Original Article
, Volume: 12( 2)Effects of Selenium on Tebuconazole-Induced Hepatotoxicity in Adult Rats
- *Correspondence:
- Ben Saad H Faculty of Medicine, Laboratory of Pharmacology, University of Sfax, 3029 Sfax, Tunisia
Tel:+216 74 241 888; Fax:+216 74 246 217; E-mail: hajer.ben.saad@hotmail.fr
Received Date: May 2, 2017 Accepted Date: May 30, 2017 Published Date: June 5, 2017
Citation: Ben-Saad H, Kammoun I, Boudawara T, et al. Effects of Selenium on Tebuconazole-Induced Hepatotoxicity in Adult Rats. Res Rev Biosci. 2017;12(2):118.
Abstract
The current study pertained to tebuconazole (TEB) toxicity in liver of adult mice and the corrective effects of selenium (Se). Male Wistar rats were divided into three groups of six each: group I served as control; group II received by intraperetoneal injection TEB alone (100 mg/Kg b.w), and group III was administered both TEB and Se (0.5 mg/kg of diet) for 30 days. Results showed that, in the TEB-treated group, antioxidant enzyme activities (superoxide dismutase and catalase), lipid peroxidation, advanced oxidation protein product, and protein carbonyl groups levels significantly increased, while glutathione peroxidase activity decreased. Se co-administration through the rats’ diet restored these parameters to near normal values. Treatment with TEB induced various histological changes in the liver, including leucocytic infiltration and cytoplasmic vacuolization, while treatment with TEB and Se led to an improvement in the histological liver picture. Our investigation revealed, therefore, that Se was effective in preventing TEB-induced hepatotoxicity.
Keywords
Tebuconazole; Selenium; Oxidative stress; Liver; Antioxidant
Abbreviations
AOPP: Advanced Oxidation Protein Product; CAT: Catalase; GPx: Glutathione Peroxidase; GSH: Glutathione; MDA: Malondialdehyde; NBT: Nitroblue Tetrazolium; PCO: Protein Carbonyl; ROS: Reactive Oxygen Species; Se: Selenium; SOD: Superoxide Dismutase; TEB: Tebuconazole Species; SOD: Superoxide Dismutase; TBARS: Thiobarbituric Acid Reactive Substances; T-Ch: Total Cholesterol; TG: Triglycerides
Introduction
Growing agricultural and industrial activities largely contribute to environmental pollution, thus affecting water systems which are the end points of contamination [1]. Among the test chemicals, tebuconazole (TEB), a systemic fungicide of the azole group with protective, curative and eradicative action, can be mentioned. The presence of TEB in stream waters has increased in recent years [2] and its concentrations detected in surface waters amount to 175-200 g/L [3]. TEB exhibited antiandrogenic and feminizing effects on rat offspring [4]. In fact, it has been extensively used because of its effective role against various mushroom, smut and bunt diseases. TEB constitutes, indeed, a potent xenobiotic, exposure to which can bring about metabolic alterations and death to various organisms. It has been classified by the US EPA as Group C-Possible Human Carcinogen [5]. Research on rats has shown that perinatal exposure to TEB produces immunological neurobehavioral and neuropathological deficiency. However, information remains scarce as regards its effects on the liver tissue [4].
Considering the relationship between fungicide exposure and oxidative stress, the focus has been put on compounds with antioxidant properties. In fact, Selenium is a trace element found in small amounts in the soil and food, and has a remarkable variability in regional distribution and bioavailability [6]. It constitutes an essential dietary component for mammals, including humans. It is also a structural component of several enzymes, including glutathione peroxidase (GPx) and thioredoxine which play a key role in cellular oxidative defense, and have been shown to be induced by oxidative stress. Selenium has been found to be implicated in a number of health issues. It is worth noting that selenium deficiency conditions also affect all selenoproteins, to a different extent, depending on their place in the selenoprotein hierarchy.
