Original Article
, Volume: 14( 1)Synthesis and Antimicrobial Activity Evaluation of Some New Thiadiazinone and Thiadiazepinone Derivatives Bearing Sulfonamide Moiety
- *Correspondence:
- Hany MM Dalloul, Department of Chemistry, Faculty of Applied Science, Alaqsa University of Gaza, P.O. Box 4051, Gaza 76888, Palestine, Tel: 0097082641601; E-mail: hmdalloul60@yahoo.com
Received: September 22, 2017; Accepted: September 27, 2017; Published: September 28, 2017
Citation: Dalloul HMM, El-Nwairy KA, Shorafa AZ, et al. Synthesis and Antimicrobial Activity Evaluation of Some New Thiadiazinone and Thiadiazepinone Derivatives Bearing Sulfonamide Moiety. Org Chem Ind J. 2017;14(1):119
Abstract
A new series of novel functionalized 1, 3, 4-thiadiazin-5-ones and 1, 3, 4-thiadiazepin-5-ones bearing sulfonamide moieties by 1, 3-dipolar cyclocondensation reaction of nitrilimines with α-mercaptoesters and mercaptosuccinic acid respectively. The structures of the newly prepared compounds were elucidated by spectral methods (IR, 1H-NMR, 13C-NMR and MS spectroscopy) and elemental analysis. The synthesized compounds were examined for their antimicrobial activity. Some of these compounds showed significant antimicrobial activity toward tested microbes.
Keywords
Nitrilimines; Sulfonamide; a-Mercaptoesters; 1, 3, 4-Thiadiazinone; 1, 3, 4-Thiadiazepinone
Introduction
Sulfonamides are medicinally effective molecules and are known to possess various types of biological activities including antibacterial [1-3], antiviral [4-7], anti-carbonic anhydrase [8,9], high-ceiling diuretic [10], hypoglycemic [11,12], antithyroid, anti-inflammatory [13] and antiglaucoma. It is also known that aromatic or heteroaromatic sulfonamides may act as antitumor agents through perturbation of cell cycle in the G1 phase, distribution of microtubule assembly or angiogenesis inhibition [14-17]. Moreover, numerous sulfonamides were found to act as antitumor agents through carbonic anhydrase CA) inhibition [18-26]. Heterocyclic compounds containing the nitrogen and sulfur were found to play important role in medicinal and pharmaceutical chemistry as these molecules have potent biological activities [27]. Among them, 1, 3, 4-thiadiazines, is a therapeutically important class of heterocyclic compounds. They are well known to show various medicinal and pharmacological applications. 1, 3, 4-Thiadiazinone derivatives have attracted a great deal of interest due to a variety of interesting biological activities. They are known as spasmolytic 30 and antibacterial agents [28-31], important matrix metalloproteinase inhibitors [32,33] and they also display cardiotonic, hypertensive [34-35] and other biological activities [36-38]. Thiadiazepines are reported for antimicrobial activity [39], antifungal activity [40] and inhibition of metalloproteinase [41].
Many literatures revealed that some condensed thiadiazepines have antidepressant [42], central nervous depressant [43], bactericidal [44,45], fungicidal and anticancer activity [46]. Recently, 1, 4, 5-dibenzo [b, f] thiadiazepine was found to show good neuroprotective properties against neurodegenerative diseases without anticholinergic effects [47]. Taking into account all previous commentaries of the biological activities of sulfonamides and in continuation of our work on the synthesis of biologically active heterocycles [48-55], efforts have been made to synthesize a series of new 1, 3, 4-thiadiazin-5-one and 1, 3, 4-thiadiazepin-5-one derivatives incorporating sulfonamide moiety via cyclocondensation reaction of nitrilimines containing moiety of sulfonamide with a-mercaptoesters and mercaptosuccinic acid which expected to show interesting biological activities.
Materials and Methods
Apparatus and chemicals
Melting points were determined using melting temperature apparatus and are uncorrected. IR spectra were measured using a Satellite 3000 Mid infrared spectrometer as potassium bromide pellets. 1H NMR and 13C NMR spectra were scanned on a Bruker AM 300 MHz spectrometer at r. t. in DMSO-d6 solution using tetramethylsilane (TMS) as internal reference. Chemical shifts are expressed in d (ppm) downfield from TMS and coupling constants are in Hertz (Hz). Mass spectra were recorded on a Shimadzu GCMS-QP1000 EX spectrometer. Elemental analysis was carried out at Cairo University, Cairo, Egypt. Ethyl mercapto acetate, mercaptosuccinic acid, triethylamine (TEA), tetrahydrofuran (THF), dicyclohexylcarbodiimide (DCC) and 1, 4-dioxane were purchased from Avocado Research Chemicals, England and used without further purification. Hydrazonoyl chlorides 1a-c was prepared by direct coupling of the appropriate sulfonamide diazonium chloride with a-chloroacetoacetanilide in sodium acetate/ethanol solution following literature procedures [50-53].
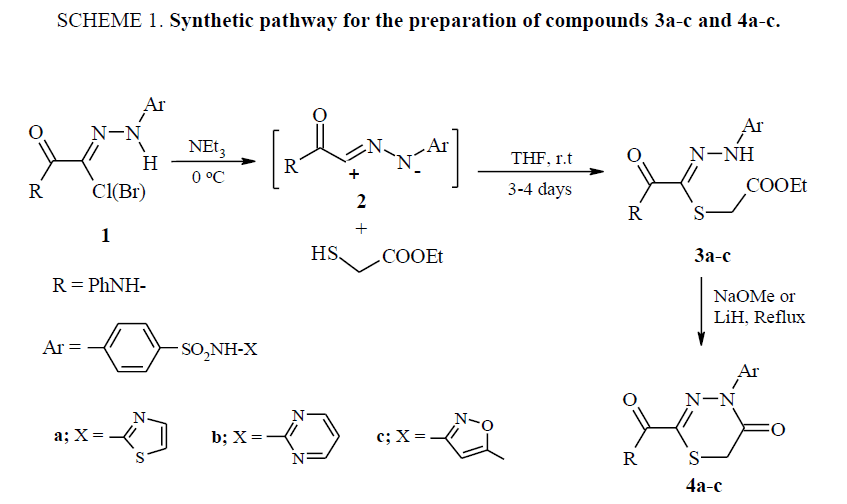
General procedure for Synthesis of 3, 5, 6-thiadiaza-4-hexenoates 3a-c
Triethylamine (5 mmol) in THF (10 ml) was drop wise added at room temperature to a stirred solution of the appropriate hydrazonoyl halide 1 (10 mmol) and ethyl mercaptoacetate (15 mmol) in tetrahydrofuran (THF) (50 ml). Stirring was continued for three days, then the solvent was removed under reduced pressure and the residual solid was washed with water (100 ml). The solid products were collected and recrystallized from ethanol to afford the desired compounds 3a-c.
