Original Article
, Volume: 5( 3)Effect of Concentration on Parameters of DSSCs was Fabricated by Alcian Blue 8GX Dye at Different Concentrations and Meh-PPV Polymer
- *Correspondence:
- Mohammed HK, Department of Sciences, Omdurman Islamic University, Sudan, Tel: +249 12 521 1983; E-mail: Kanzi_sa@yahoo.com
Received: November 11, 2016; Accepted: December 15, 2016; Published: December 26, 2016
Citation: Mohammed HK, Asim MA, Aldesogi OH, et al. Effect of Concentration on Parameters of DSSCs was Fabricated by Alcian Blue 8GX Dye at Different Concentrations and Meh-PPV Polymer. J Space Explor. 2016;5(3):109.
Abstract
Economical solar energy conversion to electricity can be boosted by the discovery of fundamentally new photovoltaic mechanism. However, in this paper reports the observation the effect of different concentration of Alcian Blue 8GX dye on the DSSCs was fabricated by depositing the polymer solution on ITO a glass manner Spin Coating method and MEH-PPV polymer photovoltaic, efficiency varied from 0.351% to 2.383%.
Keywords
Polymer; Photovoltaic; Alcian blue; 8GX dye
Introduction
It is expected that the global energy demand will double within the next 50 years. Fossil fuels, however, are running out and are held responsible for the increased concentration of carbon dioxide in the earth’s atmosphere. Hence, developing environmentally friendly, renewable energy is one of the challenges to society in the 21st century. One of the renewable energy technologies is photovoltaic (PV), the technology that directly converts daylight into electricity. PV is one of the fastest growing of all the renewable energy technologies, in fact, it is one of the fastest growing industries at present.1Solar cell manufacturing based on the technology of crystalline, silicon devices is growing by approximately 40% per year and this growth rate is increasing. This has been realized mainly by special market implementation programs and other government grants to encourage a substantial use of the current PV technologies based on silicon. Unfortunately, financial support by governments is under constant pressure [1].
In 1991, Brian O’Regan and Michael Grätzel introduced the dye sensitized solar cell (DSSC). This type of solar cell is considered as accost effective alternative for silicon solar cells. The heart of the DSSC is a high surface area TiO2 nano particulate electrode, covered with a monolayer of dye molecules. Upon photo excitation of the dye an electron is injected into the conduction band of the TiO2 . A redox couple (I-/I3-) in an electrolyte [2-3].
Materials and Methods
Were 7 sample of Alcian Blue 8GX dye different concentration and MEH-PPV polymer solar cells made on ITO a glass manner Spin Coating. Gold was fabricated on the layers to represent the anode and ITO Cathode. A clean glass plate with a thin layer of ITO (Indium Tin Oxide) is needed. The ITO acts as the first part of the solar cell, the first electrode. The fabrication process started by preparing the Alcian Blue 8GX dye different concentration (1.5, 1.4,1.3,1.2,1.1, 1 and 0.9) mg. L-1 of Alcian Blue 8GX dye dissolving by acetone and then interest on spin coated on indium tin oxide glass. The Alcian Blue 8GX dye solar cell was made on ITO glass. The ITO glasses were firstly cleaned by ethanol and distilled water. Alcian Blue 8GX dye was dissolved into acetone. Then 3mg of MEH-PPV polymer dissolved into 0.5 of high pure chloroform was deposited on Alcian Blue 8GX dye. Being inserted electrical circuit containing the (voltmeter and Ammeter and a light source Lamp with the intensity radiological” and a solar cell). Cell was offered to light and fulfilled taking the results of the current and voltages were recorded. The samples were prepared.
