Original Article
, Volume: 10( 6)Surface Modification of Cationic Dendrimers Eases Drug Delivery of Anticancer Drugs
- *Correspondence:
- Karthikeyan R , Department of Biotechnology, Acharya Nagarjuna University, Nagarjuna Nagar 522510, Guntur, India, Tel: 0863 234 6114
E-mail: rkcognosy@gmail.com
Received: September 09 2016; Accepted: September 30, 2016; Published: October 08, 2016
Citation: Karthikeyan R, Koushik OS and Kumar VP. Surface Modification of Cationic Dendrimers Eases Drug Delivery of Anticancer Drugs. Nano Sci Nano Technol. 2016;10(6):109.
Abstract
The development of dendritic architecture with well-defined size, shape and controlled exterior functionality holds promise in pharmaceutical applications such as drug delivery, solubilisation, DNA transfection and diagnosis. Highly branched, monodisperse, stable molecular level and low polydispersity with micelle-like behavior possessing nano-scale container property distinguish these structures as inimitable and optimum carrier for those applications. However reticuloendothelial system (RES) uptake, drug leakage, immunogenicity, haemolytic toxicity, cytotoxicity, hydrophobicity restricts the use of these nanostructures. PEGylation of dendrimers can generally overcome these shortcomings. Haemolytic and different cell line studies have shown reduced toxicity of PEGylated dendrimers than cationic dendrimers. PEGylation causes increased solubilisation of hydrophobic drugs in dendritic framework as well as in PEG layers. Attachments of targeting moiety on the surface of partially PEGylated dendrimer created much interest as a delivery system for crossing of biological barriers and deliver the bioactive agent near the vicinity of target site. This review focuses on the current state of the art in the field and focuses on the potential of PEGylated dendrimers in pharmaceutical area.
Keywords
PEGylation; Drug delivery; Solubilisation; Cytotoxicity; Haemolysis
Introduction
Cancer is a leading cause of death worldwide. Cancer is the second biggest cause of death in India, growing at 11% annually. From a total of 58 million deaths worldwide in 2005, cancer accounts for 7.6 million of all deaths. Deaths from cancer in the world are projected to continue rising, with an estimated 9 million people dying from cancer in 2015 and 11.4 million dying in 2030. The burden of cancer doubled globally between 1975 and 2000. It is estimated that it will double again by 2020 and nearly triple by 2030 [1].
Challenges in cancer drug therapy
The clinical outcome of treatment with many anticancer drugs has not been met with universal success as hoped. The major hurdle of current anticancer drugs is to kill tumor cells specifically without significant side effects. The theoretical basis, on which existing anticancer drugs exert their effects, relies on the higher mitotic rate in the tumor cells than that of normal cells. This often results in high systemic toxicity and the therapeutic window is narrow. Repeated dosage is thus limited. The concept of specific targeting has emerged and is crucial to help reduce uptake by normal tissue and increase the payload of the drug inside the tumor. Furthermore, as more than 40% of anticancer drug is poorly soluble in aqueous environment, the ultimate bioavailability and therapeutic efficiency can be significantly hampered. Conventionally, solvents and emulsifiers have been used to dissolve poorly water soluble anticancer drugs. They are suggested to be potentially carcinogenic and can be toxic to liver and nervous system. Polymer chemistry and technology have traditionally focused on linear polymers, which are widely in use. Linear macromolecules only occasionally contain some smaller or longer branches. In the recent past it has been found that the properties of highly branched macromolecules can be very different from conventional polymers. The structure of these materials has also a great impact on their applications. Hyper branched molecules were called dendrimers. The term originates from ‘dendron’ meaning a tree in Greek. In addition to that arborols from the Latin word ‘arbor’ also meaning a tree and the term cascade molecule is also used, but ‘dendrimer’ is the best established one [2-5].
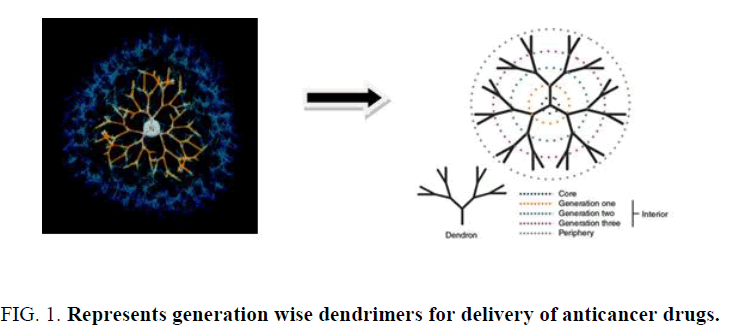
Dendrimers are highly branched synthetic polymers. It consists of center core, integral region and numerous functional end groups (Figure 1). The growth of polymer occurs in outward direction from central core by stepwise polymerization. It is characterized with numerous cavities in the core structure creating channels and cages. Precise control of size can be achieved by the extent of polymerization. The intracellular uptake of dendrimers by receptor mediated endocytosis can be aided using the conjugation of biotin. Dendrimers contribute to drug delivery either by binding the drug on the periphery as prodrug or entrapping the drug in the center core. Recent progress has been made to dendrimer formulary as a biocompatible drug carrier for cancer targeting therapy. Surface of dendrimer enables the coating with poly(ethylene) glycol (PEG) or targeting ligand for folate receptors and showed potential in improved cellular targeting for cancer therapy. Drug molecules can be incorporated into dendrimers via either complexation or encapsulation. Dendrimers are being investigated for both drug and gene delivery, as carriers for penicillin, and for use in anticancer therapy. Dendrimers used in drug delivery studies typically incorporate one or more of the following polymers: polyamidoamine (PAMAM), melamine, poly(L-glutamic acid) (PG), polyethyleneimine (PEI), poly(propylene imine) and poly(ethylene glycol) (PEG) Chitin and chitosan have also been incorporated with dendrimers.