Ethyl 4-phenylaminocarbonyl-6-[4-(thiazol-2-yl-sulfamoyl) phenyl]-3, 5, 6-thiadiaza-4-hexen-oate (3a)
M.P. 213°C-215°C (ethanol). Yield 76%. IR: ?=3365, 3346, 3270 (NH), 1718 (ester C=O), 1650 (amide C=O), 1597 (C=N), 1225 (C–S), 1150 (S=O) cm-1. 1H NMR (DMSO-d6): d=12.70 (s, 1H, SO2NH), 10.65 (s, 1H, NH), 9.96 (s, 1H, PhNH), 8.74 (d, 1H, J=9.2 Hz, thiazole), 7.93-7.04 (m, 9H, Ar-H), 6.64 (d, 1H, J=4.5 Hz, thiazole), 4.25-4.12 (q, 2H, OCH2), 3.95 (s, 2H, SCH2), 1.28-1.15 (t, 3H, CH3) ppm. 13C NMR (DMSO-d6): d=170.1 (ester C=O), 159.4 (amide C=O), 141.7 (C=N), 167.9-119.3 (Ar-C and thiazole-C), 61.3 (OCH2), 33.2 (SCH2), 13.9 (CH3) ppm. MS: m/z=519 [M+.]. Anal. Calcd. for C21H21N5O5S3 (519.62): C, 48.54; H, 4.07; N, 13.48; Found C 48.31; H, 3.98; N, 13.60.
Ethyl 4-phenylaminocarbonyl-6-[4-(pyrimidin-2-yl-sulfamoyl) phenyl]-3, 5, 6-thiadiaza-4-hexen-oate (3b)
M.P. 236°C-238°C (ethanol). Yield 75%. IR: ?=3358, 3348, 3270 (NH), 1715 (ester C=O), 1655 (amide C=O), 1595 (C=N), 1230 (C–S), 1139 (S=O) cm-1. 1H NMR (DMSO-d6): d=12.65 (s, 1H, SO2NH), 10.64 (s, 1H, N-NH), 9.93 (s, 1H, PhNH), 8.84 (d, 2H, J=8.8 Hz, pyrimidine ring), 8.02-7.07 (m, 9H, Ar-H), 6.86 (t, 1H, J=7.5 Hz, pyrimidine ring), 4.23-4.12 (q, 2H, OCH2), 3.96 (s, 2H, SCH2), 1.27-1.16 (t, 3H, CH3) ppm. 13C NMR (DMSO-d6): d=170.8 (ester C=O), 159.9 (amide C=O), 141.2 (C=N), 168.2-113.8 (Ar-C and pyrimidine-C), 61.8 (OCH2), 34.2 (SCH2), 14.1 (CH3) ppm. MS: m/z=514 [M+]. Anal. Calcd. for C22H22N6O5S2 (514.59): C, 51.35; H, 4.31; N, 16.33; Found C, 51.58; H, 4.45; N, 16.22.
Ethyl 4-phenylaminocarbonyl-6-[4-(5-methyloxazol-3-yl-sulfamoyl) phenyl]-3, 5, 6-thiadi-aza-4-hexenoate (3c)
M.P. 218°C-220°C (ethanol). Yield 73%. IR: ?=3372, 3343, 3270 (N–H), 1712 (ester C=O), 1650 (amide C=O), 1598 (C=N), 1226 (C–S), 1138 (S=O) cm-1. 1H NMR (DMSO-d6): d=12.62 (s, 1H, SO2NH), 10.46 (s, 1H, N-NH), 9.95 (s, 1H, PhNH), 7.88-7.06 (m, 9H, Ar-H), 6.24 (s, 1H, oxazole ring), 4.14-1.01 (q, 2H, OCH2), 3.89 (s, 2H, SCH2), 2.38 (s, 3H, CH3 on oxazole ring), 1.25-1.13 (t, 3H, CH3) ppm. 13C NMR (DMSO-d6): d=170.4 (ester C=O), 159.4 (amide C=O), 141.5 (C=N), 167.4-115.4 (Ar-C and oxazole-C), 61.3 (OCH2), 33.5 (SCH2), 13.8 (CH3), 12.40 (CH3 oxazole) ppm. MS: m/z=517 [M+]. Anal. Calcd. for C22H23N5O6S2 (517.59): C, 51.05; H, 4.48; N, 13.53; Found C, 50.82; H, 4.60; N, 13.65.
Cyclization of compounds 3a-c to 1, 3, 4-thiadiazin-5-ones 4a-c
Method A: Compounds 3a-c (5 mmol) were added to a methanolic solution of sodium methoxide, prepared from sodium metal (0.12 g, 5 mmol) and methanol (20 ml) with stirring. The resulting solution was refluxed for 2-3 h. After cooling the solvent was removed under vacuum and the residual solid was washed with water, dried and recrystallized from ethanol to afford 1, 3, 4-thiadi-azinones 4a-c.
Method B: Lithium hydride (0.08 g, 10 mmol) was carefully added to a stirred solution of compounds 3a-c (5 mmol) in dry THF (30 ml) at r. t. The resulting mixture was heated to reflux for ½ h. Cool and excess LiH was destroyed with drops of acetic acid. The product was extracted three times with chloroform after removing the solvent under vacuum and the combined organic extracts were dried over anhydrous MgSO4. The solvent was then removed under reduced pressure and the resulting solid product was collected and recrystallized from ethanol to give compounds 4a-c that were identical with the ones prepared by method A.
2-Phenylaminocarbonyl-4-[4-(thizol-2-yl-sulfamoyl)phenyl]-6H-1, 3, 4-thiadiazin-5-one (4a)
M.P. 251°C-253°C (ethanol). Yield 73%. IR: ?=3365, 3273 (NH), 1678 (lactam C=O), 1650 (amide C=O), 1610 (C=N), 1149 (S=O), 684 (C-S) cm-1. 1H NMR (DMSO-d6): d=11.61 (s, 1H, SO2NH), 10.12 (s, 1H, PhN–H), 8.76 (d, 1H, J=9.1 Hz, thiazole), 7.87-7.03 (m, 9H, Ar-H), 6.63 (d, 1H J=4.5 Hz, thiazole), 3.90 (s, 2H, CH2) ppm. 13C NMR (DMSO-d6): d=161.5 (lactam C=O), 158.5 (amide C=O), 143.3 (C=N), 166.7-119.2 (Ar-C and thiazole-C), 26.2 (CH2) ppm. MS: m/z=473 [M]+. Anal. Calcd. for C19H15N5O4S3 (473.55): C, 48.19; H, 3.19; N, 14.79; Found C, 48.41; H, 3.30; N, 14.67.
