Abstract
Computational and FTIR-spectroscopic study on the reactivity of some polyphenolic compounds with HCHO
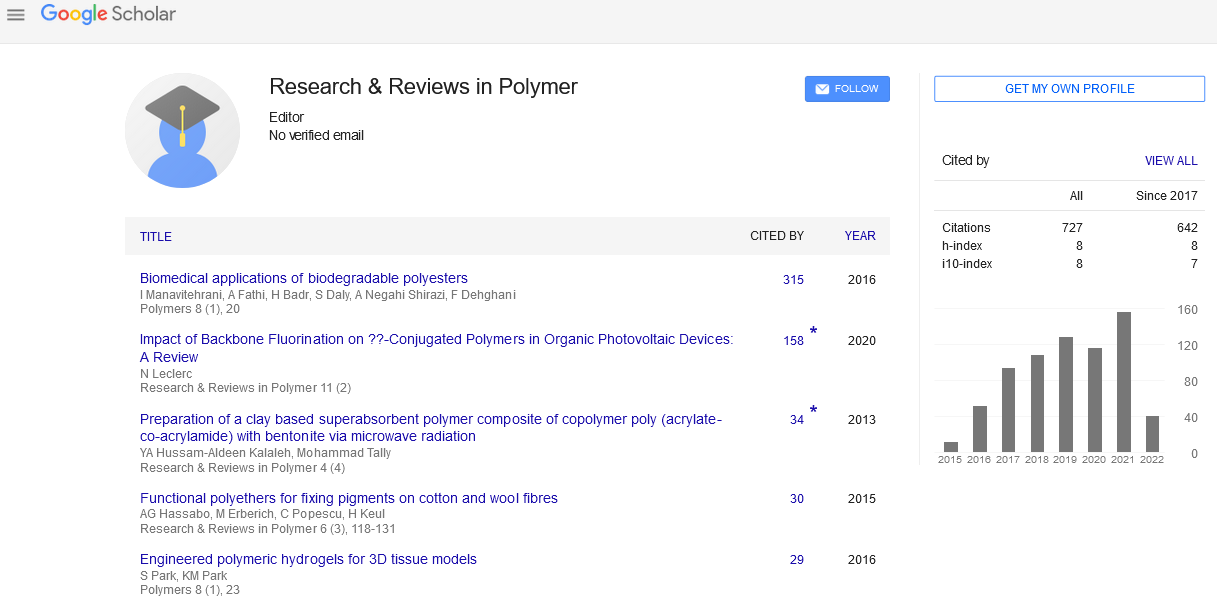
Author(s): S.Arasaretnam, L.KarunanayakeA semi-empirical computational approach was used to study the reaction of some polyphenolic compounds with formaldehyde in an aqueous alkaline system. Mulliken charges were calculated for the reactive sites on the aromatic rings of the polyphenolic compounds to predict their reaction pathways with formaldehyde. Semi-empirical calculations atRHF/PM3modelwere performed on a series of polyphenolic compounds with different chain lengths using HyperChem and GAMESS softwares. The results were compared with the experimental Fourier TransformInfra Red (FT-IR) data. The results indicate that the initial gallic acid formaldehyde resin forms predominantly via CH2OCH2 links. Itwas found that C3-C6 link is formed between the B-ring of the two flavanoid units and the link formed is a CH2OCH2 type link. Based on analysis study the negative charges at reactive sites C3' and C6' of catecholic B-ring complexed with Zn2+ ions have increased, affinity of the reactive sites towards formaldehyde