Original Article
, Volume: 16( 1)Shungite Waste of Mining Production in Modified Concretes Regional Case Study
- *Correspondence:
- Yestemessova AS Kazakh Leading Academy of Architecture and Civil Engineering (KazGASA), Ryskulbekov Street 28, Almaty 050043, The Republic of Kazakhstan, Kazakhstan, Tel: +77017456437; E-mail: axayayestemessova@yahoo.com
Received Date: December 20, 2017 Accepted Date: December 26, 2017 Published Date: January 02, 2017
Citation: Yestemessova AS, Yesselbayeva AG, Yestemessova SM. Shungite Waste of Mining Production in Modified Concretes Regional Case Study. Int J Chem Sci. 2017;16(1):233
Abstract
The increasing regulatory requirements for concretes and modifying additives, as well as the need for environment protection and the recycling of industrial waste stimulate the creation of energy-efficient and environmentally friendly materials. One of the latest scientific achievements is the making of modified concretes with special properties. The concrete production technology significantly improved by using the chemical additives, special modifiers, as well as active and inert fillers. Kazakhstan has the richest mining resource base. Still, most of modifiers imported at high prices and with limited choice. The market of domestic modifiers based on local raw materials is underdeveloped. Shungite waste formed during extraction and technological processing used as a modifying mineral additive to concrete. Shungite can provide qualitatively new properties to concrete and reinforced concrete products and structures. The authors analyze its influence on the main parameters of concrete and characterize the processes in the solidifying silicate system with cement-dispersed schungite particles. These particles increase the strength of concrete and reduce the duration of its heat and moisture treatment.
Keywords
Shungite; Concrete; Mineral additives; Strength of concrete; Hydration of cement stone
Introduction
Kazakhstan has huge reserves of mineral resources, as well as mining wastes. The current growth in the mining industry is associated with the growing amount of mining waste. The resulting ecological consequences require maximum recycling of such waste. The Law of the Republic of Kazakhstan "On Subsoil and Subsoil Use" [1] obliged the introduction of resource and energy-saving technologies. The construction industry is one of the most resource-demanding sectors of industry. The existing experience of using mining waste has shown its obvious technological, environmental and economic advantages vs.the traditional fillers. The unique properties of shungite are due to its nano-structure and the composition from species of carbon-silicon shale with a particular form of natural carbon and silica.
The extraction of shungite creates a lot of dust. This worsens the environmental situation in the region and requires processing of shungite waste. The shungite waste products used as mineral additive (MA) and modifier of concrete to increase the strength of concrete and reduce the duration of its heat and moisture treatment. The article studies the influence of the mineral additive from the waste of Koksu shungite field, Almaty region, Kazakhstan, on the properties of concrete.
Theory
The use of mineral raw materials and mining waste in a finely divided form as fillers for traditional concretes impeded by a significant increase in cement consumption. However, in the new generation of concretes, the use of fine production wastes as an MA with a super- hyper plastifier reduces the consumption of cement and the cost of concrete [2,3].
The prerequisites for the use of shungite (taurite) as an MA for concretes are:
? Its unique properties due to its Nano-structure and the composition from species of carbon-silicon shale with a particular form of natural carbon and silica,
? Significant waste from shungite production and the need for its processing due to dust emission which worsens the ecological situation in the region of production, and
? A sufficient resource bases. Only in Almaty region of Kazakhstan, the probable reserves of taurite field exceed 620 M tons Therefore, taurite has a promising and long-term environmental and nano-structural potential in construction market if used as a modifying MA to concretes [4-7].
Materials and Methods
The adhesive for concretes was Portland cement PTs 400-D20 (Shymkent Cement JSC). The studied cement met the requirements of GOST 30515-2013 Cements. General specifications [8], and GOST 10178 (2002) Portland cement and Portland blast furnace slag cement. Specifications and was categorized by steaming efficiency as Cement Group I. The fine filler was the natural sand for construction purposes produced by Kapshagay Tas- Kum LLP, the town of Kapshagay, Almaty Region.