2-Phenylaminocarbonyl-4-[4-(pyrimidin-2-yl-sulfamoyl) phenyl]-6H-1, 3, 4-thiadiazin-5-one (4b)
M.P. 241°C-243°C (ethanol). Yield 72%. IR: ?=3375, 3273 (NH), 1680 (lactam C=O), 1640 (amide C=O), 1615 (C=N), 1170 (S=O), 683 (C-S) cm-1. 1H NMR (DMSO-d6): d=11.60 (s, 1H, SO2NH), 10.14 (s, 1H, PhNH), 8.86 (d, 2H, J=8.8 Hz, pyrimidine), 7.97-7.11 (m, 9H, Ar-H), 6.89 (t, 1H, J=7.5 Hz, pyrimidine), 3.91 (s, 2H, CH2) ppm. 13C NMR (DMSO-d6): d=162.2 (lactam C=O), 159.5 (amide C=O), 143.6 (C=N), 167.9-110.3 (Ar-C and pyrimidine-C), 26.6 (CH2) ppm. MS: m/z=468 [M]+. Anal. Calcd. for C20H16N6O4S2 (468.52): C, 51.27; H, 3.44; N, 17.94; Found C, 51.46; H, 3.35; N, 18.05.
2-Phenylaminocarbonyl-4-[4-(5-methyloxazol-3-yl-sulfamoyl)phenyl]-6H-1, 3, 4-thiadia-zin-5-one (4c)
M.P. 232°C-234°C (ethanol). Yield 71%. IR: ?=3382, 3272 (NH), 1675 (lactam C=O), 1645 (amide C=O), 1612 (C=N), 1153 (S=O), 682 (C-S) cm-1. 1H NMR (DMSO-d6): d=11.58 (s, 1H, SO2NH), 10.13 (s, 1H, PhNH), 7.78-7.10 (m, 9H, Ar-H), 6.34 (s, 1H, oxazole ring), 3.92 (s, 2H, CH2), 2.35 (s, 3H, CH3) ppm. 13C NMR (DMSO-d6): d=160.8 (lactam C=O), 158.6 (amide C=O), 143.4 (C=N), 166.9-115.6 (Ar-C and oxazole-C), 26.3 (CH2), 12.4 (CH3). ppm. MS: m/z=471 [M]+. Anal. Calcd. for C20H17N5O5S2 (471.52): C, 50.95; H, 3.63; N, 14.85; Found C, 51.15; H, 3.52; N, 14.97.
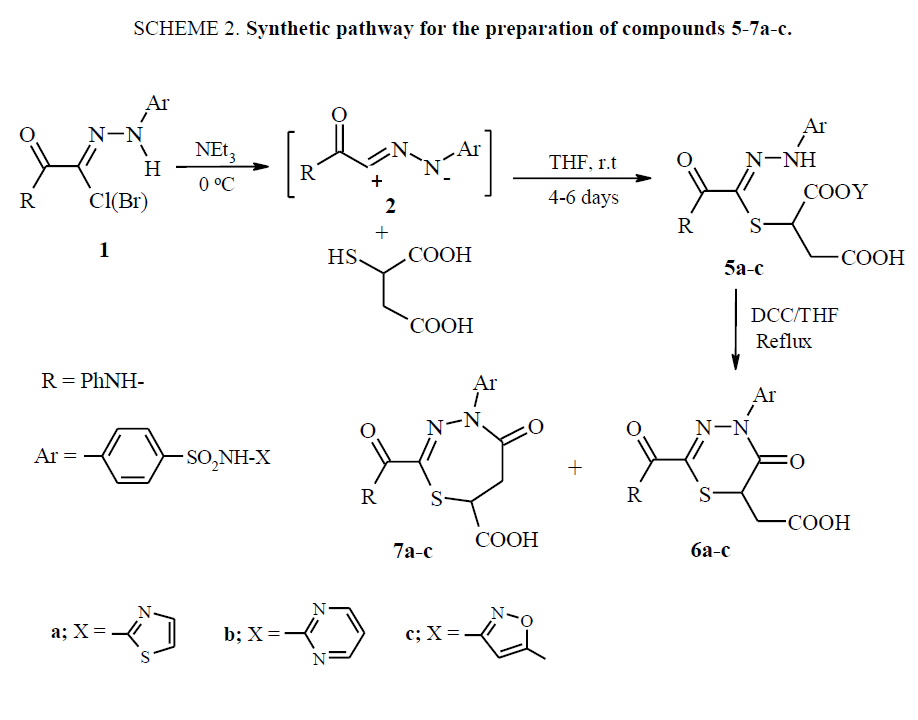
Synthesis of compounds 5a-c (general procedure): Reaction of nitrilimines with mercaptosuccinic acid
To a mixture of the appropriate hydrazonoyl halide 1a-c (10 mmol) and mercaptosuccinic acid (7.50 g, 50 mmol) in dry tetrahydrofuran or 1, 4-dioxane (100 ml), triethylamine (5 mL, 50 mmol) was added at room temperature and the reaction mixture was controlled by TLC. The stirring continued until the starting substrates were completely consumed (4-6 days). The precipitated salt was filtered off, the solvent was removed under reduced pressure and the residue was partitioned between ethyl acetate and water. The aqueous layer was extracted with ethyl acetate and the combined organic layers were extracted with saturated NaHCO3 solution and dried over MgSO4. The solvent was evaporated under vacuum and the crude residue was treated with ethanol, where 5a-c could be isolated by slow evaporation, or immediately cyclized to 6, 7.
2-{2-Anilino-2-oxoethanehydrazonoyl-N-[4-(thiazol-2-yl-sulfamoyl) phenyl]} thiosuccinic acid 5a
White solid, yield 73%, M.P. 216°C-218°C, 1H NMR (DMSO-d6) d: 3.66 (d, 2H, J=6.7 Hz, CH2), 3.76 (t, 1H, J=6.7 Hz, CH), 6.64 (d, 1H, J=4.5 Hz, thiazole), 7.24-8.22 (m, 9H, Ar-CH), 8.78 (d, 1H, J=9.2 Hz, thiazole), 9.86 (NH anilino), 10.52 (s, 1H, ArNH), 12.70 (s, 1H, SO2NH). 13C NMR (DMSO-d6) d: 39.7 (CH2), 42.3 (CH), 125.2-141.6 (Ar-C), 145.9 (C=N), 159.6 (C=O amide), 171.8, 172.5 (COOH). IR (KBr) v/cm-1: 1237 (C-S), 1621 (C=N), 1654 (C=O amide), 1723, 1734 (C=O), 2539, 3240 (OH), 3265, 3347 (NH). MS, (m/z): 549 [M]+. Analysis (% calculated/found) for C21H19N5O7S3 (Mw 549.61) C: 45.89/46.15, H: 3.48/3.62, N: 12.74/12.63.
