis of xonotlite whiskers by hydrothermal method.
Hydrothermal synthesis reaction was carried out according to the reaction conditions in TABLE 1. The temperature was raised from room temperature to the reaction temperature and the heating up time was controlled, and heat preservation is carried out according to the preset heat preservation time conditions of each reaction. After the reaction, it was cooled to room temperature, and the sample of xonotlite whisker is obtained after filtration, washing and drying.
Experimental equipment: The experimental used a GSHA-0.5 (300°C, 10 MPa) permanent magnet rotary stirring autoclave that made in China.
Characterization of experimental samples
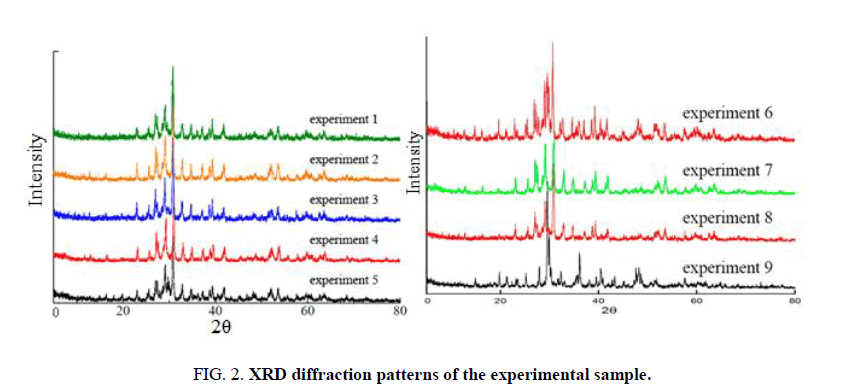
The samples obtained from 9 groups of experiments were tested by X-Ray Diffraction (XRD) and Scanning Electron Microscope (SEM). According to the analysis of XRD spectrum (FIG. 2), the samples of 9 groups of experiments basically have the characteristic peaks of xonotlite whiskers, but some samples (experiments 1, 6, 8) have mixed impurity peaks in the samples. This shows that not all of them have synthesized the target product xonotlite whisker. According to the analysis of the atlas and the experimental conditions, it can be seen that when the reaction temperature is fixed the Ca/Si is 1.0, and the sample with a higher water-solid ratio and a longer holding time has a lower impurity. While the Ca/Si is 1.0, the water-solid ratio is 50, and the reaction time is 6 hours, the higher the reaction temperature in the samples under different reaction temperature conditions, the lower the impurity content in the sample.
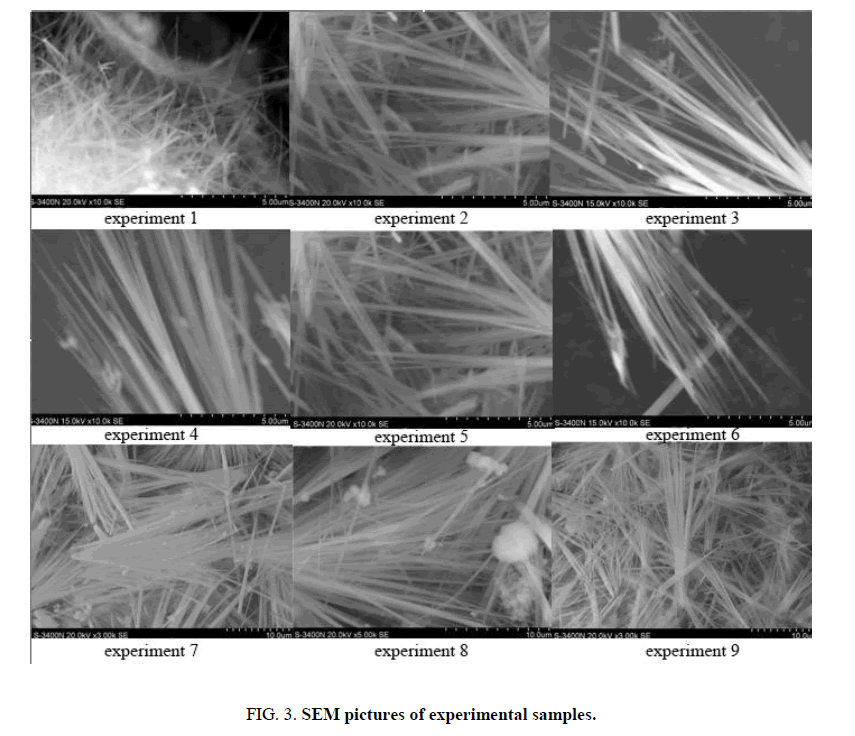
According to the electron micrograph of the samples (FIG. 3), the 9 groups of samples basically have the micro morphology of whiskers, and the aspect ratio is more than 100. The specific analysis shows that the whiskers obtained in experiments 1, 6, and 8 were found to have obvious flocs attached between the whiskers and on the surface, and the main components of the flocs are tobermorite and high alkalinity silicate hydrates. The main reasons for the presence of impurities are the low water-solid ratio of the reaction, the uneven concentration of silicon-calcium in the reactant, and the imbalance of the calcium-silicon ratio. Therefore, other impurity products appeared during the reaction. The roots of whiskers from experiments 1, 5 and 9 are connected and exist in the form of clusters. The whiskers were not fully developed and the diameter of the whiskers was not uniform. The main reason is that the reaction has a shorter holding time and the reaction is not thorough. In addition, through the analysis of the microscopic morphology of the whiskers obtained through the above experiments, and combined with the situation of whiskers obtained in experiment 2, it can be determined that the whiskers obtained when the raw material Si/Ca is 1.0 have better development and lower impurity content. If the calcium silicon ratio is too low, the reaction product will appear tobermorite, if the calcium silicon ratio is too high; the reaction product will contain high alkalinity hydration products. It will affect the performance of the whisker purity and volume density, heat resistance and other products.