The bulk density of sand-1392 kg/m3, the true density-2.68 g/cm3, the size module-2.2. The coarse filler was gravel produced by KazTasProm LLP, the town of Kapshagay, Almaty Region. The shatter grade of gravel-1000, the bulk density-1428 kg/m3, the content of dust and clay particles-0.1%. The MA to the concrete was shungite under the brand name "Taurite" by Koksu Mining Company. Taurite is a fine divided material with particle size of 0-1 mm to 20 μm produced by mechanical activation and enriching at the temperature of 400°? to 600°?. The properties of ??-? grade shale taurite provided in Table 1.
| Parameters | Value |
|---|---|
| Weight content of silicone dioxide (SiO2), % | 84.93 |
| The content of water-soluble substances, % | 0.63 |
| pH of the aqueous suspension | 7.36 |
| Bulk density, kg/m3 | 695 |
| Humidity, % | 2 |
Table 1. Main properties of ??-? grade shale taurite.
| Parameters | Value |
|---|---|
| Weight content of silicone dioxide (SiO2), % | 79.51 |
| The content of water-soluble substances, % | 0.75 |
| pH of the aqueous suspension | 8.3 |
| Bulk density, kg/m3 | 504 |
| Humidity, % | 0.7 |
Table 2. Main properties of ??-D-? grade shale taurite. This table contains the parameters of ??-D-? mineral additive with the particle size of 5 µm.
| Parameter | Value |
|---|---|
| Weight content of silicone dioxide (SiO2), % | 52.76 |
| The content of water-soluble substances, % | 0.73 |
| pH of the aqueous suspension | 8.28 |
| Bulk density, kg/m3 | 674.3 |
| Humidity, % | 2.0 |
Table 3. Main properties of ?K-? grade shale taurite. Contains the parameters of ?K-? mineral additive with the particle size of 0-1 mm.
| Parameter | Value |
|---|---|
| Weight content of silicone dioxide (SiO2), % | 52.76 |
| The content of water-soluble substances, % | 0.73 |
| pH of the aqueous suspension | 8.28 |
| Bulk density, kg/m3 | 674.3 |
| Humidity, % | 2.0 |
Table 4. Main properties of ?K-D-? grade shale taurite. It contains the parameters of ?K-D-? mineral additive with the particle size of 10 µm.
Portland cement and all cement stone samples studied using physicochemical methods: X-ray phase and differential-thermal analysis. X-ray diffraction patterns obtained on DRON-3M diffractometer with a copper anticathode in the angular interval 4-64 degrees. Samples in the form of a fine-grained powder pressed into a plexiglass cuvette. The thermograms taken from the Q-1500D derivato graph from 10°? to 1000°C with a temperature rise rate of 100°C per minute.
Results and Discussion
Water demand of cement composites with MAs reduced by the combined use of MAs with superplasticizers with a considerable water-reducing effect. Table 5 provides the compositions of concretes with MAs and polymethylene-naphthalene- sulfonate additive of Kratasol brand.
| Material, kg | W/C ratio |
||||||||
|---|---|---|---|---|---|---|---|---|---|
| Mix | Cement | Gravel | Sand | MA TC-A |
MA TC-D-A |
MA TK-A |
MA TK-D-A |
Superplasticizer Kratasol |
|
| 1 | 380 | 990 | 875 | - | - | - | - | - | 0.58 |
| 2 | 380 | 990 | 875 | 19 | - | - | - | - | 0.63 |
| 21 | 380 | 990 | 875 | 19 | - | - | - | 3.8 | 0.56 |
| 3 | 380 | 990 | 875 | - | 11.4 | - | - | - | 0.68 |
| 31 | 380 | 990 | 875 | - | 11.4 | - | - | 3.8 | 0.65 |
| 4 | 380 | 990 | 875 | - | - | 19 | - | - | 0.64 |
| 41 | 380 | 990 | 875 | - | - | 19 | - | 3.8 | 0.56 |
| 5 | 380 | 990 | 875 | - | - | - | 11.4 | - | 0.68 |
| 51 | 380 | 990 | 875 | - | - | - | 11.4 | 3.8 | 0.64 |
Table 5. Compositions of concretes with taurite as a mineral additive.
The analysis of Table 5 shows that the composition and content of MAs influence the W/C ratio of the concrete mix. i.e., coarse additives without plasticizer require water content within 239 to 243 L/m3, with superplasticizer – the average of 01 L/m3, fine additives without superplasticizer – the average of 258 L/m3, with superplasticizer – 243 to 247 L/m3.