The present investigation was, therefore, undertaken to elucidate the effect of tebuconazole on the liver tissue of adult rats, as well as the protective role of selenium.
Materials and Methods
Animals
Adult rats purchased from the Central Pharmacy of Tunisia were housed at ambient temperature 21 ± 3°C and given commercial diet and tap water ad libitum. The general guidelines for the use and care of living animals in scientific investigation were closely followed [7].
Experimental design
Eighteen male rats of Wistar strain were randomly divided into three groups of six each. The group one represented controls which received by intraperitoneal injection oil corn as vehicle; the second group received (100 mg/kg b.w) of TEB by intraperitoneal injection, and animals of the third group (TEB+Se) were administered TEB (100 mg/kg b.w) with 0.5 mg/kg of Se added to their diet. TEB and Se doses and manner of administration were chosen on the basis of available literature data [8,9]. A 30-day treatment was then carried out.
Protein quantification
Liver protein content was determined according to Lowry et al. [10] using Bovine serum albumin as standard.
Malondialdehyde (MDA) measurement
Liver MDA concentrations were measured according to the method of Draper and Hadley [11]. An aliquot of liver homogenate and trichloroacetic acid was mixed and centrifuged at 2500 rpm. A volume of 1 mL thiobarbituric acid reagent (0.67%) was added to 500 μL supernatant and heated at 90°C for 15 min. The mixture was then cooled and measured spectrophotometrically at 532 nm. MDA values are expressed as nmol of MDA/g of liver.
Advanced oxidation protein product (AOPP)
AOPP levels were determined according to the method of Kayali et al. [12]. An amount of 0.4 mL of extract was treated with 0.8 mL phosphate buffer (0.1 M; pH 7.4). Two minutes later, a volume of 0.1 mL, 1.16 M potassium iodide was added to the tube followed by 0.2 mL acetic acid. The reaction mixture absorbance was immediately measured at 340 nm. AOPP concentration is expressed as μmol/mg protein.
Protein carbonyl (PCO) content
Liver PCO content was measured following the method of Reznick and Packer [13]. It was calculated based on the DNPH molar extinction coefficient (ε 2.2 × 104 cm-1 M-1), and expressed as micromoles per milligram of protein.
Liver glutathione (GSH) contents
The contents of GSH in the liver were determined in accordance with the method described by Ellman [14] and modified by Jollow et al. [15] based on the development of a yellow color when DTNB (5,5-dithiobis-2 nitrobenzoic acid) was added to compounds containing sulfhydryl groups. Briefly, 3 mL of sulfosalicylic acid were added to 500 μL of liver homogenate in a phosphate buffer. The mixture was then centrifuged at 2500 xg for 15 minutes, and the Ellman reagent added to 500 μL of supernatant. Absorbance was measured at 412 nm. GSH content is expressed as mg/g of liver.
Antioxidant enzyme activities
Enzyme activities were determined in liver homogenates diluted in a phosphate buffer.
Total superoxide dismutase activity (SOD)
The estimation of SOD activity was done spectrophotometrically analyzed at 560 nm, according to the method of Beauchamp [16]. The reaction mixture comprised 50 mM of liver homogenates in potassium phosphate buffer (pH 7.8), 13 mM Lmethionine, 0.1 mM EDTA, 2 mM riboflavin and 75 mM nitroblue tetrazolium (NBT). The blue color developed in the reaction was measured at 560 nm. SOD activity units are expressed as the amount of enzyme required inhibiting NBT reduction by 50%, and the activity is expressed as units per mg of protein.
CAT activity was assayed by spectrophotometrically analyzed at 240 nm according to Aebi method [17]. The enzymatic reaction was triggered by the addition of a 20 μl aliquot of the homogenized tissue and the substrate (H2O2) to an 0.5 M concentration in a medium containing 100 mM phosphate buffer, pH 7.4. Absorbance changes were recorded at 240 nm. CAT activity is expressed as μmoles H2O2 consumed/min/mg protein.