PEGylation
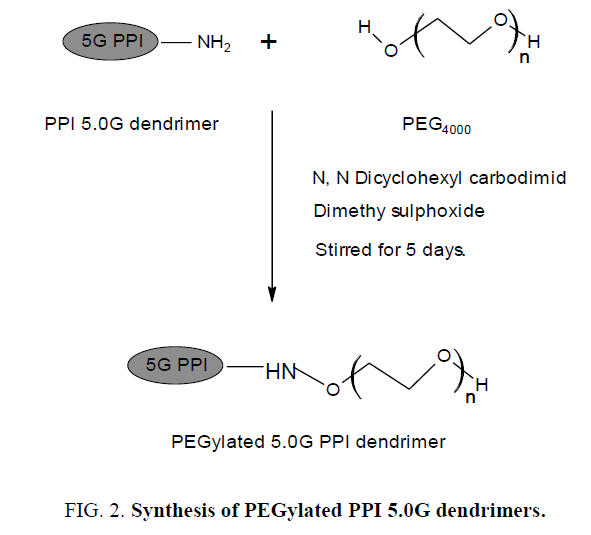
It is the process of covalent attachment of polyethylene glycol (PEG) polymer chains to another molecule, normally a drug or therapeutic protein. PEGylation is routinely achieved by incubation of a reactive derivative of PEG with the target molecule. The covalent attachment of PEG to a drug or therapeutic protein can "mask" the agent from the host's immune system, and increase the hydrodynamic size of the agent which prolongs its circulatory time by reducing renal clearance (Figure 2). PEGylation can also provide water solubility to hydrophobic drugs and proteins [6].
PEGylated dendrimers in cancer technology
PEGylated dendrimers in cancer warfare resemble mini-submarines, which offer an effectual mode to lug high payloads relatively unharmed, through blood to hit cancer. PEGylated dendrimers not only drastically augment drug loading and solubilisation, but also annihilate the drawbacks associated with the naked dendrimeric scaffolds viz; hemolytic toxicity, uncontrolled drug-outflow, macrophageal uptake, short half-life etc. PEGylation appreciably improves the stability kinetics and makes them efficacious towards extended delivery of bioactive species. This PEGylation can also be supportive in targeting the system to the active sites of action, with reduced immunogenicity, anti-genecity and toxicity by shielding the system against destructive mechanisms of the body. Often this concept helps in avoiding situations as seen in gene therapy. PEGylated dendrimers in cancer technology had been studied extensively and this plethora of existing studies can be discussed more eloquently by aligning them under following headings [7].
PEGylated dendrimers in solubilisation and controlled release of chemotherapeutics
A poorly water-soluble anticancer candidate manifests several in-vivo consequences: hampered bioavailability, raised probability of food effect, unfinished release from the formulation, and also greater interpatient variability. Poor water solubility also often accounts for in vitro obstacles such as limited choices of delivery technology, complicated dissolution testing, and/or poor in vitro: in vivo correlation. Poorly soluble but effective anticancer drugs such as cisplatin and MTX motivated the development of new delivery devices to overcome the obstacles on the way to their solubilisation. A number of technologies have been applied for solubilizing anticancer entities, ranging from traditional particle size reduction via comminution, spray drying, and micellar solubilisation to cyclodextrin-mediated inclusion complexes. Though Cyclodextrin- and micelle-mediated enhances solubility, but the high cost and nephrotoxicity of the former and the disruption by dilution of the micellar structure of the latter limit the use of these techniques. At present, PEGylated dendrimers are an innovative class of dendrimers with the additional advantage of attached PEG-chains, which not only enhances their solubilisation ability but also augments surface crowding and thereby enables controlled release from the dendrimeric scaffold. An early model demonstrating the advantage of the stepwise synthesis and the controlled multivalency of PEGylated dendrimers for drug delivery was put forward wherein it was possible by using a careful synthetic approach to attach both hydrophobic drugs and polyethylene oxide (PEO) moieties to the dendrimer periphery in a controlled manner. Hence this approach can be considered a hybrid of drug-conjugated and PEGylated dendrimers. In addition, the potential of dendritic unimolecular micelles for drug delivery using both the container and sustained drug releasing properties of such systems. released. Since drug release is accomplished by dialysis, targeted delivery would be difficult to attain, but sustained release would be easier to achieve. Furthermore, as the encapsulation via the dendrimer varied appreciably depending on the drug and the dendrimer configuration, this method would be quite tricky to make universal for all drugs.