The following compositions of concrete analyzed to determine the effect of mineral additives and Kratasol superplasticizer on the mobility of the concrete mix and its changes over time: 1) No additives; 2) with TC-A; 21) with TC-A + Kratasol; 3) with TC-D-A; 31) with TC-D-A + Kratasol; 4) with TK-A; 41) with TK-A + Kratasol; 5) with TK-D-A; 51) with TK- D-A + Kratasol. The plasticizing effect at different amount of imposed mineral and plasticizing additives was different. After 1 hour, the mobility of concrete mix no.1 went down from grade 5 to grade 3; concrete mix no.2 – from grade 5 to grade 4; the mobility of concrete mix no. 21 has not changed; concrete mix no. 3 – from grade 5 to grade 4; concrete mix no.31 – from grade 5 to grade 4; concrete mix no. 4 – from grade 5 to grade 4; the mobility of concrete mix no.41 has not changed; concrete mix no.5 – from grade 5 to grade 3; concrete mix no. 51 – from grade 5 to grade 4. The samples left for hardening at room temperature for one day. After demolding, one part of cubic concrete samples left for hardening for 3, 7, 14, 28 days under normal humidity, while the other part molded and after, two hours placed in a humid-heat treatment chamber (700°?).
The diluting effect of Kratasol superplasticizer can significantly reduce the amount of mixing water and obtain high-strength concretes with the given mobility of concrete mixture. In this series, we investigated the same compositions of concrete mixes: 1) no additives; 2) with TC-A; 21) with TC-A + Kratasol; 3) with TC-D-A; 31) with TC-D-A + Kratasol; 4) with TK-A; 41) with TK-A + Kratasol; 5) with TK-D-A; 51) with TK-D-A + Kratasol. The concrete compressive strength study results provided in Table 6.
| Mix | W/C ratio |
Re-moldability | Compressive strength, MPa | |||||
|---|---|---|---|---|---|---|---|---|
| Hardening under normal humidity, days | Hardening under heat and humidity, days | |||||||
| Cone slump, cm | 3 | 7 | 28 | 12 h | 1 | 28 | ||
| 1 | 0.58 | 20 | 13.2 | 19.8 | 32.9 | 10.6 | 22.5 | 33.4 |
| 2 | 0.63 | 22 | 14.1 | 20.2 | 33.7 | 13.2 | 23.7 | 34.2 |
| 21 | 0.56 | 23 | 15.9 | 25.4 | 40.8 | 14.8 | 23.8 | 41.4 |
| 3 | 0.68 | 22 | 17.5 | 26.9 | 41.3 | 14.4 | 25.7 | 41.8 |
| 31 | 0.65 | 23 | 18.2 | 28.6 | 42.7 | 14.8 | 29.0 | 41.6 |
| 4 | 0.64 | 21 | 13.5 | 20.1 | 34.1 | 12.4 | 22.8 | 33.6 |
| 41 | 0.56 | 23 | 15.2 | 22.4 | 34.5 | 14.7 | 22.6 | 33.9 |
| 5 | 0.68 | 22 | 14.4 | 21.3 | 33.8 | 12.8 | 22.7 | 34.9 |
| 51 | 0.64 | 23 | 14.1 | 22.3 | 34.0 | 13.5 | 23.4 | 34.3 |
Table 6. The strength of concretes with the added taurit and Kratasol super-plasticizer in different hardening conditions.