Results
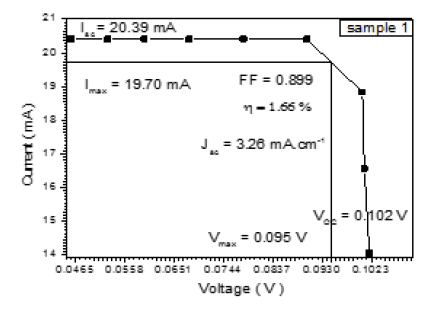
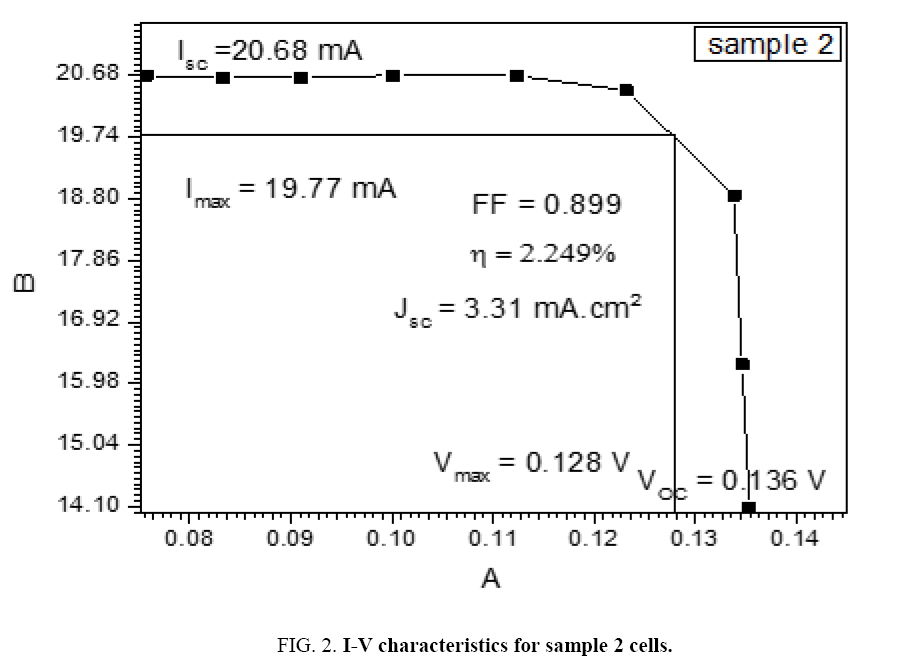
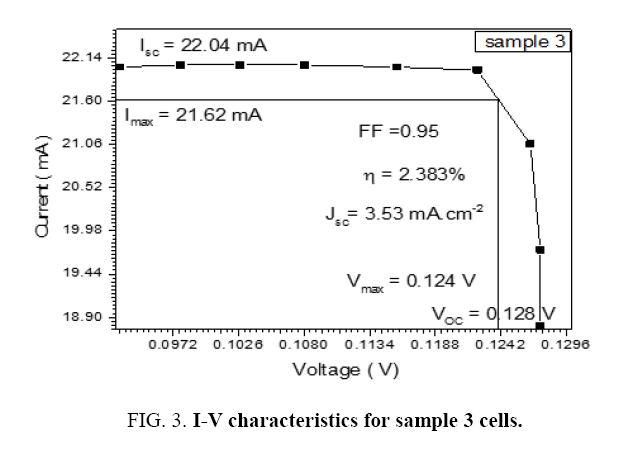
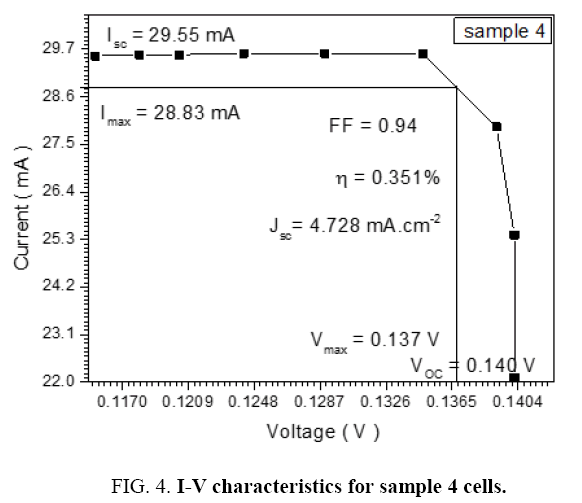
The DSSCs was fabricated by depositing the polymer solution on ITO a glass manner Spin Coating method for eight types of Alcian Blue 8GX dye different concentration and MEH-PPV polymer, DSSCs parameters was calculated from the (I-V) characteristics, the figures below i.e. Tables 1-7 from sample 1 to sample 7 show these (I-V) characteristics.