2-{2-Anilino-2-oxoethanehydrazonoyl-N-[4-(pyrimidin-2-yl-sulfamoyl) phenyl]} thiosuccinic acid 5b
White solid, yield 72%, M.P. 220°C-222°C, 1H NMR (DMSO-d6) d: 3.67 (d, 2H, J=6.7 Hz, CH2), 3.74 (t, 1H, J=6.7 Hz, CH), 6.86 (t, 1H, J=7.5 Hz, pyrimidine), 7.06-7.98 (m, 9H, Ar-CH), 8.82 (d, 2H, J=8.8 Hz, pyrimidine), 9.88 (NH anilino), 10.51 (s, 1H, ArNH), 12.65 (s, 1H, SO2NH). 13C NMR (DMSO-d6) d: 39.7 (CH2), 42.3 (CH), 125.2-141.6 (Ar-C), 145.9 (C=N), 159.5 (C=O amide), 171.8-172.5 (COOH). IR (KBr) v/cm-1: 1236 (C-S), 1623 (C=N), 1656 (C=O amide), 1723, 1734 (C=O), 2533-3240 (OH), 3248-3341 (NH). MS, (m/z): 544 [M]+. Analysis (% calculated/found) for C22H20N6O7S2 (Mw 544.57) C: 48.52/48.75, H: 3.70/3.57, N: 15.43/15.55.
2-{2-Anilino-N-[5-(methyloxazol-3-yl-sulfamoyl) phenyl]-2-oxoethanehydrazonoyl} thiosuccinic acid 5c
White solid, yield 70%, M.P. 230°C-232°C, 1H NMR (DMSO-d6) d: 2.36 (s, 3H, CH3 of oxazole), 3.68 (d, 2H, J=6.7 Hz, CH2), 3.76 (t, 1H, J=6.7 Hz, CH), 6.21 (s, 1H, oxazole proton), 7.08-7.78 (m, 9H, Ar-CH), 9.86 (NH anilino), 10.54 (s, 1H, ArNH), 12.62 (s, 1H, SO2NH). 13C NMR (DMSO-d6) d: 21.6 (CH3), 39.7 (CH2), 42.3 (CH), 125.2-141.6 (Ar-C), 145.9 (C=N), 159.7 (C=O amide), 171.8, 172.5 (COOH). IR (KBr) v/cm-1: 1238 (C-S), 1625 (C=N), 1665 (C=O amide), 1723, 1734 (C=O), 2534-3227 (OH), 3241, 3334 (NH). MS, (m/z): 547 [M]+. Analysis (% calculated/found) for C22H21N5O8S2 (Mw 547.57) C: 48.26/48.05, H: 3.87/4.02, N: 12.79/12.65.
General procedure for compounds 6 and 7 and cyclization of compounds 5
To a stirred solution of compounds 5-a-c in THF (30 ml) was added 1 equivalent DCC in THF (10 ml) at room temperature. The stirring continued until the starting substrates were completely consumed (2 h to 3 h). The precipitate urea salt was filtered off and the remaining solution was evaporated under reduced pressure. The viscous or crude solid was dissolved in hot ethanol and by slow cooling and evaporation of ethanol the desired cyclic compounds 6a-c and 7a-c were obtained as a mixture which chromatographed on preparative TLC plates, using Merck silica gel 60 HF254 as the adsorbent and CHCl3/EtOAc (5:1) as solvent.
5-Oxo-{2-Phenylaminocarbonyl-4-[4-(thiazol-2-yl-sulfamoyl)phenyl]-5,6-dihydro-4H-1,3,4-thiadiazin-6-yl} acetic acid 6a
Yellow solid, yield 64%, M.P. 196°C-198°C, 1H NMR (DMSO-d6) d: 2.51 (s, 3H, CH3), 3.61 (d, 2H, J=7.1 Hz, CH2), 4.59 (t, 1H, J=7.1 Hz, CH), 6.62 (d, 1H, J=4.5 Hz, thiazole), 7.16-7.98 (m, 9H, Ar-CH), 8.77 (d, 1H, J=9.1 Hz, thiazole), 9.95 (PhNH), 12.45 (s, 1H, SO2NH). 13C NMR (DMSO-d6) d: 24.7 (CH3), 32.5 (CH2), 34.3 (CH), 126.3-139.2 (Ar-C), 144.6 (C=N), 157.8 (C=O amide), 159.8 (C=O lactam), 171.4 (COOH). IR (KBr) v/cm-1: 1248 (C-S), 1626 (C=N), 1660 (C=O amide), 1723 (C=O), 2550-3200 (OH). MS, (m/z): 531 [M]+. Analysis (% calculated/found) for C21H17N5O6S3 (Mw 531.59) C: 47.45/47.63, H: 3.22/3.35, N: 13.17/13.30.
5-Oxo-{2-Phenylaminocarbonyl-4-[4-(pyrimidin-2-yl-sulfamoyl) phenyl]-5, 6-dihydro-4H-1, 3, 4-thiadiazin-6-yl} acetic acid 6b
Pale yellow solid, yield 61%, M.P. 183°C-185°C, 1H NMR (DMSO-d6) d: 3.68 (d, 2H, J=7.1 Hz, CH2), 4.55 (t, 1H, J=7.1 Hz, CH), 6.87 (t, 1H, J=7.5 Hz, pyrimidine), 7.11-7.89 (m, 9H, Ar-CH), 8.84 (d, 2H, J=8.8 Hz, pyrimidine), 9.93 (PhNH), 12.65 (s, 1H, SO2NH). 13C NMR (DMSO-d6) d: 32.7 (CH2), 34.5 (CH), 126.6-139.7 (Ar-C), 143.7 (C=N), 157.9 (C=O amide), 159.5 (C=O lactam), 171.6 (COOH). IR (KBr) v/cm-1: 1247 (C-S), 1624 (C=N), 1655 (C=O amide), 1723 (C=O), 2535-3230 (OH). MS, (m/z): 526 [M]+. Analysis (% calculated/found) for C22H18N6O6S2 (Mw 526.55) C: 50.18/50.35, H: 3.45/3.33, N: 15.96/16.12.
4-{[5-(Methyloxazol-3-yl-sulfamoyl) phenyl]-5-oxo-2-phenylaminocarbonyl-5, 6-dihydro-4H-1, 3, 4-thiadiazin-6-yl} acetic acid 6c
White off solid, yield 63%, M.P. 246°C-248°C, 1H NMR (DMSO-d6) d: 2.36 (s, 3H, CH3 of oxazole), 3.61 (d, 2H, J=7.1 Hz, CH2), 4.59 (t, 1H, J=7.1 Hz, CH), 6.21 (s, 1H, oxazole proton), 7.06-7.84 (m, 7H, Ar-CH), 9.93 (PhNH), 12.70 (s, 1H, SO2NH). 13C NMR (DMSO-d6) d: 32.2 (CH2), 33.7 (CH), 126.6-139.7 (Ar-C), 143.8 (C=N), 157.8 (C=O amide), 159.8 (C=O lactam), 171.9 (COOH). IR (KBr) v/cm-1: 1224 (C-S), 1626 (C=N), 1660 (C=O amide), 1721 (C=O), 2540-3235 (OH). MS, (m/z): 529 [M]+. Analysis (% calculated/found) for C22H19N5O7S2 (Mw 529.55) C: 49.90/50.15, H: 3.62/3.50, N: 13.22/13.11.