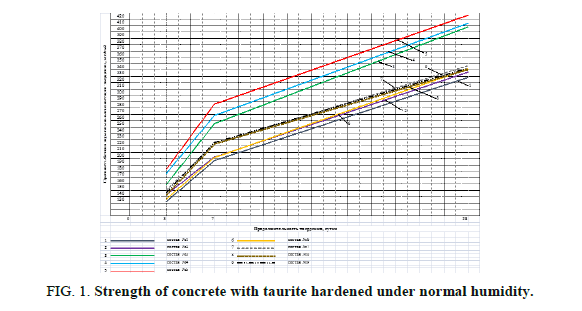
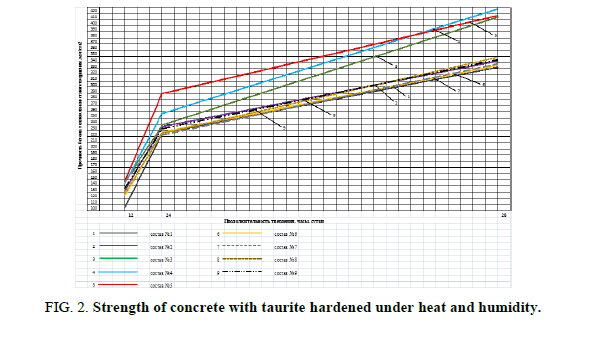
Table 6 shows the highest strength of the mixes nos. 3, 4 and 5, which contain ??-? and ??-D-? and Kratasol. Figures 1 and 2 show the increase of concrete strength hardened under normal humidity and under heat and humidity.
The analysis of Figure 1 shows that the strength of mixes nos.2-51 has increased in comparison to the control mix no.1: after 3 days – by 2.7-15.3%, after 7 days-by 1-27%, and after 28 days-by 2.5-30%. The analysis of Figure 2 shows that the strength of mixes nos. 2-51 has increased in comparison to the control: after 12 hours-by 5. 5-12.9%, 8%, and 28 days-by 0.6-25%.
Figure 1: Strength of concrete with taurite hardened under normal humidity.
Figure 2: Strength of concrete with taurite hardened under heat and humidity.
The authors have analyzed the processes taking place in the cement-MA system. Identification of products of hydration and hardening of silicate systems has shown a significant influence of Mas on phase composition of hardening systems. The microstructure of naturally solidified solution after 3 days showed that the MA particles exposed to the alkaline components of the cement-water mixture already at the initial stage of hydration of the hardening silicate system.
Because of this exposure, the surface of the MA particles is hydrated with the thickness of the hydration layer of 1-5 μm. The hydrated part of the grains forms around them the rim not separated from the residual main part. In some cases, the intermediate cementing phase penetrates into the grain structure of the mineral component.
The analysis of the solution microstructure after hardening showed that:
There is an initial mechanical interaction between the MA and the clinker components due to the roughness of the particle surfaces;
1. The amorphous structure of SiO2 microparticles acts as crystallization sites for hydrates such as calcium silicate hydrate (CSH). This explains the formation of an additional amount of crystallites that initiate the setting process. Besides, SiO2 reacts with portlandite [??(??)2] in the following reaction:
SiO2 + C?(??)2 → ?S?
2. In the hardening silicate system, the microfiller grains are mainly hydrated creating a rim 1 μm ... 5 μm thick around the residual part;
3. The cement constituents are hydrated releasing the hydrated phase which actively affects the microfiller grains;
4. Active hydration and surface hydration of glass waste grains produce an intermediate hydrate layer between them, which binds together the cement stone and filler;
5. Such position of the intermediate hydrated layer between the grains of the microfiller and the cement stone promotes a significant hardening and consolidation of the solution.