GPx activity was determined according to the method of Flohe and Gunzler [18]. GPx catalyzes by reduced glutathione oxidation cumene hydroperoxide. Briefly, 200 μl of the homogenized tissue were added to 200 μl of the reduced glutathione reductase (4 mM) and 100 μl of 100 mM phosphate buffer, pH 7.4. In the presence of nicotinamide adenine dinucleotide phosphate reduced form (NADPH), the oxidized reduced glutathione is immediately converted to the reduced form with a concomitant oxidation of NADPH- NADP+. Decrease in absorbance at 340 nm is measured, and the enzyme activity was expressed as nmol of oxidized GSH/min/mg protein.
Histopathological examination
Liver samples were placed in a 10% buffered formalin solution, embedded in paraffin, sectioned at a 5 mm thickness, and stained with hematoxylin-eosin for histological studies. Four slides were prepared from each liver. All sections were assessed for the degree of tubular and glomerular injury.
Statistical Analysis
The data were analyzed using the statistical package program Stat view 5 Software for Windows (SAS Institute, Berkley, CA). Statistical analysis was performed using one-way. Analysis of Variance (ANOVA) followed by Fisher’s Protected Least Significant Difference (PLSD) test as a post hoc test for comparison between groups. All values were expressed as means ± S.D. Differences were considered significant if p<0.05.
Results
Lipid peroxidation
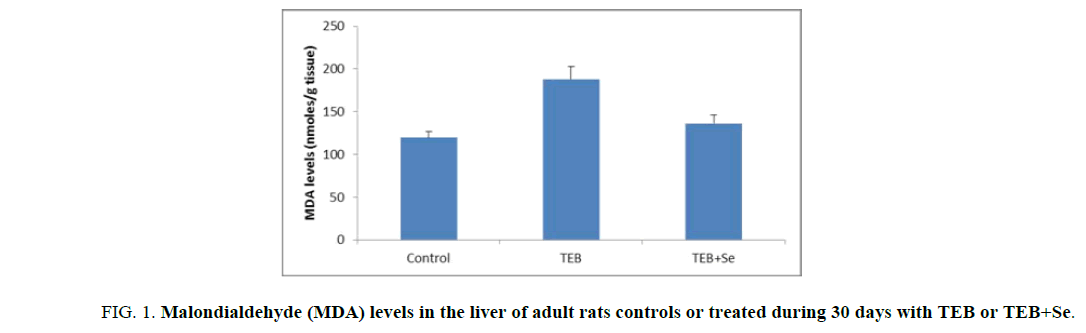
Our results revealed, in TEB-treated rats, a significant increase in lipid peroxidation level in the liver, as evidenced by the enhanced MDA levels. In fact, in the liver homogenate of TEB-treated rats, MDA levels significantly increased (175.86 nmol/g tissue) fold compared to control group (126.37 nmol/g tissue). Supplementation of Se to the diet of (TEB+Se) group decreased MDA contents in the liver (146.43 nmol/g tissue) when compared to TEB group (Figure. 1).
Figure 1: Malondialdehyde (MDA) levels in the liver of adult rats controls or treated during 30 days with TEB or TEB+Se.
Values are expressed as means ± S.D for 6 animals in each group. Comparisons are made between:
TEB and TEB+Se groups vs. control group: **p<0.01.
TEB+Se group vs. TEB group: +: p<0.05.