5-Oxo-2-phenylaminocarbonyl-4-[4-(thiazol-2-yl-sulfamoyl)phenyl]-4,5,6,7-tetrahydro-1,3,4-thiadiazepine-7-carboxylic acid 7a
Yellow solid, yield 57%, M.P. 232°C-234°C, 1H NMR (DMSO-d6) d: 3.64 (d, 2H, J=6.9 Hz, CH2), 4.89 (t, 1H, J=6.9 Hz, CH), 6.62 (d, 1H, J=4.5 Hz, thiazole), 7.16-7.91 (m, 9H, Ar-CH), 8.76 (d, 1H, J=9.2 Hz, thiazole), 9.89 (PhNH), 11.75 (s, 1H, SO2NH). 13C NMR (DMSO-d6) d: 24.7 (CH3), 31.9 (CH2) 36.8 (CH), 126.6-139.7 (Ar-C), 144.3 (C=N), 160.5 (C=O ring), 171.4 (COOH), 193.6 (CH3C=O), IR (KBr) v/cm-1: 1208 (C-S), 1624 (C=N), 1692 (RC=O), 1723 (C=O), 2560-3210 (OH). MS, (m/z): 531 [M]+. Analysis (% calculated/found) for C21H17N5O6S3 (Mw 531.59) C: 47.45/47.65, H: 3.22/3.35, N: 13.17/13.30.
5-Oxo-2-phenylaminocarbonyl-4-[4-(pyrimidin-2-yl-sulfamoyl)phenyl]-4,5,6,7-tetra-hydro-1,3,4-thiadiazepine-7-carboxylic acid 7b
Yellow solid, yield 56%, M.P. 279°C-281°C, 1H NMR (DMSO-d6) d: 3.46 (d, 2H, J=6.9 Hz, CH2), 4.66 (t, 1H, J=6.9 Hz, CH), 6.85 (d, 1H, J=7.5 Hz, pyrimidine), 7.14-7.98 (m, 9H, Ar-CH), 8.86 (d, 2H, J=8.8 Hz, pyrimidine), 9.87 (PhNH), 11.78 (s, 1H, SO2NH). 13C NMR (DMSO-d6) d: 31.7 (CH2), 36.6 (CH), 126.6-139.7 (Ar-C), 144.7 (C=N), 160.8 (C=O ring), 171.4 (COOH), 187.6 (CH3C=O). IR (KBr) v/cm-1: 1208 (C-S), 1624 (C=N), 1665 (RC=O), 1723 (C=O), 2520-3230 (OH),. MS, (m/z): 526 [M]+. Analysis (% calculated/found) for C22H18N6O6S2 (Mw 526.55) C: 50.18/49.90, H: 3.45/3.55, N: 15.96/16.11.
4-[5-(Methyloxazol-3-yl-sulfamoyl)phenyl]-5-oxo-2-phenylaminocarbonyl-4,5,6,7-tetra-hydro-1,3,4-thiadiazepine-7-carboxylic acid 7c
White solid, yield 53%, M.P. 254°C-256°C, 1H NMR (DMSO-d6) d: 2.35 (s, 3H, CH3 of oxazole), 3.48 (d, 2H, J=6.9 Hz, CH2), 4.61 (t, 1H, J=6.9 Hz, CH), 6.21 (s, 1H, oxazole proton), 7.07-7.84 (m, 9H, Ar-CH), 9.88 (PhNH), 11.90 (s, 1H, SO2NH). 13C NMR (DMSO-d6) d: 31.4 (CH2), 36.7 (CH), 126.6-139.7 (Ar-C), 144.4 (C=N), 160.8 (C=O ring), 171.3 (COOH), 176.2 (RC=O). IR (KBr) v/cm-1: 1208 (C-S), 1624 (C=N), 1660 (RC=O), 1723 (C=O), 2530-3235 (OH). MS, (m/z): 529 [M]+. Analysis (% calculated/found) for C22H19N5O7S2 (Mw 529.55) C: 49.90/50.15, H: 3.62/3.55, N: 13.22/13.35.
Results and Discussion
Hydrazonoyl halides have been widely used for preparation of different heterocyclic compounds. In recent years, cyclocondensations using nitrilimines have received considerable attention because they have been shown to be an efficient synthetic tool for the preparation of various thia-aza heterocycles. The reactive nitrilimines are found to react with °-sulfanyl alkanoic acids or ethyl sulfanylacetate yielding acyclic adducts (4-arylhydrazono-5-oxo-3-thiahexanoic acid or ethyl 6-aryl- 4-aroyl-3, 5, 6-thiadiaza-4-hexenoate) which underwent cyclization to 1, 3, 4-thiadiazinone rings in the presence of dicyclohexylcarbodiimide (DCC) or lithium hydride, or methanolic sodium methoxide 50. In the present study, the nitrilimines 2a-c having sulfonamide moieties were generated in situ from the respective hydrazonoyl chlorides 1a-c, are found to react readily with ethyl mercaptoacetate for 3-4 days at room temperature gave acyclic electrophilic addition products (3, 5, 6-thiadiaza-4-hexenoates) 3a-c SCHEME. 1.
Cyclization to the corresponding 1, 3, 4-thiadiazin-5-ones 4a-c did not observe. The 3, 5, 6-thiadiaza-4-hexenoates 3a-c were cyclized intramolecularly to the corresponding 2, 4-disubstituted 1, 3, 4-thiadiazin-5-ones 4a-c by heating them with methanolic sodium methoxide (NaOMe) or lithium hydride (LiH) SCHEME. 1.
Similarly, the mercaptosuccinic acid reacts with reactive nitrilimines 2a-c for 4 to 6 days at room temperature yielding acyclic electrophilic addition products 5a-c SCHEME. 2. Acyclic adducts 5a-c underwent cyclization upon loosing water molecule, to the corresponding (4-Heterylsulfamoyl-phenyl-2-phenylaminocarbonyl-5-oxo-5, 6-dihydro-4H-1, 3, 4- thiadiazin-6-yl)-acetic acid 6a-c and 1, 3, 4-thiadiazepine-5-ones 7a-c, in the presence of dicyclohexylcarbodiimide (DCC) in refluxing tetrahydrofuran (THF) SCHEME. 2. The antimicrobial activities of the synthesized compounds 4a-c, 6a-c and 7a-c were investigated.