The effects of polymeric architecture on the solubilisation and release of paclitaxel, a poorly water-soluble drug. The effect of the density of ethylene glycol chains on the solubility enhancement of paclitaxel was investigated. This group synthesized the poly (oligo ethylene glycol) methacrylate (OEGMA), star shaped poly(OEGMA), and polyglycerol 3.0, 4.0, and 5.0 G dendrimers. Poly (OEGMA) increased the paclitaxel solubility, but a much more significant effect was observed with the five-arm star poly (OEGMA). The aqueous solubility produced by 10% star-shaped poly(OEGMA), and by 3.0, 4.0, and 5.0 G polyglycerol dendrimers, respectively, were 130-, 270-, 370-, and 434-fold greater than the unenhanced paclitaxel solubility in water. These data are sufficient to conclude that polyglycerol dendrimers are much more effective in increasing the paclitaxel solubility than are the others under study. This is likely due to the increased local density of the ethylene glycol unit. Even with relatively similar molecular weight (Mw: 1690) and concentration (50 wt%) of the 3.0 G dendrimers to PEG2000 (Mw: 2000), the paclitaxel solubility was raised 11-fold over that of PEG2000. Polyglycerol dendrimers dissolved in water at high concentrations without significantly increasing the viscosity and, at 80 wt%, were found to increase the solubility of paclitaxel 10000-fold. This proposed star dendritic polymer could be modified further in the future to serve as a useful tool for both oral and parenteral delivery of paclitaxel and other poorly water-soluble drugs [8].
PEGylated dendrimers in targeted drug delivery
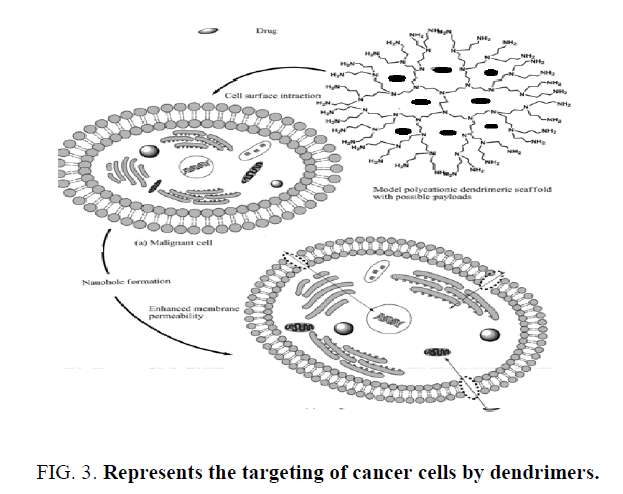
Dendritic architecture provides a great opportunity for trans-barrier (Gastrointestinal tract or Blood Brain Barrier) delivery of drugs and peptides. PAMAM dendrimers, shown have much higher rates of transport across the rat intestinal tissue as compared to linear polymers particularly those bearing –COOH group at their periphery, have shown that PEG-polyester dendrons of particular molecular weight and particular generation can show exceptionally high rates of exocytosis following internalization by the endothelial cell line ECV304. The synthesized novel, branched PEG-based dendritic architectures for studying their cytotoxicity by preparation of Oregon green (OG) conjugates and defining the effect of structure on their cellular uptake (Figure 3). Five PEG-based den-dimeric structures were synthesized using monodisperse Fmoc-amino PEG propionic acid as a monomer, and cadaverine, tris(2-aminoethyl) amine or lysine as the branching unit. The dendrimers were modified by diamino, bis-PEG (Mw 1300); tri amino, tris-PEG (Mw 1946); tetra amino, tetra- PEG (Mw 3956); monocarboxy, diamino, bis -PEG (Mw 1346); and monocarboxy, tetra amino, tetra-PEG (Mw 3999). These products had either –NH2 or both –NH2 and –COOH groups at their periphery. All five PEG-based dendritic structures showed progressive uptake by endothelial-like cell line (ECV304 cells), in this tetramino, tetra-PEG showed highest rate of internalization in first 20 min. The polyester dendritic system based on 2,2-bis(hydroxymethyl)propanoic acid as monomer unit. For the assembly of dendrimer, a monoprotected tri-sphenolic core was prepared by reacting an excess of 1, 1, 1-tris (4-hydroxyphenyl) ethane with benzyl chloroformate. To this core the G-4 dendron were attached. Dendritic structures were PEGylated and conjugated Doxorubicin and targeting moiety 4-methoxyphenyl acetic acid. These systems were shown to have their intended use as drug carriers, including water solubility, nontoxicity, and stability of the polymeric backbone. Branched poly (L-glutamic acid) (PG) containing multiple PG chains centered on a PAMAM dendrimer or PEI cores. Unlike conventional linear PG, branched PG possessed multiple terminal amino groups which make possibility of attaching multiple targeting moieties selectively to the termini of branched PG. PEG conjugation to the chain ends of branched PG was demonstrated in the presence of side chain carboxyl groups. Additionally, folic acid, a model targeting moiety, and the near infrared dye indocyanine green, a model diagnostic agent, were effectively conjugated to the terminal amino groups and the side chain carboxyl groups of branched PG, correspondingly. These conjugates resulted in reduced non-specific interaction and caused selective binding to tumor cells expressing folate receptors [9].