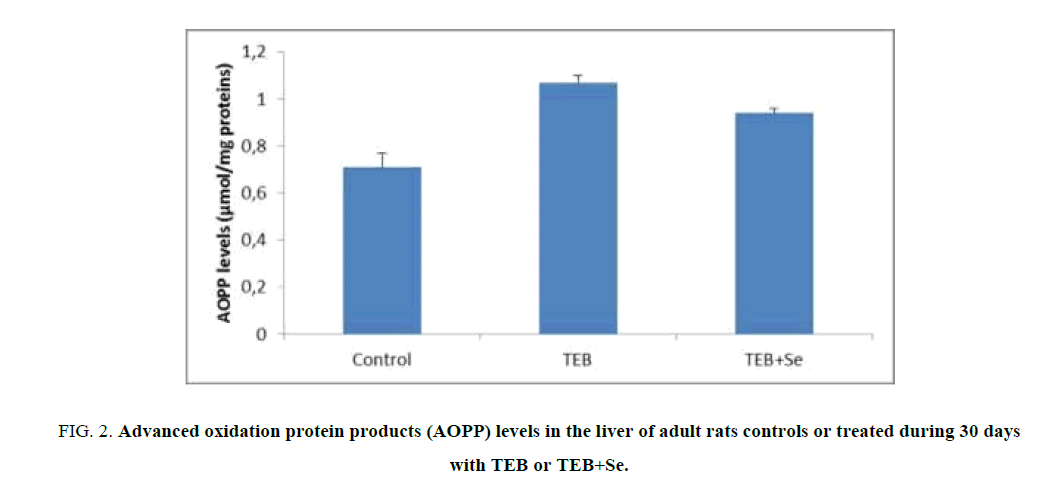
Liver AOPP levels
Figure. 2 shows values of AOPP, a marker of protein oxidative damage in the liver tissue of control and treated rats. A significant increase of AOPP levels in liver homogenates was observed in TEB-treated rats (1.07 μmol/mg proteins) when compared to controls (0.71 μmol/mg proteins). Supplementation of Se in the diet of TEB-treated rats resulted in a decrease of liver AOPP levels (0.94 μmol/mg proteins) when compared to those of TEB-treated rats.
Figure 2: Advanced oxidation protein products (AOPP) levels in the liver of adult rats controls or treated during 30 days with TEB or TEB+Se.
Values are expressed as means ± S.D for 6 animals in each group.
Comparisons are made between:
TEB and TEB+Se groups vs. control group: * p<0.05; ** p<0.01.
Liver PCO levels
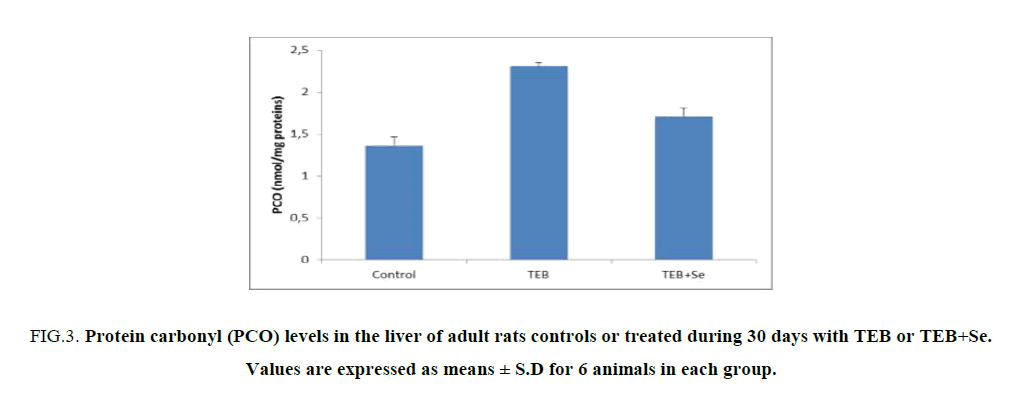
The levels of PCO in the liver tissue were increased (2.31 nmol/mg proteins) in TEB-treated rats when compared to controls (1.36 nmol/mg proteins) (Figure. 2). Supplementation of Se in the diet of TEB-treated rats resulted in a decrease of liver PCO levels (2.72 nmol/mg proteins) when compared to those of TEB-treated rats (Figure. 3).
Figure 3: Protein carbonyl (PCO) levels in the liver of adult rats controls or treated during 30 days with TEB or TEB+Se. Values are expressed as means ± S.D for 6 animals in each group.