| Voltage (V) | Current (mA) |
|---|---|
| 0.04556 | 20.40674 |
| 0.05248 | 20.40674 |
| 0.05941 | 20.40674 |
| 0.06784 | 20.40674 |
| 0.07814 | 20.40674 |
| 0.09012 | 20.40674 |
| 0.10061 | 18.83226 |
| 0.10098 | 16.56598 |
| 0.10192 | 14.05543 |
Table 1: I-V reaction for sample 1.
| Voltage (V) | Current (mA) |
|---|---|
| 0.07566 | 20.68402 |
| 0.08323 | 20.65762 |
| 0.09099 | 20.65762 |
| 0.10011 | 20.68402 |
| 0.11232 | 20.68402 |
| 0.12319 | 20.45528 |
| 0.13386 | 18.8585 |
| 0.13465 | 16.27199 |
| 0.13543 | 14.09018 |
Table 2: I-V reaction for sample 2.
| Voltage (V) | Current (mA) |
|---|---|
| 0.09275 | 22.02823 |
| 0.09785 | 22.05352 |
| 0.10273 | 22.05352 |
| 0.10803 | 22.05352 |
| 0.11561 | 22.02823 |
| 0.12227 | 21.98974 |
| 0.12664 | 21.06488 |
| 0.12747 | 19.74633 |
| 0.12747 | 18.79509 |
Table 3: I-V reaction for sample 3.
| Voltage (V) | Current (mA) |
|---|---|
| 0.11541 | 29.54531 |
| 0.118 | 29.57522 |
| 0.12037 | 29.57522 |
| 0.12418 | 29.60264 |
| 0.12898 | 29.60264 |
| 0.13477 | 29.60264 |
| 0.13912 | 27.90762 |
| 0.14019 | 25.40748 |
| 0.14019 | 22.1022 |
Table 4: I-V reaction for sample 4.
| Voltage (V) | Current (mA) |
|---|---|
| 0.14802 | 23.0165 |
| 0.15214 | 23.0165 |
| 0.15874 | 23.00601 |
| 0.16462 | 22.99457 |
| 0.17216 | 22.98409 |
| 0.181 | 22.95073 |
| 0.1863 | 22.11584 |
| 0.18713 | 20.95117 |
| 0.18724 | 20.02859 |
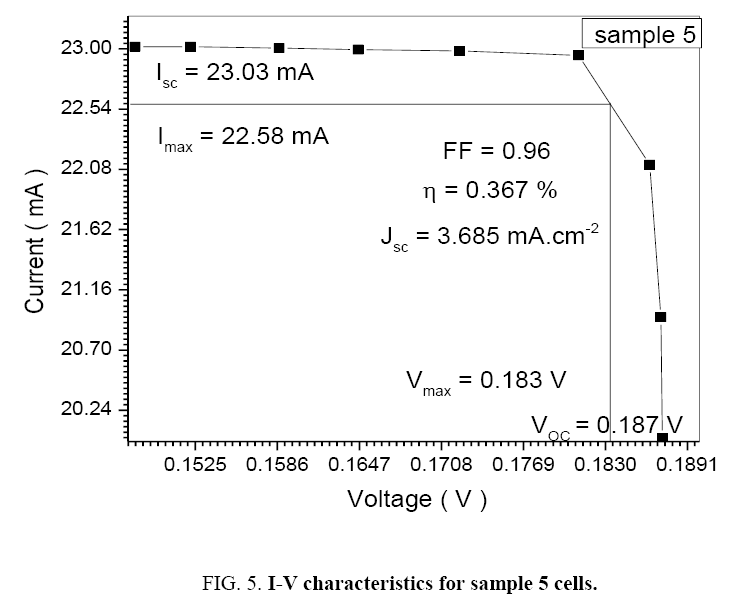
Table 5: I-V reaction for sample 5.
| Voltage (V) | Current (mA) |
|---|---|
| 0.14298 | 25.96862 |
| 0.16625 | 25.96862 |
| 0.19567 | 25.94751 |
| 0.22505 | 25.94751 |
| 0.26216 | 25.94751 |
| 0.29875 | 25.90704 |
| 0.3237 | 24.64897 |
| 0.32817 | 23.02669 |
| 0.32928 | 20.59326 |
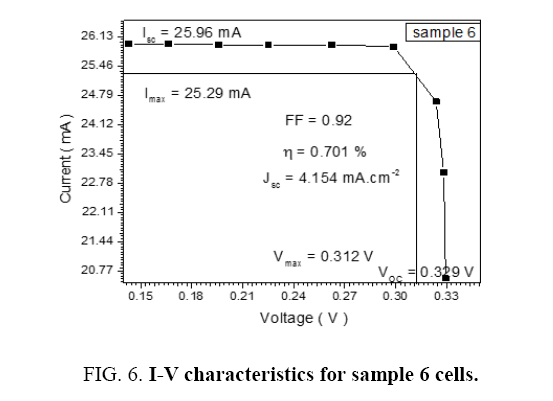
Table 6: I-V reaction for sample 6.