PEGylated dendrimers enhance long circulation of system in blood
A scantily water-soluble anticancer candidate manifests several in vivo consequences viz; hampered bioavailability, raised probability of food effect, unfinished release from the formulation as well as higher inter-patient variability. Scanty water solubility often accounts for quite a few in vitro obstacles; such as inadequate choices for delivery technology, complicated dissolution testing and/or poor in vitro: in vivo correlation. Scantily soluble, but competent anticancer drugs such as cisplatin and methotrexate motivated the development of new delivery devices to overcome the obstacles on the way to their solubilisation. A number of technologies have been applied for solubilizing anticancer entities, ranging from traditional particle size reduction via comminution, spray drying, micellar solubilisation to cyclodextrin mediated inclusion complexes. Extensive literature reporting cyclodextrin and micelle mediated solubility enhancement is available. However high costs and nephrotoxicity of former and disruption of micellar structure of later upon dilution limit their use. At present, PEGylated dendrimers represents an innovated class of dendrimers with additional advantage of attached PEG-chains, which not only enhances the solubilizing ability but also augments surface crowding thereby imparts controlled release property to the dendrimeric scaffold. An early model demonstrating the advantage of the stepwise synthesis and the controlled multivalency of PEGylated dendrimers for drug delivery was put forwarded where upon by using a careful synthetic approach it is made possible to attach both hydrophobic model drugs and PEO moieties to the dendrimer periphery in a controlled manner. Hence this concept can be assumed to be a hybrid of drug conjugated and PEGylated dendrimers. The potential of dendritic unimolecular micelles in drug delivery systems employing the container and sustained drug releasing property. The effect of the density of ethylene glycol chains on the solubility enhancement of paclitaxel. This group synthesized the poly (oligo ethylene glycol) methacrylate (OEGMA), star shaped poly OEGMA and poly glycerol 3.0, 4.0, 5.0 G dendrimers. Poly (OEGMA) increased the paclitaxel solubility, but a much more significant effect was observed with the five-arm star poly (OEGMA). The aqueous solubility increased by 10%-star shaped poly OEGMA, 3.0, 4.0 and 5.0 G polyglycerol dendrimers were respectively 130, 270, 370 and 434 folds more than the paclitaxel solubility in water. This data is enough to state that poly glycerol dendrimers were much more effective than others under study, in increasing the paclitaxel solubility. This is likely due to the increased local density of ethylene glycol unit. Even with relatively similar molecular weight (Mw: 1690) and concentration (50 wt. %) of the 3.0 G dendrimers to PEG2000 (Mw:2000), the paclitaxel solubility was raised 11-fold higher than that of PEG2000. Polyglycerol dendrimers dissolved in water at high concentrations without significantly increasing the viscosity and, at 80 wt. %, were found to increase the solubility of paclitaxel 10,000-fold. This proposed star dendritic polymer could further be manipulated in future to serve as a useful tool for both oral and parenteral delivery of paclitaxel and other poorly water-soluble drugs. synthesized 4.0 G, 5.0 G polyglycerol dendrimers were used to investigate the effect of dendritic architecture and generation on the aqueous solubilisation of paclitaxel, a poorly water-soluble drug. The paclitaxel solubility in all the solutions of polyglycerol dendrimers, even below 10-wt%, was much higher than that in PEG400 which is commonly employed as a co solvent or a hydrotropic agent. Increase in the paclitaxel solubility was found to be a function of dendrimer generation. 1H NMR spectra of paclitaxel before and after mixing with PGDs in D2O suggested that the aromatic rings and some methyne groups of paclitaxel were surrounded by polyglycerol dendrimers, hence providing an excellent alternative skill for hydrotropic solubilisation of poorly soluble drug. With an aim of achieving both solubilisation and sustained release benefits, synthesized dendrimeric construct by combining the chain ends of 3.0 G and 4.0 G PAMAM dendrimers with poly (ethylene glycol) monomethyl ether via urethane bond.
The anticancer drugs methotrexate, a practically water insoluble drug and adriamycin, a hydrophobic entity was encapsulated within the hydrophobic interior of the PEGylated PAMAM dendrimer set. Talent of the system to encapsulate these anticancer entities improved with improving dendrimer generation and chain extent of PEG grafts. Among the PEG attached dendrimers, the highest ability was achieved with the 4.0 G-MPEG-2000, which could retain 6.5 adriamycin or 26 methotrexate molecules per dendrimer molecule. It was felt that adriamycin was solubilized and complexed on the chain surface of MPEG, while in case of methotrexate, an acidic drug encapsulation efficiency is raised due to increased electrostatic interaction owing to acid base feedback between methotrexate and dendrimer. The methotrexate loaded PEG dendrimers released the drug slowly in an aqueous solution of low ionic strength. However, in isotonic solutions, methotrexate and adriamycin were readily released. Since the drug release is accomplished by dialysis, targeted delivery would be difficult to attain, but sustained release would be easier to achieve. Furthermore, as the encapsulation via the dendrimer varied appreciably depending on the drug and the dendrimer configuration, this method would be quite tricky to make universal for all drugs. Apart from drug attachment, dendrimers also provide the right of entry into various new polymer architectures with potentiality to provide some extraordinary service as drug delivery device.