Results and Discussion
In summary, after analyzing the electron micrograph of the experimental product and the diffraction spectrum, it can be known that under the same conditions, when the water-solid ratio of the whisker synthesis is higher and the holding time is longer, the whisker grows more fully and length diameter ratio is larger. While the raw material ratio of Ca/Si is 1.0, the reaction is the most sufficient, the microstructure of the whisker is more ideal, and the content of impurities is the least. In theory, the initial calcium to silicon ratio of xonotlite is 1:1, but there are often some unreacted siliceous materials in actual production. In addition, since the solubility of Ca(OH)2 and SiO2 is very different, it is necessary to adjust the calcium to silicon ratio according to the type of raw material. According to the hydrothermal synthesis, when the reaction temperature was 160°C, the hydrothermal product was calcium silicate hydrate C-S-H, and when the temperature was raised to 180°C, most of the products were still spherical agglomerated C-S-H hydrate gel. At the same time, tobermorite began to form. When the temperature was raised to 200°C, the xonotlite phase began to appear. This shows that the synthesis of xonotlite should be realized under the condition of high temperature and high pressure.
Conclusion
After purification and impurity removal, the fly ash desilication solution and lime milk completely satisfied the requirements of preparing xonotlite whiskers. The proportion of reaction raw materials has a great influence on the morphology of the synthetic xonotlite whiskers. Hydrothermal synthesis conditions have a great influence on the morphology of xonotlite whiskers. When the reaction temperature is higher and the holding time is longer, the growth of xonotlite whiskers is more favorable. The xonotlite whiskers prepared by the experiment have bending, continuous growth, bundling and winding phenomena. After analysis, the reason for these may be the excessive alkalinity of the reaction system.
Conflict of Interest
Authors declared no conflict of interest.
References
- Xiaolan H, Guozheng L. Research on whisker/polymer matrix composites. Mater Sci Tech. 2002;10:442-8.
- Erfan C, Yajuan T, Benlian Z. Research progress of whisker reinforcement and its composite materials. Polym Mat Sci Eng. 2002;18:1-9.
- Jinsheng P, Yonghua C. Whisker and its application. J Compos Mater. 1995;12:1-7.
- Xiaoyan M, Guozheng L, Qiaoying J. Application of whiskers in composite materials. Mat Guide. 2001;15:44-6.
- Zhaoyu X. New progress in research and application of whiskers. Chem Tech Develop. 2005;34:11-7.
- Yanagisawa K, Feng Q, Yamasaki N. Hydrothermal synthesis of xonotlite whiskers by ion diffusion. J Mater Sci Lett. 1997;16:889-91.
- Milestone NB, Ghanbari AK. Hydrothermal processing of xonotlite based compositions. Adv Appl Ceram. 2007;6:302-8.
- Udawatte CP, Yanagisawa K, Kamakura T, et al. Solidification of xonotlite fibers with chitosan by hydrothermal hot pressing. Mater Lett. 2000;45:298-301.
- LiX K, Chang J. A novel hydrothermal route to the synthesis of xonotlite nanofibers and investigation on their bioactivity. J Mater Sci. 2006;41:4944-7.
- Takahashi K, Yamasaki N, Mishima K, et al. Coating of pulp fiber with xonotlite under hydrothermal conditions. J Mater Sci Lett. 2002;21:1521-3 .
- Lin KL, Chang J, Chen GF, et al. A simple method to synthesize single-crystalline β-wollastonite nanowires. J Cryst Growth. 2007;300:267-71.
- Cao JX, Liu F, Lin Q, et al. Hydrothermal synthesis of xonotlite from carbide slag. Prog Nat Sci. 2008;18:1147-53.
- Youkun Z, Chaohui K, Xiao L, et al. Treatment and recycling of calcium carbide slag. Polyvinyl Chloride. 2001;1:52-4.
- Fei L, Lingke Z, Jianxin C, et al. Effect of heat treatment conditions of calcium carbide slag on synthetic xonotlite. J Mater Heat Treat. 2008;6:36-40.
- Cao JX, Liu F, Lin Q, et al. Effect of calcination temperature on mineral composition of carbide slag, lime activity and synthesize xonotlite. Ky Eng Mater. 2008;368:1545-7.
- Xiaoling X, Zhongjia M, Wei W. Some basic conditions for the production of light calcium silicate board. China Build Mater Tech. 1997;6:1-9.
- Junmin S, Dexin H. Formation and characteristics of fly ash and its application prospects. Beijing: Chem Industr Press. 1999;20:10-4.
- Huanqiang H, Tingda J. Utilization technology of fly ash. Beijing: Chem Industr Press. 2001;22:1-29.
- Qinhai H, Hui Z, Guanghui B, et al. Research progress of fine utilization of high aluminum fly ash. Chem Prog. 2011;30:1613-7.