Comparisons are made between:
TEB and TEB+Se groups vs. control group: ***p<0.001.
TEB+Se group vs. TEB group: +: p<0.05.
Antioxidant enzyme activities liver GSH levels
A significant increase of GSH levels in liver (+50%) was evident in rats exposed to TEB (Table 1). Supplementation of Se decreased GSH levels in (TEB+Se) group when compared to TEB group.
| Parameters and treatment | GPX | GSH | Catalase | SOD |
|---|---|---|---|---|
| Controls | 7.10 ± 1.02 | 319.55 ± 28.08 | 403.44 ± 27.98**+ | 152.38 ± 11.36 |
| TEB | 4.09 ± 0.81*** | 479.69 ± 19.4*** | 403.44 ± 27.98**+ | 93.29 ± 11.16*** |
| TEB+Se | 5.26 ± 0,86**+ | 403.44 ± 27.98**+ | 8.36 ± 0.33*+ | 117.72 ± 13.43*+ |
| GPx: nmol of GSH oxidized/min/mg protein. CAT: nmol H2O2/min/mg protein. GSH: μg/g tissue TEB and TEB+Se groups vs. control group: *: p<0.05; **: p<0.01; ***: p<0.001. TEB+ Se group vs. TEB group: +: p<0.05. |
||||
Table 1. Enzymatic antioxidant activities (glutathione peroxidase, catalase and superoxide dismutase) and the glutathione levels in the heart of adult rats controls or treated during 30 days with TEB or TEB+Se.
Enzymatic antioxidant status in liver
Antioxidant enzyme activities of CAT, SOD and GPx of control and treated groups are represented in Table 1. TEB treatment led to a significant increase in CAT activity by 47% in TEB group, compared to those of control group, while GPx and SOD activities were significantly lower (-73% and -63% respectively) in TEB group than that of control.
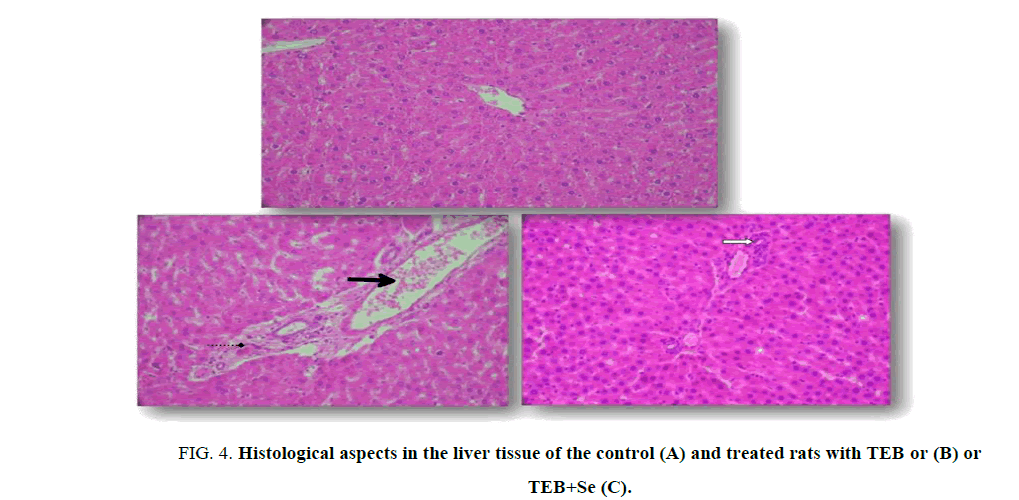
Liver histopathology
Light microscopic examination indicated a normal structure of the liver in the controls (Figure. 4A). Exposure to TEB induced degenerative changes in the organ. In fact, TEB causes infiltration of inflammatory leucocyte cells and hepatocyte vacuolization (Figure. 4B and 4C). The severe liver damages significantly decreased when selenium was added in the diet of TEB-treated rats compared with those treated only with TEB (Figure. 4C).