For case in point, the design and preparation of new polyester dendrimers and poly (ethylene oxide) hybrid systems for drug delivery and related therapeutic applications along with its accumulation studies in solid tumors. These systems consist of covalently attached polyester dendrons, where one dendron provides multiple functional handles for the attachment of therapeutically active moieties, while the other is used for attachment of solubilizing poly (ethylene oxide) chains. These new carriers are nontoxic and biodegradable in vitro, and bio distribution studies in vivo have shown that carriers with a molecular weight of more than 40,000 are generally long circulating with half-lives greater than 24 h. The carrier excreted at a slower rate into the urine by glomerular filtration, probably as a consequence of their decreased flexibility and ability to diffuse through pores relative to linear polymers. Substantial levels of long circulating bow-tie polymers showed its accumulation in subcutaneous B16F10 solid tumors via the EPR effect, establishing promising claim of these carriers in cancer technology. In view of the fact that macromolecules conjugated with polyethylene glycol acquire higher hydrophilicity resulting in a longer half-life in circulation and lower immunogenicity, the concept of PEGylated dendrimers to develop as MRI contrast agents employing 2.0 G and 3.0 G PAMAM dendrimers. They synthesized macrocycle 1-(4-isothiocyanatobenzyl) amido-4, 7, 10-triacetic acid-tetraazacyclododecane (D03A-bz-NCS) and coupled to the terminal amine sites of starburst PAMAM dendrimers (n-SBDs) creating macromolecular polychelates. Also, gadolinium ion was added to the dendrimer polychelates. The resulting complex n-SBD-GdD03As were water-soluble and mono-disperse. There was an increase in blood elimination half-life with molecular weight ranging from 11 min for 3-SBD-(GdD03A), (22 kDa) to 115 min for the 5-SBD-(GdD03A), (61.8 kDa), when studied in rat model. The seven-day liver retention increased from 1% to 40% over the same molecular weight range. They also studied the effects of grafting PEG moieties onto n-SBD-GdD03As polychelates. Blood elimination half-lives increased significantly (range 33-1219 min) and the seven-day liver uptake was also dropped to 1% to 8% of the injected dose. Addition of covalently bound PEG to the n-SBD-GdD03As surface significantly improves the biological performance of the contrast agent. The synthesized two novel conjugates for MRI contrast agents from 4.0 G PAMAM dendrimers, 2-(p-isothiocyanatobenzyl)-6- methyl-diethylenetriamine pentaacetic acid (1B4M), with one or two PEG molecules with a molecular weight of 20,000 Da to yield PEG2-PAMAM-(1B4M-Gd) and PEG1-PAMAM-(1B4M-Gd) conjugate. Then they evaluated the pharmacokinetics, excretion, and properties as vascular MRI contrast agents and compared with those of non-PEGylated counterparts. PEG2-G4D (1B4MGd) conjugate remained in the blood for significantly longer time and showed less accumulation in the liver and kidney than the other two preparations. In conclusion, the major positive effects of PEG conjugation on a PAMAM constructed MRI contrast agent were found to be prolonged retention in the circulation and decreased accumulation in the organs [10-14].
Role of PEGylated dendrimers in reducing cytotoxicity and haemolysis
Regardless of the extensive interest in the pharmaceutical and biomedical applications of dendrimers, there is contradictory evidence regarding their biological safety. Dendrimer related toxicity is dependent on the chemistry of the core, while more strongly influenced by the nature of the dendrimeric surface functionality. Cationic dendrimers bearing –NH2 termini display concentration and usually generation dependent cytotoxicity. Cationic PAMAM dendrimers have been shown to be hemolytic, a property that was associated with their cationic nature. When cationic PAMAM dendrimers were incubated with V79 Chinese hamster lung fibroblast for 24 h they caused decrease in cell viability or changed integrity of cell structure. The cytotoxicity of cationic PAMAM dendrimers is thought to be the result of the interaction between positively charged dendrimers and negatively charged cell surfaces. The cytotoxicity study of the cationic melamine dendrimers having surface groups like amine, guanidine, carboxylate, sulfonate or phosphonate showed that cationic dendrimers were much more cytotoxic than anionic or PEGylated dendrimers. Increase in the branching or generation and covering the surface with biocompatible terminal groups like PEG are being widely used to synthesize less toxic biocompatible dendrimers. The influence of surface modification on the cytotoxicity of PAMAM dendrimers was examined. Using Caco-2 cells (human intestinal adenocarcinoma cell line). Dendrimers were modified by PEG-2000 onto the surface of cationic PAMAM dendrimers (G2, G3, and G4). They compared the effect of whole and half generation PAMAM dendrimers on the surveillance of Caco-2 cells and investigated the effect of cytotoxicity of dendrimer surface modified by PEG. The PEGylation (PEG 2000) of PAMAM dendrimers was achieved by the addition of tresylated poly-ethylene glycol monomethyl ether (mPEG-OT) to an aqueous solution of PAMAM dendrimer (G4), by adjusting pH to 8.0 with 0.1 M HCl. The modification of cationic dendrimers with PEG shields the positive charge on the dendrimer sur-face and leads to a decrease in cytotoxicity [15-21].