Spectroscopical data for compounds 3-7a-c
The assignment of structures 3-7a-c is based on their analytical and spectroscopic data. Physical properties, molecular ion peaks and elemental analysis are presented in the experimental section. The characteristic data of compounds 3-7a-c are given in detail in the experimental section. All compounds gave satisfactory combustion analysis for the proposed structures which were confirmed on the basis of their spectroscopic data. For compounds 3a-c, the electron impact (EI) mass spectra displayed the correct molecular ions (M+.) in accordance with the suggested structures. Their IR spectra showed three NH absorption bands at the region 3360 cm-1-3200 cm-1 SCHEME. 2.
The carbonyl absorption of the ester and amide groups appeared in the regions 1720 cm-1 to 1710 cm-1 and 1655 cm-1 to 1650 cm-1, respectively. The C–S stretching band appeared in the region 1230 cm-1 to 1220 cm-1 and SO2 of sulfonamide group bands appeared around 1150 cm-1 and 1060 cm-1. The 1H NMR spectra of compounds 3a-c showed signals of the ethyl protons at d=1.3 ppm-1.1 ppm (t, 3H, CH3) and 4.2 ppm-4.1 ppm (q, 2H, OCH2), indicating clearly that the ethyl group of the ester was not lost and that the compounds have acyclic structure. Also the N–NH proton appeared as a singlet at d=10.6 ppm- 10.4 ppm. The 13C NMR spectra illustrate that compounds 3a-c have the assigned acyclic structures. The carbonyl carbon of the ester group appeared at about d=170 ppm and the signals of the CH2 and CH3 carbon atoms of the ethoxy group appeared at about d=61and 14 ppm, respectively. The methylene carbon of S–CH2 appeared at about d=34 ppm-33 ppm and the signal at d ˜ 141 ppm is attributed to the C=N carbon atom. Structure elucidation of the obtained thiadiazinones 4a-c was achieved as follows: their mass spectra displayed the correct molecular ion peaks [M+] in accordance with the suggested structures and showed the loss of an ethoxy group from the acyclic adducts 3a-c via ethanol elimination. Their IR spectra support the formation of the thiadiazinone ring by the absence of N-NH (around 3340 cm-1) and C=O (around 1710 cm-1) vibration bands of the ester and the appearance of a new absorption band for a lactam (C=O of the thiadiazinone ring) in the region 1680 cm-1 to 1670 cm-1. The 1H NMR spectra of compounds 5a-c showed all the signals of the proposed structures, indicating the disappearance of ethyl (CH2CH3) and N-NH protons.
Finally, the 13C NMR data illustrated that compounds 4a-c have the assigned cyclic structure by the absence of signals for ester group carbons (170 ppm, 61 ppm, 14 ppm) and the presence of the signal at d ˜ 161 ppm which is typical for a lactam group. Furthermore, the signal of the methylene carbon (d ˜ 34 ppm) of the thioester moiety in the acyclic adducts 3a-c is shifted up field to d ˜ 26 ppm in compounds 4a-c, whereas the signal of the C=N carbon is recorded at d ˜ 143 ppm. For compounds 5a-c, their IR spectra are characterized by the 3NH bands in the region 3370 cm-1 to 3220 cm-1, a broad hydroxyl bands in the region 3200 cm-1 to 2520 cm-1 indicating the carboxyl group, a strong and broad carbonyl of the carboxyl groups band in the region 1730 cm-1 to 1720 cm-1, a C=N band at 1630 cm-1 to 1610 cm-1 and a C-S stretching band appeared in the region 1240 cm-1 to 1220 cm-1. The 1H NMR spectra of compounds 5a-c showed characteristic signals of the aliphatic and aromatic protons, especially the triplet at 4.4 ppm-4.2 ppm for the proton at C-3 and a doublet at 3.9 to 3.7 ppm for the protons at C-2. Also the N-NH proton appeared as singlet at 10.4 to 10.6 ppm.
Structure elucidation of the obtained 1, 3, 4-thiadiazin-5-ones 6a-c and 1, 3, 4-thiadiazepin-5-ones 7a-c were achieved by their analytical and spectral data summarized in the experimental section. Their mass spectra displayed the correct molecular ion peaks [M+] in accordance with the suggested structures. The IR spectra of those compounds 6a-c and 7a-c support the formation of the cyclic structures by the absence of NH band and the appearance of a new absorption band for a lactam (C=O of the ring) in the region 1670 cm-1 to 1680 cm-1. Their 1H NMR spectra showed all the signals of the proposed structures, indicating the disappearance of the signal of the proton of NNH. Finally, also the 13C NMR data illustrated that compounds 6a-c and 7a-c have the assigned cyclic structure by the presence of the signal at 159-160 ppm which is typical for a lactam group, whereas the signal of the C=N carbon is recorded at 143-144 ppm.
Antimicrobial activity
All organisms used in this study were standard strains were obtained from the Microbiology laboratory (Al-Aqsa University) and included bacterial strain such as Enterococci, Escherichia coli, Staphylococcus aureus, Klebsiella spp, Proteus spp and fungi strain such as Aspergillus niger, Candida albicans. The MIC of Tetracycline and fluconazole was determined concurrently as reference for antibacterial and antifungal activities, respectively TABLE 1. Control DMSO was carried out with each experiment. Three sulfonamide moieties substituents were placed on the thiadiazinone and thiadiazepinone rings in order to study their effects on an antimicrobial activity in vitro. Most of the synthesized compounds were screened in vitro for their antimicrobial activity against a variety of bacterial and fungal strains, employing the nutrient agar disc diffusion method52 at 1 mg/ml-100 mg/ml using dimethyl sulfoxide (DMSO) as solvent control and measuring the average diameter of the inhibition zone in mm. The results reviled that most of tested compounds exhibited good degree of activity against different strains of bacteria and fungi compared with well-known antibacterial and antifungal drugs such as tetracycline and fluconazole respectively. The results are tabulated in TABLE 1. According to National Committee on Clinical Laboratory Standards (NCCLS) (2004)53, zones of inhibition for tetracycline and fluconazole less than 14 mm were considered resistant, between 15 mm and 18 mm were considered weakly affective and more than 19 mm were considered affective. From the obtaining results, it found that these compounds possess various antimicrobial activities towards all the microorganisms tested. The results confirm that, the antimicrobial activity is strongly dependent on the substituents on triazinone and thiadiazinone rings and the presence of sulfonamide moieties enhance the activity than the reference drug TABLE 1.
| Comp. No. |
Antibacterial activity | Antifungal activity | |||||
|---|---|---|---|---|---|---|---|
| En. | E. coli | S. aureus | K. spp | P. spp | C. alb. | A. niger | |
| 4a | 18 | 16 | 17 | 15 | 14 | 18 | 16 |
| 4b | 19 | 19 | 15 | 17 | 14 | 19 | 18 |
| 4c | 16 | 16 | 17 | 16 | 15 | 16 | 19 |
| 6a | 18 | 19 | 19 | 18 | 13 | 19 | 17 |
| 6b | 17 | 18 | 18 | 19 | 16 | 19 | 16 |
| 6c | 17 | 15 | 18 | 19 | 18 | 18 | 17 |
| 7a | 18 | 18 | 16 | 16 | 19 | 15 | 13 |
| 7b | 16 | 16 | 19 | 18 | 16 | 16 | 17 |
| 7c | 15 | 17 | 18 | 15 | 13 | 17 | 19 |
| DMSO | -- | -- | -- | -- | -- | -- | -- |
TABLE 1. Antimicrobial results of the tested compounds.