| Current (mA) | Voltage (V) |
|---|---|
| 25.06891 | 0.16547 |
| 25.09003 | 0.17148 |
| 25.09003 | 0.17866 |
| 25.09003 | 0.19031 |
| 25.09003 | 0.20252 |
| 25.09003 | 0.21785 |
| 23.50821 | 0.22813 |
| 20.91114 | 0.23027 |
| 19.57214 | 0.23027 |
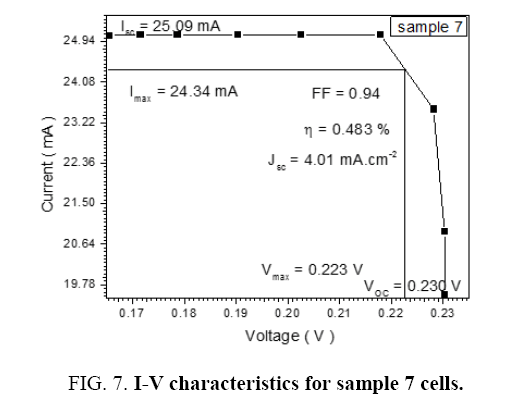
Table 7: I-V reaction for sample 7.
| Sample concentration (mg. L-1) | Isc mA | Imax (mA) | Vmax (V) | Voc (V) | FF | Jsc (mA/cm2) | h % |
|---|---|---|---|---|---|---|---|
| 1.5 | 20.39 | 19.70 | 0.095 | 0.102 | 0.899 | 3.26 | 1.66 |
| 1.4 | 20.68 | 19.77 | 0.128 | 0.136 | 0.899 | 3.31 | 2.249 |
| 1.3 | 22.04 | 21.62 | 0.124 | 0.128 | 0.95 | 3.53 | 2.383 |
| 1.2 | 29.55 | 28.83 | 0.137 | 0.140 | 0.94 | 4.728 | 0.351 |
| 1.1 | 23.03 | 22.58 | 0.183 | 0.187 | 0.96 | 3.685 | 0.367 |
| 1 | 25.96 | 25.29 | 0.312 | 0.329 | 0.92 | 4.154 | 0.701 |
| 0.9 | 25.59 | 24.34 | 0.223 | 0.23 | 0.94 | 4.01 | 0.483 |
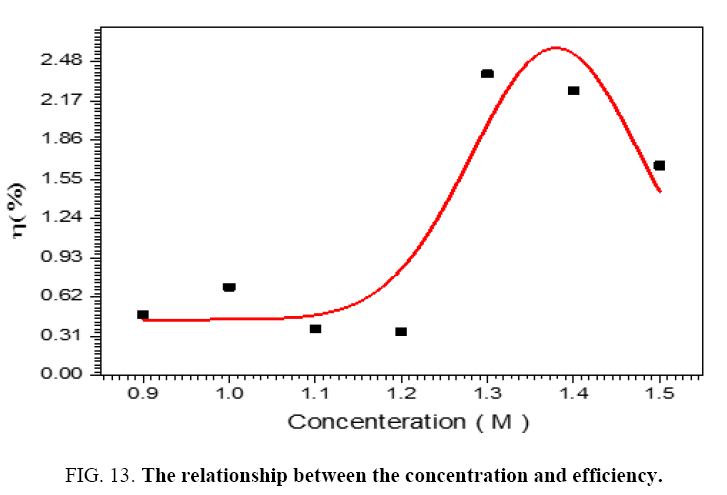
Table 8: The parameters of the DSSCs sensitized with different concentration.