PEGylated dendrimers: In maintenance of in vivo stability
Dendrons based on aspartic acid units and Ara-C conjugated via its amine group by various linkers including amides and carbamates. This stratagem was found to exhibit improvement in the in vivo stability and blood residence time of the drug and to increase its stability towards degradation. It should be noted that in contrast to PEGylation of active bioactive moiety PEGylated drug loaded system imparts a number of advantages including retention of bioactivity, bioenvironmental protection heading up this pool with dendrimers. The synthetic strategies for conjugating 1-Beta-D arabinofuranosylcytosine (Ara- C) via amino groups to carboxy groups of dendron. But the basic problem with the system was low loading efficiency of the polymer, which, in turn, was tried to improve by functionalizing the hydroxyl functions of PEG with a bicarboxylic amino acid yielding a tetra functional derivative. As a general rule, large size body tends to show slow renal filtration. High molecular weight polymers (>20,000 Dalton) have been widely used as soluble drug carriers to improve drug targeting and therapeutic efficacy; dendritic polymers represent one of outstanding candidate. Hence, one move toward increasing the half-life is to make the dendrimer larger. PEO is a biocompatible polymer. One hybrid performance was made covalently conjugated doxorubicin via a hydrazone linkage to a high molecular weight 3-arm poly (ethylene oxide)-dendrimer hybrid. Their goal was to have a long circulating in vivo stable conjugate with drug release property as a function of pH. As expected drug release from this conjugate was more rapid when exposed to conditions of pH<6. The cytotoxicity of the doxorubicin-polymer conjugate determined on multiple cancer lines in vitro was found to be reduced though was not eliminated completely, indicating that some amount of active doxorubicin was also escaping from the drug polymer conjugate under physiological conditions. At a standstill, the outcomes showed that the serum half-life of doxorubicin attached to high molecular weight polymer had been significantly increased; in addition, little doxorubicin-polymer conjugate gets accumulated in vital organs, when compared to that of free doxorubicin. pH responsive and controlled release of drug from the encapsulating micellar compartment suggested another novel approach. Hybrids of poly (ethylene oxide) and either a polylysine or polyester dendron with attached pH responsive hydrophobic acetal groups on the periphery of dendrimeric scaffold by highly acid-sensitive cyclic acetals. At mildly acidic pH, loss of hydrophobic groups upon acetal hydrolysis triggers micellar disruption followed by payload release. To demonstrate the potential of these systems for controlled release, the release of Nile Red as a "model payload" was examined. At pH 7.4, the fluorescence of construct encapsulated Nile Red was relatively constant, indicating it was retained in the micelle, while at pH 5, the fluorescence decreased which is consistent with its release into the aqueous milieu. The rate of release showed strong correlation with the rate of acetal hydrolysis, which in turn often controls the release rate [22-27].
PEGylated dendrimers: towards biocompatibility of anticancer drugs and the system
It is at the moment well clear to all that dendrimers possess a great potential to be exploited as a drug delivery device in cancer chemotherapy. But before proceeding towards the next generation designing it is highly compulsory that biocompatibility plus toxicity of dendrimers as well as the chemotherapeutic loaded dendrimer be fully understood and cured, aimed at developing and exploring the use of PEGylated PAMAM dendrimers for delivery of poorly water-soluble anti-cancer drug, 5-fluorouracil (5-FU). This PEGylated system was typified with improved hemolytic toxicity and rate of drug release; along with well-expected enhanced loading and solubilizing capacity. The hematological studies showed that there was significant increase in WBC and lymphocyte count in the case of non-PEGylated systems. Upon PEGylation of dendrimers, the hemolysis of the RBCs was found to decline significantly below 5%. This may be due to inhibition of interaction of RBCs with the charged quaternary ammonium ion in the presence of PEG chains. The blood level was much prolonged and was detectable up to 12 h. The mean residence time for both non-PEGylated and PEGylated dendrimer–drug complexes as compared to plain drug solution was recorded to be 6.024 and 13.31 folds, respectively; suggesting significant prolonged release. The 5-FU entrapment in PEGylated dendrimers increased significantly by about 12 folds, due to supplementary sealing of dendrimeric periphery by MPEG, which prevented drug release by enhancing drug complexation, probably by steric and electronic effects of the supplementary functional groups made available by MPEG.