Figure 4: Histological aspects in the liver tissue of the control (A) and treated rats with TEB or (B) or TEB+Se (C).
Optic microscopy: Hematoxylin-eosin, X 400.
Arrows indicate:  Leucocyte inflammatory cells;
Leucocyte inflammatory cells; Congested central veins;
Congested central veins; Hepatocyte vacuolization.
Hepatocyte vacuolization.
Discussion
The current study aimed to probe into the protective effect of Selenium against TEB-inflicted rat liver damage. MDA constitutes one of the metabolic products of lipid peroxides generated by lipid oxidation reaction induced by oxygen-free radicals in tissues. Our investigation revealed an increased MDA content in the liver of the TEB-treated group. Moreover, the increased free radicals generation could lead to protein modifications, giving rise to a carbonyl group formation into side chains and/or a reduction of sulphhydryl groups in susceptible amino acids [19].
It also revealed that ROS, probably generated by TEB treatment, induced an increase in PCO and AOPP levels, markers of protein oxidative injury. Our results demonstrated that treatment with Se prevented lipid peroxidation and protein oxidation in rats, and thereby diminished TEB-induced liver damage. Lipid peroxidation prevention might be derived from Se capability to scavenge ROS.
In addition, it could be suggested that the elevated lipid peroxidation level induced by TEB treatment might be brought about by the decrease in antioxidant enzyme activities.
GSH is one of the essential cell function-regulating compounds. The increase of the GSH level observed in the TEB-treated rats, compared with control, indicated that GSH depletion resulted in an enhanced lipid peroxidation [20]. The activity of GSH antioxidant enzymes was significantly diminished in the Se group, as compared with control. Reduction in GSH activity might be due to the decreased availability of its substrate and reduced glutathione [20].
GPx is a GSH-dependent antioxidant enzyme [20]. The biochemical function of Se containing GPx is to reduce lipid hydroperoxides to their corresponding alcohols, and also to reduce free hydrogen peroxide to water [21]. SOD catalyzes superoxide anion dismutation into hydrogen peroxide, which is then detoxified to hydrogen peroxide by Catalase, which is a common enzyme found in nearly all living organisms and whose functions include catalyzing hydrogen peroxide decomposition to oxygen and water [22]. Treatment with TEB alone caused significant changes in GPx, CAT and SOD activities, while treatment with Se remarkably improved theses parameters.
Investigating liver dysfunction was correlated with hepatic histological studies. In fact, TEB treatment caused inflammatory leucocytes infiltration, vacuolization and necrosis in the liver. These alterations could result from ROS generation which interacted with biological target molecules, thus causing liver injury and TEB-induced membrane distribution. Se supplementation enhanced liver structure regeneration.
In fact, normal liver cell architecture was shown after co-administration of Se in the TEB-treated rat’s diet. Results suggested that Se could reduce TEB-induced liver injury. Indeed, Se acted as an antioxidant and inhibited the oxidative processes of lipids and apo-proteins in cell membranes as reported by previous studies [23,24].
Conclusion
To conclude, the current study demonstrated that TEB enhances lipid peroxidation and protein oxidation associated with increased levels of GSH and altered antioxidant enzyme activities (GPx, SOD and CAT), and induces impairments of histological aspects in the liver of adult mice. Our results also showed that TEB-induced hepatotoxicity leads to liver injury as a result of ROS generation. Se supplementation in TEB-treated rats improved oxidative stress. Accordingly, it should be confirmed that the supplementation of a natural antioxidant may act as a protective agent against pesticide toxicity.
References
- Fu J, Mai B, Sheng G, et al. Persistent organic pollutants in environment of the Pearl River Delta, China: An overview. Chemosphere. 2003;52:1411-22.
- Montuelle B, Dorigo U, Berard A, et al. The periphyton as a multimetric bioindicator for assessing the impact of land use on rivers: An overview of the ArdiSres-Morcille experimental watershed. Hydrobiologia. 2010;657:123-41.