Discussion
The percentage power conversion efficiency (PCE) or of any solar cell device is simply the ratio of power output (Pout) versus power input, (Pin), power input (Pin) depend upon the incident light flux (Iο), and Power output implicit properties of the device itself ;namely the short- circuit current (Isc) open-sir cut voltage (Voc) and fill-factor (FF). The short-circuit current density (Jsc) is typically reported to allow comparison between devices whose dimensions may vary (Jsc=Isc/area). The FF is determined by the ratio maximum obtainable power/theoretical obtainable power where the theoretical obtainable power is the product Isc, Voc (Isc and Voc being zero at open-circuit and short-circuit condition respectively with grade A solar-cells typically having [4-8].
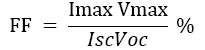 (1)
(1)
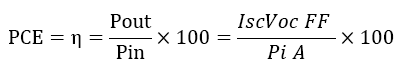 (2)
(2)
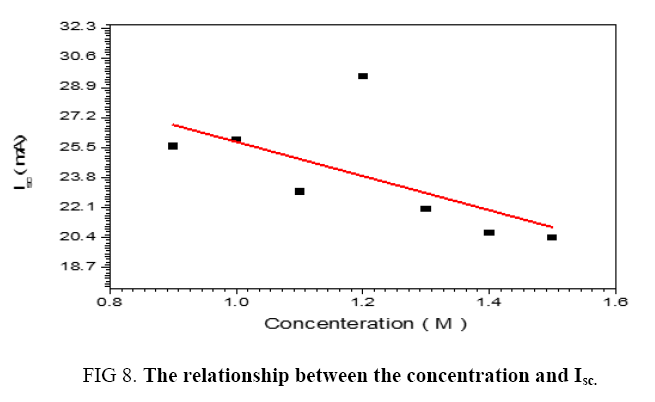
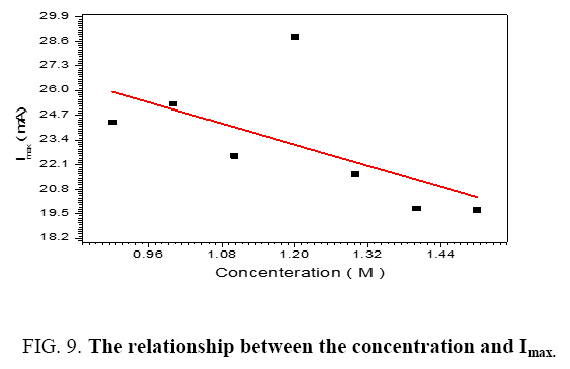
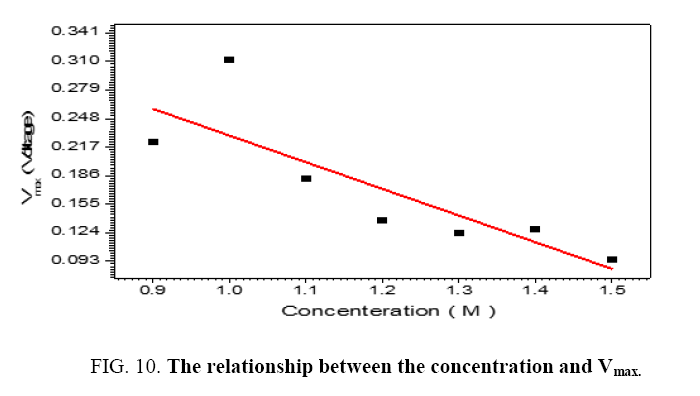
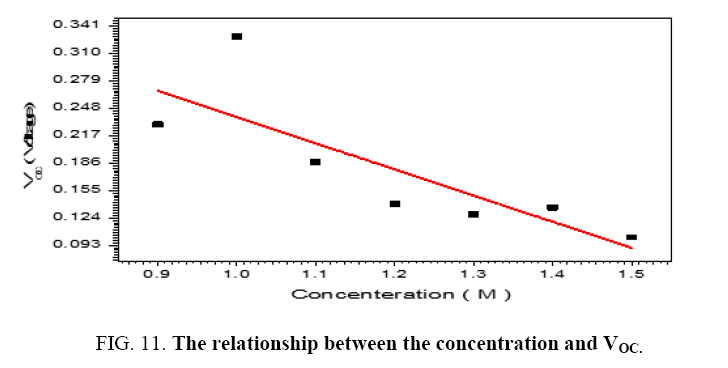
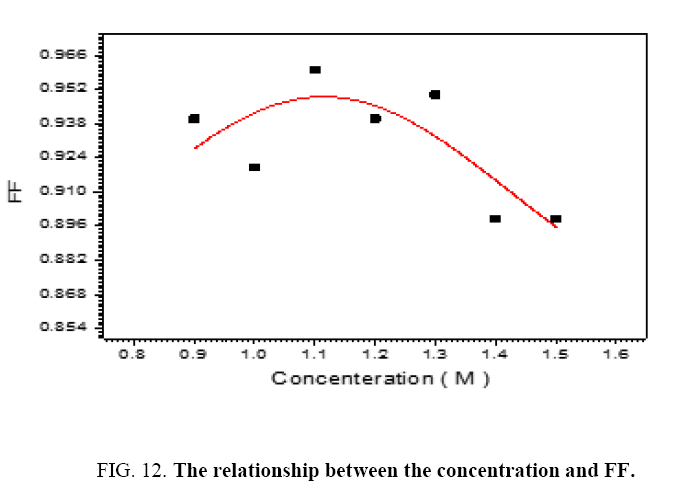
The performance of DSSC sensitizers (was fabricated by Alcian Blue 8GX dye different concentration and MEH-PPV polymer) was evaluated by Isc, Voc, fill factor (FF) from equation (1), and energy conversion efficiency (η) from equation (2). Figure 1 to Figure 7 shows the I-V curves of the DSSCs fabricated using was fabricated by Alcian Blue 8GX dye different concentration and MEH-PPV polymer. All the parameters of the DSSCs sensitized with different concentration are listed in Table 8. As displayed in Table 8, Isc varied from 20.39 to 29.55 mA, Voc changed from 0.102 to 0.329 V, and the FF of the fabricated DSSC ranged between 0.899 and 0.95. The best performance was obtained from the DSSC sensitized by 1.3 mg. L-1 concentrations, where the efficiency of both of the cells reached 2.383%, because it is more stable under concentration conditions and show the effect of concentration on all parameters in Figure 8 to Figure 13 . The effects of concentration of Alcian Blue 8GX dye on the (Isc, Imax, Vmax, and VOC) decreased with concentration increased but FF and η expiation relationship.