The average release rate for PEGylated dendrimeric system was found to be 0.679%, which was nearly 1/6th times that from non-PEGylated types. Such systems were found to be a suitable runner for extended delivery of anti-cancer drug, in vitro and in vivo, prepared the library of six surface modified dendrimers based on melamine that varied in the chemistry of the surface group (amine, guanidine, carboxylate, sulfonate, phosphonate, and PEGylated) that were evaluated for hemolytic potential and cytotoxicity. Cationic dendrimers were found to be more cytotoxic and hemolytic than anionic or PEGylated dendrimers. The group also reported that the PEGylated dendrimer when assayed for in vivo acute toxicity in mice showed no toxicity, neither mortality nor abnormal blood chemistry. Thus these mini-submarines have been proved to be an efficient move toward reducing drug leakage, hemolytic toxicity, renal filtration and raising in vivo stability and biocompatibility. But, still the purpose of efficient cancer chemotherapy has not reached its goal completely, due to lack of selective, specific and least toxic delivery mode, so as to bring dendrimers as generally regarded as safe (GRAS) level [28-37]. Hence to fulfill the requirements of this concept some innovative classes of dendrimers came into being such as Liposomal “locked in” dendrimers represented in Table 1.
| S.No | Type of scaffold | Bioactive studied | Outcomes of study |
|---|---|---|---|
| 1 | PEGylated PAMAM | MTX/adriamycin | Solubilisation and sustained release benefits. |
| 2 | PEGylated PAMAM | 1-(4-isothiocyanatobenzyl) amido-4,7,10-triaceticacid-tetraazacyclododecane and GadoliniumTU-DTPA | Effect of PEG grafting on biological half-life.Assess blood residence and bio distributionpattern. |
| 3 | PEGylated PAMAM | Paclitaxel | To investigate the effect of the density of ethylene glycol chains on the solubility enhancement. |
| 4 | star-shaped poly OEGMA; PGDs | Paclitaxel | Solubilisation was more in case of PGDs as compared to PEG400, a frequently used solubilizing agent |
| 5 | PGDs | - | Cationic dendrimers were found to be more cytotoxic and haemolytic than anionic or PEGylated dendrimers. |
| 6 | Dendrimers based on melamine | Ara-C | To improve in vivo stability, blood residence time and drugs stability. |
| 7 | PEO-dendrimer hybrids | Ara-C | To improve in vivo stability, blood residence time and drugs stability. |
| 8 | PEO-dendrimer hybrids | Doxorubicin | To enhance drug loading, in vivo stability and residence time. |
| 9 | PEO-dendrimer hybrids | 5-fluorouracil | To have a long circulating, in vivo stable, pH responsive drug releasing carrier. |
| 10 | PEGylated PAMAM dendrimer | Nile Red (model construct) | To reduce hemolytic toxicity and rate of drug release; along with enhanced solubilizing and loading capacity. |
| 11 | Either polylysine or polyester dendron | - | The rate of release showed strong correlation with the rate of acetal hydrolysis (pH responsive hydrophobic group) which in turnoften controls the release rate to develop a polymer drug delivery. It showed selective accumulation in solid tumours via EPR effect. |
Table 1: Status of PEGylated Dendrimers in different level of treatment.
Conclusion
PEGylation has emerged as an important tool for optimization of drug therapy. PEGylated dendrimers offer even more promising strategies because of the possibility of sur-face modification and hence interacting with changed functional groups. Encapsulation and/or attachment of a variety of molecules, both hydrophilic and hydrophobic may solve some intriguing problems in drug therapy. Prolonged circulation and sustained release, ability to cross biological barriers, delivering the drug at or near the target site, low polydispersity and cytotoxicity, enhanced solubilisation propensity and finally the nanoscopic size make PEGylated dendrimers an attractive tool for pharmaceutical application.
References
- Jemal A, Siegel R, Xu J, et al. Cancer Statistics, 2010. CA Cancer J Clin. 2010;60(5):277-300.
- Fauci AS. Harrison’s principles of internal medicine. 23rd ed.New York: McGraw Hill Medical, USA; 2008.
- Mu L, Feng SS. PLGA/TPGS nanoparticles for controlled release of paclitaxel effects of the emulsifier and drug loading ratio. Pharm Res. 2003;20(11):1864-72.
- Berlin K, Edling C, Persson B, et al. Cancer incidence and mortality of patients with suspected solvent- related disorders. Scand J Work Environ Health. 1995;21(5):362-7.
- Maeda H. The enhanced permeability and retention (EPR) effect in tumor vasculature: the key role of tumor-selective macromolecular drug targeting. Adv Enzyme Regul. 2001;41:189-207.
- Storm G, Belliot SO, Daemen T, et al. Surface modification of nanoparticles to oppose uptake by the mononuclear phagocyte system. Adv Drug Deliv Rev. 1995;17(1):31-48.
- Kallay N, Zalac S. Stability of nanodispersions: a model for kinetics of aggregation of nanoparticles. J Colloid Interface Sci. 2002;253(1):70-6.
- Moghimi SM, Hunter AC, Murray CJ. Nanomedicine: current status and future prospects. Faseb J. 2005;19:311-30.
- Li S.D, Huang L.Pharmacokinetics and biodistribution of nanoparticles. Mol Pharm. 2008;5(4):496-504.
- Pirollo KF, Zon G, Rait A, et al. Tumor-targeting nanoimmunoliposome complex for short interfering RNA delivery.Hum Gene Ther. 2006;17(1):117-24.