Conclusion
New series of novel functionalized 1, 3, 4-thiadiazinones 5a-c, 6a-c and 1,3,4-thiadiazepinones 7a-c containing benzenesulfonamide moiety were synthesized using hydrazonoyl halides as a precursor of nitrilimines and evaluated for their in vitro antibacterial and antifungal activities. From the screening results, it found to possess various antimicrobial activities towards all the microorganisms tested. The results confirm that, the antimicrobial activity is strongly dependent on the nature of the substituents on triazinone and thiadiazinone nucleus. The methoxzolyl, pyrimidinyl and thiazolyl groups generally led to improvements in activity against both bacteria and fungi strains. Shortly, the present study can lead medicinal chemists to design and synthesize similar compounds with enhanced biological activity in future.
Acknowledgements
The authors are great thankful to the Qatar Charity for the financial support of this research through Ibhath grant (GCC-07-06).
References
- Gadad AK, Mahajanshetti CS, Nimbalkar S, et al. Synthesis and antibacterial activity of some 5-guanylhydrazone/thiocyanato-6-arylimidazo[2, 1-b]-1, 3, 4-thiadiazole-2-sulfonamide derivatives. European Journal of Medicinal Chemistry. Eur J Med Chem. 2000;35(9):853-57.
- Misra VS, Saxena VK, Srivastava RJ. Synthesis and amoebicidal activity of some new n-(2-nitro-4, 5-dimethoxybenzoyl) glycine hydrazones. Ind Chem Soc. 1982;59:781.
- Zani F, Vicini P. Antimicrobial Activity of Some 1, 2-Benzisothiazoles Having a Benzenesulfonamide Moiety. Arch Pharm. 1998;331:219-23.
- Scozzafava A, Owa T, Mastrolorenzo A, et al. Anticancer and antiviral sulfonamides. Curr Med Chem. 2003;10(11):925-53.
- Stranix BN, Lavallee JG, Sevigny G. Lysine sulfonamides as novel HIV-protease inhibitors: N-Acyl aromatic ??-amino acids. Bioorg Med Chem Lett. 2006;16(13):3459-462.
- Beale JM Jr. Anti Infective Agents. In: JH Block, JM Beale Jr, editors. Wilson and Gisvold’s Text Book of Organic Medicinal and Pharmaceutical Chemistry. 11th ed. Lippincott William and Wilkins: Philadelphia; 2004:217-81.
- Dogruer D, Urlu S, Onkol T, et al. Synthesis of some pyridazine derivatives carrying urea, thiourea and sulfonamide moieties and their antimicrobial activity. Turk J Chem. 2010;34(1):57-65.
- Brzozowski Z, S1awi_nski J, Sa?czewski F, et al. Carbonic anhydrase inhibitors: Synthesis and inhibition of the human cytosolic isozymes I and II and transmembrane isozymes IX, XII (cancer-associated) and XIV with 4-substituted 3-pyridinesulfonamides. Eur J Med Chem. 2010;45:2396-404.
- Renzi G, Scozzafava A, Supuran CT. Carbonic anhydrase inhibitors: Topical sulfonamide antiglaucoma agents incorporating secondary amine moieties. Bioorg Med Chem Lett. 2000;10:673-76.
- Maren TH. Electrocoupling of vibrating quartz plates. Physiol Rev. 1967;47:595-81.
- Maren TH. Relations between structure and biological activity of sulfonamides. Ann Rev Pharmacol Toxicol. 1976;16:309-27.
- Drew J. Drug discovery: A historical perspective. Science 2000;287:1960-964.
- Li JJ, Anderson D, Burton E, et al. 1, 2-Diarylcyclopentenes as selective cyclooxygenase-2 inhibitors and orally active anti-inflammatory agents. Med Chem. 1995;38:4570-578.
- Sa?czewski F, Innocenti A, S1awinski J, et al. Carbonic anhydrase inhibitors: Inhibition of human cytosolic isozymes I and II and tumor-associated isozymes IX and XII with S-substituted 4-chloro-2-mercapto-5-methyl-benzenesulfonamides. Bioorg Med Chem. 2008;16:3933-40.
- Garaj V, Puccetti L, Fasolis G, et al. Carbonic anhydrase inhibitors: Novel sulfonamides incorporating 1, 3, 5-triazine moieties as inhibitors of the cytosolic and tumour-associated carbonic anhydrase isozymes I, II and IX. Bioorg Med Chem Lett. 2005;15:3102-108.
- Sa?czewski F, S1awi_nski J, Kornicka A, et al. Carbonic anhydrase inhibitors. Inhibition of the cytosolic human isozymes I and II and the transmembrane, tumor-associated isozymes IX and XII with substituted aromatic sulfonamides activatable in hypoxic tumors. Bioorg Med Chem Lett. 2006;16:4846-851.
- Supuran CT, Scozzafava A, Casini A. Carbonic anhydrase inhibitors. Med Res Rev. 2003;23:146-89.
- Supuran CT. Carbonic anhydrases: Catalytic and inhibition mechanisms, distribution and physiological roles. In: Carbonic Anhydrase, Its Inhibitors and Activators. CRC Press: Boca Raton; 2004:1-23.
- Kivela AJ, Kivela J, Saarnio J, et al. Carbonic anhydrases in normal gastrointestinal tract and gastrointestinal tumours. J World Gastroenterol. 2005;11:155-63.
- Supuran CT, Scozzafava A. Carbonic anhydrases as targets for medicinal chemistry. Bioorg Med Chem. 2007;15:4336-350.
- Talley JJ, Brown DL, Carter JS, et al. 4-[5-Methyl-3-phenylisoxazol-4-yl]-benzenesulfonamide, Valdecoxib: A Potent and Selective Inhibitor of COX-2. J Med Chem. 2000;43:775-77.
- Thun MJ, Henley SJ, Patrono CJ. Nonsteroidal anti-inflammatory drugs as anticancer agents: Mechanistic, pharmacologic and clinical issues. Natl Cancer Inst. 2002;94:252-66.
- Wykoff CC, Beasley J, Watson H, et al. Gene array of VHL mutation and hypoxia shows novel hypoxia-induced genes and that cyclin D1 is a VHL target gene. Cancer Res. 2000;60:7075-083.