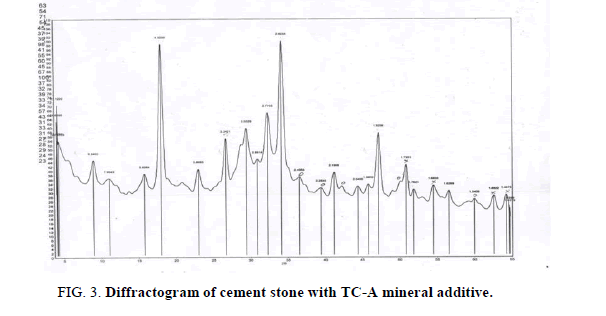
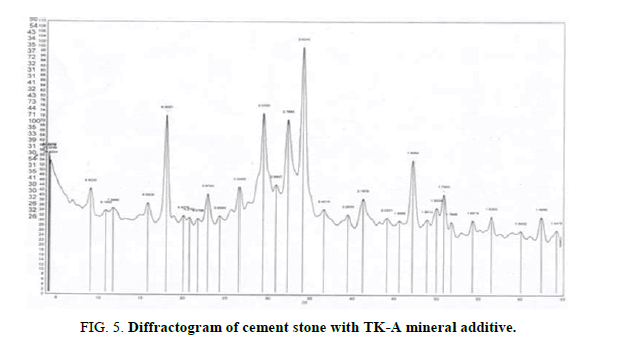
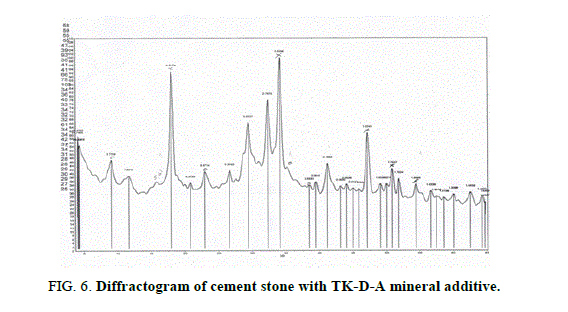
Figures 3-6 show the X-ray diffractograms of compositions based on cement and MAs like TC-A, TC-D-A, TK-A, and TK-D-A after 28 days of natural hardening. Diffractograms of hardening with TC-A (Figure 3) contain the remaining lines of minerals of from the original Portland cement: tricalcium silicate C3S with lines (3.037-2.77 Å), dicalcium silicate β-C2S (2.89 Å), as well as the lines of quartz (3.34-2.456-2.284-1.980- 1.540 Å) and hydration products: ??(??)2 (4.923-2.625-1.925-1.795-1.683-1.482 Å); CaCO3 (3.032-2.28 Å) and ettringite (9.84-5.6 Å). Differential-thermal end effect at 120°? is associated with dehydration of ettringite, and the end effect at 520? ? – with dehydration of ??(??)2.
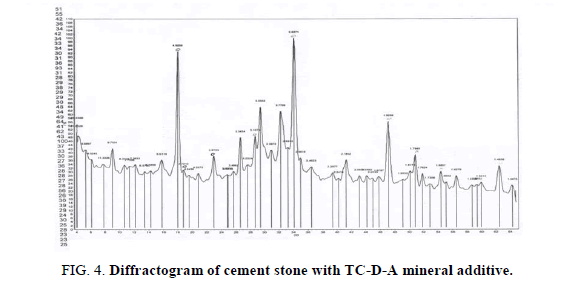
Diffractograms of hardening with TC-D-A (Figure 4) contain the lines of tricalcium silicate C3S (2.772-2.185-1.85 Å), dicalcium silicate β-C2S (2.88 Å), C3A (2.69 Å), quartz (4.25-3.34-2, 46-2.28-1.817-1.541 Å) and hydration products: ??(??)2 (4.92-3.10 -0.2.62-1.92-1.88-1.48 Å) and ettringite (9.75-5.61-3.87-3.466-3.22-2.56-2.18 Å). Differential-thermal endoeffect at 100°? and 120°? is associated with dehydration of ettringite and the transition to CaO, and the endo effect at 500°?-with dehydration of ??(??)2.
Diffractograms of hardening with TK-A (Figure 5) contain the lines of tricalcium silicate C3S (3.035-2.76 Å), dicalcium silicate β-C2S (2.89 Å), as well as the lines of quartz (4.27-3.346- 2.451-2.283-1.988-1.540-1.447 Å) and hydration products: ??(??)2 (4.923-2.624-1.926- 1.795-1.687-1.485-1.447 Å), CaCO3 (3.03-1.861 Å), and ettringite (9.803-5.59-3.874-2.20. Å). On the differential-thermal curve, the end effects at 100°? and 120°? are associated with dehydration of ettringite and the transition to CaO, at 500°?-with dehydration of ??(??)2, at 800°?-with decomposition of calcite.