- Begley DJ. ABC transporters and the blood-brain barrier. CurrPharm Des. 2004;10(12):1295-312.
- Domenech J, Alba M, Morera JM, et al. Gastric, intestinal and colonic absorption of metoprolol in rat. Br J ClinPharmacol. 1985;19(S2):S85-9.
- Snoeck V,Coxa E, Verdonck F, et al. Influence of porcine intestinal pH and gastric digestion on antigenicity of F4 fimbriae for oral immunisation. VeterMicrobiol. 2004;98(1):45-53.
- Prajakta D, Ratnesh J, Chandan K, et al. Curcumin loaded pH-sensitive nanoparticles for the treatment of colon cancer. J Biomed Nanotechnol. 2009;5(5):445-55.
- Margolin JF, Poplack DG, Steuber CP.Acute lymphoblastic leukemia. In:Pizzo P, Poplack D, eds. Principles and Practice of Pediatric Oncology,Philadelphia: Lippincott-Raven, USA; 1997.
- Golub T, Weinstein H, Grier H. Acute myelogenous leukemia. In:Pizzo P, Poplack D, eds. Principles and Practice of Pediatric Oncology, Philadelphia: Lippincott-Raven, USA; 1997.
- Cheson BD, Cassileth PA, Head DR, et al. Report of the National Cancer Institute sponsored workshop on definitions of diagnosis and response in acute myeloid leukemia. J ClinOncol. 1990;8(5):813-9.
- Smith M, Chen T, Simon R. Age-specific incidence of acute lymphoblastic leukemia in U.S. children: in utero initiation model. J Natl Cancer Inst. 1997;89(20):1542-4.
- Gale KB, Ford AM, Repp R, et al. Backtracking leukemia to birth: identification of clonotypic gene fusion sequences in neonatal blood spots. Proc Natl Acad Sci USA. 1997;94(25):13950-4.
- Ford AM, Bennett CA, Price CM, et al. Fetal origins of the TEL-AML1 fusion gene in identical twins with leukemia. Proc Natl Acad Sci USA. 1998;95(8):4584-8.
- Ries L, Kosary C, Hankey B. SEER Cancer Statistics Review, 1973-1994. Bethesda, MD: National Cancer Institute. 1997.
- Hess JL, Zutter MM, Castleberry RP, et al. Juvenile chronic myelogenous leukemia. Am J ClinPathol. 1996;105(2):238-48.
- Arico M, Biondi A, Pui CH. Juvenile myelomonocytic leukemia. Blood. 1997;90(2):479-88.
- WHO. Global Programme on Evidence for Health Policy Discussion. Cancer incidence, mortality and survival by site for 14 regions of the world. Geneva: World Health Organization; 2010.
- Hill JM, Meehan KR.Chronic myelogenous leukemia: curable with early diagnosis and treatment. Postgrad Med. 1999;106(3):149-59.
- Mitus AJ, Rosenthal DS. The adult leukemias. In: Murphy GP, Lawrence W, Lenhard RE, eds. American Cancer Society Textbook of Clinical Oncology, 2ndEd, Atlanta: American Cancer Society, USA; 1995.
- Boyer K.Adult Leukemias - Part 1: Acute Myeloid Leukemia. Inter J Ace Phy Ass 1996;1(1).
- Ghaddar HM, Estey EH. Acute myelogenous Leukemia. In:Pazdur R, ed. Medical Oncology - A Comprehensive Review. 2nd Ed. New York:PRR, USA; 1995.
- Duncan R. The dawning era of polymer therapeutics. Nat Rev Drug Discovery. 2003;2(5):347-60.
- Li YY, Cunin F, Link JR, et al. Polymer replicas of photonic porous silicon for sensing and drug delivery applications. Science. 2003;299(5615):2045-7.
- Dalby MJ, Berry CC, Riehle MO, et al. Attempted endocytosis of nano-environment produced by colloidal lithography by human fibroblasts. Exp Cell Res. 2004;295(2):387-94.
- Couvreur P, Barratt G, Fattal E, et al. Nanocapsule technology: a review.Crit Rev Ther Drug Carrier Syst. 2002;19(2):99-134.
- Lu Y, Chen SC. Micro and nano-fabrication of biodegradable polymers for drug delivery. Adv Drug Delivery Rev. 2004;56(11):1621-33.
- Frenot A, Chronakis IS. Polymer nanofibers assembled by electrospinning.CurrOpin Colloid In Sci. 2003;8:064-75.
- Tomalia DA, Baker H, Dewald JR, et al. A new class of polymers: Starburst- dendritic macromolecules.PolymJ.1985;17:117-32.
- Newkome GR, Yao ZQ, Baker GR. et al. Cascade molecules: A new approach to micelles.Aarborol J Org Chem. 1985;50(11):2003-4.
- Yellepeddi VK, Kumar A, Palakurthi S. Biotinylated poly(amido)amine (PAMAM) dendrimers as carriers for drug delivery to ovarian cancer cells in vitro. Anticancer Res. 2009;29(8):2933-43.