- Parkkila S, Rajaniemi H, Parkkila A, et al. Carbonic anhydrase inhibitor suppresses invasion of renal cancer cells in vitro. Proc Natl Acad Sci. USA 2000;97:2220-224.
- Svastova E, Hulikova A, Rafajova M, et al. Hypoxia activates the capacity of tumor-associated carbonic anhydrase IX to acidify extracellular pH. FEBS Lett. 2004;577:439-45.
- Cecchi A, Hulikova A, Pastorek J, et al. Carbonic anhydrase inhibitors. Design of fluorescent sulfonamides as probes of tumor-associated carbonic anhydrase IX that inhibit isozyme IX-mediated acidification of hypoxic tumors. J Med Chem. 2005;48:4834-841.
- Argyropoulou I, Geronikaki A, Vicini P, et al. Synthesis and biological evaluation of sulfonamide thiazole and benzothiazole derivatives as antimicrobial agents. In: Zani F, editor. Synthesis and biological evaluation of sulfonamide thiazole and benzothiazole derivatives as antimicrobial agents. Arkivoc. 2009:89-102.
- Demirbas N, Demirbas A, Karaoglu S, et al. Synthesis and Biological Activities of New 1, 2, 4-Triazol-3-one Derivatives. Arkivoc 2005:75-91.
- Zircle C, Kaiser C. In: Medicinal Chemistry. editor, Burger A; Wiley-Interscience: New York; 1970:1410.
- Shakya AK, Mishra P, Patnaik GK, et al. Synthesis and biological evaluation of novel 2-[substituted acetly]-amino-5-alkyl]-amino-5-alkyl-1, 3, 4-thiadiazoles. Arch Pharm Res. 1998;21:753.
- Pawar MJ, Burungale AB, Karale BK, et al. Synthesis, reactivity and electronic structure of multifarious, five-membered heteroaryl and heteroaroyl azides. Arkivoc. 2009:97.
- Schroder J, Henke A, Wenzel H, et al. Structure-based design and synthesis of potent matrix metalloproteinase inhibitors derived from a 6H-1, 3, 4-thiadiazine scaffold. J Med Chem. 2001;44:3231.
- Tschesche H, Schroder J, Pat EP. 3D Structure and Drug Design. Appl. 2002:127-50.
- Sugawara H, Endoh M. (-)-Enantiomer EMD 57439 antagonizes the Ca2+ sensitizing effect of (+)-enantiomer EMD 57033 on diastolic function but not on systolic function in rabbit ventricular cardomyocytes. Jpn J Pharmacol. 1999;80:55.
- Himmel HM, Amos GJ, Wettwer E, et al. Effects of the calcium sensitizer [+]-EMD 60263 and its enantiomer [-]-EMD 60264 on cardiac ionic currents of guinea pig and rat ventricular myocytes. J Card Pharm. 1999;33:301.
- Dogan HN, Duran A, Rollas S, et al. Synthesis of new 2, 5-disubstituted-1, 3, 4-thiadiazoles and preliminary evaluation of anticonvulsant and antimicrobial activities. Bioorg Med Chem. 2002;10:2893.
- Carvalho SA, Da-Silva E, Santa-Rita RM, et al. Synthesis and antitrypanosomal profile of new functionalized 1, 3, 4-thiadiazole-2-arylhydrazone derivatives, designed as non-mutagenic megazol analogues. Bioorg Med Chem Lett. 2004;14:5967.
- Swamy SN, Basappa, Priya BS, et al. Synthesis of pharmaceutically important condensed heterocyclic 4, 6-disubstituted-1, 2, 4-triazolo-1, 3, 4-thiadiazole derivatives as antimicrobials. Eur J Med Chem. 2006;41:531.
- Kalluraya B, Gururaja RE, Ganesha R. One pot reaction: Synthesis, characterization and biological activity of 3-Alkyl/Aryl-9-substituted-1, 2, 4-triazolo [3, 4-b] [1, 3, 4] quinolinothiadiazepines. Indian J Chem. 2003;42:211.
- Dandia A, Singh R, Khaturia S. Microwave enhanced solid support synthesis of fluorine containing benzopyrano-triazolo-thiadiazepines as potent anti-fungal agents. Bioorg Med Chem. 2006;14:1303.
- Duan J, Chen L, Chemey RJ, et al. P1, P2'-Linked macrocyclic amine derivatives as matrix metalloproteinase inhibitors PCT. Int Appl WO. 1999;41:246.
- Albrecht WL, Sweet FW.US Pat 1976;3:954-83. Chem Abstr.1976;781-72.
- Albrecht WL, Jones W. US Pat 1976;3:954-81. Chem Abstr.1976.
- Molina P, Alajarin M, Perez MJ, et al. Synthesis of 6, 7-dihydro-5H-1, 2, 4-triazolo[3, 4-b] [1, 3, 4] thiadiazines by a C–C ring cyclization under mild conditions. J Chem Soc Perkin Trans I. 1987:1853.
- Reddy V, Reddy K. Naproxen in heterocyclic chemistry: Novel syntheses of triazoles, triazolothiadiazines, triazolothiadiazoles and triazolothiadiazepine bearing an asymmetric carbon atom and radiostability of the biologically active compounds. Chem Pharm Bull. 2010;58(8):1081;
- Ammar YA, Ghorab MM, El-Sharief, et al. Heteroatom Chem. 2002;13:199.
- Kalluraya B. Ph D Thesis, Bangalore University, India, 1987.
- Gonzalez-Munoz GC, Arce MP, Lopez B, et al. Old phenothiazine and dibenzothiadiazepine derivatives for tomorrow’s neuroprotective therapies against neurodegenerative diseases. Eur J Med Chem. 2010;45:6152.
- Dalloul HM. Can Chem Trans. 2015;3:108-17.
- Hany Dalloul M, Khaled El-nwairy A, Ali Shorafa Z, et al. On reactions of thiadiazinones: Synthesis of new 6-arylidene-1, 3, 4-thiadiazin-5-ones. Inter J Adv Res Chem S. 2016;3:1-9.
- Thaher BA, Otto HH. On reactions of thiadiazinones: Synthesis of new 6-arylidene-1, 3, 4-thiadiazin-5-ones Monatsh. Chem. 2002;133:1011.
- Turnidge J, Bordash G. Clinical Laboratory Standards Institute, “Methods for dilution antimicrobial susceptibility tests for bacteria grow aerobically”, Approved Standard M7-A4, Clinical and Laboratory Standards Institute, Wayne, Pa, USA, 2005.
- Irobi ON, Moo-Young, M Anderson WA. Inter J Pharm. 1996;34:87-90.
- Grayer RJ, Harborne JB. Phytochemistry. 1994;37:19-42.
- Richard A, Van Enk. Susceptibility testing of drugs and organisms not in NCCLS guidelines. National Committee on Clinical Laboratory Standards (NCCLS), Ph.D. Bronson Methodist Hospital Kalamazoo, Michigan, 2004.