Diffractograms of hardening with TK-D-A (Figure 6) contain the lines of tricalcium silicate C3S (3.037-2.76-2.32-2.188-1.853-1.762-1.48Å), dicalcium silicate β-C2S (2.89 Å), as well as the lines of quartz (4.27-3.34-2.28-1.821-1.539 Å) and hydration products: ??(??)2 (4.917- 2.625-1.924-1.793-1.684 Å), ????3 (3.037-2.28-2.093Å), and ettringite (9.73-3.871-2.77- 2.18 Å). On the differential-thermal curve, the endoeffects at 120°? and 150°? are associated with dehydration of ettringite and the transition to CaO, at 520°? – with dehydration of ??(??)2, at 800°C
An indication of the hydration reaction in the system is the presence of the 4.92Å line belonging to portlandite ??(??)2. The latter always accompanies the process of hydration of cement, the hydrolysis of minerals of Portland cement clinker (calcium silicates). The 5.6 Å line is due to ettringite ?3?.3??SO.32?2? which is usually formed in the early stages of portland cement hydration.
ray phase analysis demonstrates a positive influence of MAs like TC-A, TC-D-A, TK-A, and TK-D-A on the hydration of clinker minerals. This is evidenced by an increase in the intensity of the ??(??)2 line with the interplanar distance of 4.92 Å and the appearance of the 2.63 Å line, also related to ??(??)2. The thermograms have recorded the endo effects at 500°C and 520°C associated with the removal of water from ??(??)2. Thermo-gravimetric analysis of cement stone with MAs confirms the data of X-ray analysis of the effect of the above additives on hydration processes. The cement hydration by mass loss on day 28 reaches 23%.
Conclusion
Thus, the authors have established the possibility of using the new mineral additive to concrete fine divided taurite with the size of particles from 0-1 mm to 10 μm produced by mechanical activation and enriching at a temperature of 400-600°?. The study showed that the introduction of taurite MA and naphthalene superplasticizer Kratosol improves the strength of normal-moisture hardening concretes in comparison to the control mix.
For the concretes hardened under normal humidity, the strength after 3 days has increased by 2.7-15.3%, after 7 days-by 1-27%, and after 28 days-by 2.5-30%. For the concretes hardened under heat and humidity, the strength after 12 hours has increased in comparison to the control mix by 5.5-12.9%, after 1 day-by 0.3-19.8%, and after 28 days-by 0.6-25%. The authors have established the optimum “soft” heat and humidity concrete treatment mode with isothermal heating temperature not more than 700°?.
The authors described the specifics of behavior of dispersed particles of mineral additive from shungite waste in hardening silicate systems. The surface of the particles of the mineral filler is susceptible to hydration that contributes to the production of concrete and mortar with improved physical and mechanical properties. The amorphousness of SiO2 particles in the mineral filler creates crystallization sites for hydro aluminates and calcium hydro silicates. The mineral additives influence the rate of hydration processes of hardening of silicate systems. The main products of hydration of the modified mixtures are ettringite, portlandite and gel-like silicate phase. The mineral additives TC-A, TC-D-A, TK-A and TK-D-A act as the inhibitors of structure formation promoting the formation of interdependent aluminosilicate and polymer phases.
References
- Anisimov SN, Kononova OV, Minakov Yu A, et al. Investigation of the strength of heavy concrete with plasticizing and mineral additives. Modern issues of science and education. 2015.
- Dvorkin LI. Construction materials from industrial waste. Rostov on Don: Phoenix. 2007.
- Yezersky VA, Kuznetsova NV. Improving the properties of fine-grained concrete with the help of complex mineral additives. Construction Materials. 2015;6:4-6.
- Zhurinov MZ, Bayeshov AB, Zhumabay IM, et al. Investigation of the sorption properties of the Koksu schungites. Proceedings of the Republican Scientific and Theoretical Conference "Ecology, Knowledge, Science and Society" to the 60th anniversary of Professor A. Bayeshov. Kentau. 2006;pp:6-12.
- Lukutssova NP, Pykin AA. Theoretical and technological aspects of production of micro- and nano-dispersed additives for concrete from shungite-bearing rocks. Monograph. Publishing House of the Belarusian State Medical University, Bryansk. 2013.
- Izotov VS, Sokolova Yu A. Chemical additives for concrete modification: Monograph. Kazan State Architecture and Construction University. Paleotype Publishing House, Moscow. 2006.
- Interstate Standard GOST 10178-2002. Portland cement and Portland blast furnace slag cement. Specifications. UDC 691.542:066.354 Group G12. Ministry of Construction Materials of the USSR, Moscow. 1987.
- Interstate Standard GOST 30515-2013. Cements. General specifications. MKS 91.100.10. Standartinform, Moscow. Russia, 2015.