- Elsaesser D, Schulz R. Mitigation of fungicide pollution in vegetated agricultural surface waters: GIS modeling and monitoring in the field. In: Conference Proceedings from the SETAC Europe 18th Annual Meeting. SETAC, Warsaw. 2008; 406-7.
- Taxvig C, Hass U, Axelstad M, et al. Endocrine-disrupting activities in vivo of the fungicides tebuconazole and epoxiconazole. Toxicol Sci. 2007;100:464-73.
- EPA (Environmental Protection Agency). Chemicals evaluated for carcinogenic potential. Office of Pesticide Programs, U.S. Environmental Protection Agency, Washington, DC. 2006.
- Montgomery R. Determination of glycogen. Arch Biochem Biophys. 1957;67:378-81.
- Council of European Communities, Council instructions about the protection of living animals used in scientific investigations. Official Journal of the European Communities (JO 86/609/CEE). 1986;358:1-18.
- Moser VC, Barone S, Smialowicz RJ, et al. The effects of perinatal tebuconazole exposure on adult neurological, immunological, and reproductive function in rats. Toxicol Sci. 2001;62:339-52.
- Ognjanovic BI, Markovic SD, Pavlovic SZ, et al. Effect of chronic cadmium exposure on antioxidant defense system in some tissues of rats: Protective effect of selenium. Physiol Res. 2008;57:403-11.
- Lowry OH, Rosenbrough NJ, Farr AL. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951;193:265-75.
- Draper HH, Hadley H. Malondialdehyde determination as index of lipid peroxidation. Method Enzymol. 1990;86:421-31.
- Kayali H, Young VE, Barkey K. Empire to Nation: Historical perspectives on the making of the modern world. Editeur: Rowman and Little field Publishers Series: World Social Change. 2006.
- Reznick AZ, Packer L. Oxidative damage to proteins: Spectrophotometric method for carbonyl method. Enzymol. 1994;233:357-63.
- Ellman G. Tissue sulfhydryl groups. Arch Biochem Biophys. 1959;82:70-77.
- Jollow DJ, Mitchell JR, Zampaglione N. Bromobenzene-induced liver necrosis. Protective role of glutathione and evidence for 3, 4-bromobenzene oxide as the hepatotoxic metabolite. Pharmacology. 1974;11:151-169.
- Beauchamps C. Superoxide dismutase: Improved assays and assay applicable to acrylamide gels. Anal Biochem. 1971;44:276-87.
- Aebi H. Catalase in vitro method. Enzymol. 1984;105:121-26.
- Flohe L, Gunzler WA. Assays of glutathione peroxidase method. Enzymol. 1984;105:114-21.
- Halliwell B, Gutteridge JMC. Comments on review of free radicals in biology and medicine, 2nd ed. In: Barry Halliwell and John MC Gutteridge, editors. Free Radic Biol Med. 1992;12:93-95.
- Karthikeyan K, Sarala-Bai BR, Niranjali DS. Cardioprotective effect of grape seed pro-anthocyanidins on isoproterenol-induced myocardial injury in rats. Int J Cardiol. 2007;115: 326-33.
- Ran Q, Liang H, Ikeno Y, et al. Reduction in glutathione peroxidase increases life span through increased sensitivity to apoptosis. J Gerontol Biol Sci Med Sci. 2007;62:932-42.
- Gaetani G, Ferraris A, Rolfo M, et al. Predominant role of catalase in the disposal of hydrogen peroxide within human erythrocytes. Blood. 1996;87:1595-99.
- McPherson A. Selenium vitamin E and biological oxidation. In: Cole DJ, Garnsworthy PJ, editors. Recent advances in animal nutrition. Oxford: Butterworth and Heinemann. 1994;3-30.
- Murray RK, Granner DK, Mayes PA, et al. Harper’s biochemistry (A Lange medical book). International Edition. Appleton and Lang Lange Medical Publications, Albany, New York. 988;574-76.