Conclusion
In this research, DSSCs were assembled using depositing the polymer solution on ITO a glass manner Spin Coatingas Nano crystalline TiO2 photo electrodes. Photovoltaic parameters of the fabricated DSSCs were determined under 180 W/cm2 illumination. The best performance was obtained for the DSSC sensitized with the Alcian Blue 8GX dyes concentration
(1.3 mg. L-1 ) and MEH-PPV polymer, where the efficiency of the cell reached 2.383% because it is more stable under concentration conditions.
Acknowledgments
Financial support of this paper was provided by Omdurman Islamic University Faculty of Science and Al Neenlen University Faculty of Science and Technology Department of Physics.
Conflict of Interest
The authors declare no conflict of interest.
References
- Janssen R. Introduction to polymer solar cells. Departments of Chemical Engineering & Chemistry and Applied Physics, Eindhoven, University of Technology, The Netherlands: 2005.
- Sariciftci NS, Smilowitz L, Heeger AJ, et al. Photo-induced electron transfer from conducting polymers onto buckminsterfullerene. Science.1992;258:1474-6.
- Grätzel M. Dye-sensitized solar cells. Journal of Photochemistry and Photobiology. 2003;4:145-53.
- Oku T, Motoyoshi R, Fujimoto K, et al. Structures and photovoltaic properties of copper oxides/fullerene solar cells. J Phys Chem. Solids. 2011;72:1206-11.
- Alam MM, Jenekhe SA. Efficient solar cells from layered nanostructures of donorand acceptor conjugated polymers. Chem Mater. 2004;16:4647-56.
- Green, MA. Emery K, Hishikawa, Y, et al. Solar cell efficiency tables’ version35. ProgPhotovolt Res. Appl. 2010;18:144-50.
- Song MY, Kim KJ, Kim DY. Enhancement of photovoltaic characteristics using aPEDOT interlayers in TiO2/MEHPPV heterojunction devices. Sol Energy Mater. 2005;85:31-9.
- Abdalsakhi SMH, Abd-AllaDM, AbdElgani R, et al. Using gum arabic in making solar cells by thin films instead of polymers. IOSR Journal of Applied Physics. 2016;8(1):27-32.