Original Article
, Volume: 13( 3)Microelements in Coals: Basic Carriers, Paragenesises and Distribution of Gross Contents in the Basic Carriers
- *Correspondence:
- Admakin LA, Darioush Sharafie, Russian Prospecting Institute of Coal Deposits, 2nd Baumanskaya Str., 9/23 Building 1, Rostovon Don, Moscow, Tel: 989125214247; E-mail: admakin.leonid@ya.ru
Received: January 20, 2018; Accepted: February 07, 2018; Published: February 12, 2018
Citation: Admakin LA. Microelements in Coals: Basic Carriers, Paragenesises and Distribution of Gross Contents in the Basic Carriers. Phys Chem Ind J. 2018;13(1):119.
Abstract
On the example of coal deposits of Transbaikalia, the main constituents of coals are characterized, the inherited and newly formed macerals are distinguished. The characteristic of the main carriers of microelements in coals is given: organic matter, mineral impurity, initial plant mass. A method for isolating paragenesis of trace elements from associations is proposed, which is of great importance in the evaluation of rare metals in fossil fuels. Specific examples of the isolation of paragenesis of microelements from associations in coals of various rare-metality are given. The statistical method of decomposition of gross contents of microelements on the basic carriers is substantiated. It is concluded that the infrared-organic concentrations of microelements played a decisive role in the formation of rare metal fossil fuels.
Keywords
Microelements; Organic system; Geochemical regime; Paragenesis; Biogeochemical component; Abundance ratio; Distribution function; Infiltrate-organic concentration
Introduction
In coal, as physical and chemical mixtures of organic and mineral substances, trace elements are more complex than in mineral systems. In coal geochemistry there are no fundamental principles governing the distribution of trace elements that act in the inorganic system [1], but only probabilistic trends. Nevertheless, the problem of concentration and distribution of microelements in coal attracts close attention from both the geochemical and technological points of view, since coals serve as a source of industrial extraction of some of them. In anomalous amounts in the coals of some deposits, Ge, U and Ga, extracted from the ash, can be present. A wide range of potentially valuable (V, Sc, Li, B), with known extraction technologies, and a number of associated (Hg, Mo, W, Pb, Cu, Ag, Au, Tl) are considered. On the other hand, many trace elements are toxic and, thus, create environmental problems in the extraction and processing of coals. At the same time, the degree of environmental danger increases due to the cumulative effect of polyelement associations of trace elements accumulating in coals in different forms. Many of them are relatively easy to volatile, so the effect of environmental hazards is created in all technological processes: coal mining, enrichment, combustion and coking. In the areas of such enterprises, soils, surface and groundwater, the air basin is systematically polluted, which progresses and has a detrimental effect on the state of the triad "plant-animal-human."
Trace elements are distributed in the coals quite unevenly. According to their contents, not only the coals of basins and deposits, but also the seams and their local areas differ, and the genetic and geochemical features of such differences are not always obvious. But, one thing is certain: microelements in coal are in different forms of communication - organic and mineral, and, as studies show, there is no correspondence between these contents and dependence on ash content [2,3].
The main carriers of microelements in fossil fuels
Fossil coals are a mixture of organic and mineral material in a wide range. These components in their sources and forms of finding trace elements are independent substances. The organic substance itself is a complex substrate, consisting of organic components - macerals, differing in composition, specific properties, peculiarities of origin and behavior in geochemical processes. In this respect, they are divided into two groups [4]:
1. Inherited, or macerals of the exinite group;
2. Newly formed, or macerals of vitrinite and inertite groups.
Their names speak for themselves. The concept of inheritance means that some object of the system, characterized by its own composition and structure, which, when the system goes from one state to another, remains unchanged. The inherited substances retain their composition, structure, and behave conservatively in physicochemical processes. Therefore, they are inert to the stage of humification, in particular, they are characterized by low concentrations of microelements, even in coals with anomalous contents. These contents are of the same size, or close to the content in modern plant analogs.
The macerals of the exinite group are inherent, which are characterized by a relatively high stability with changing parameters such as temperature, pressure, geochemical regime, which are set by the external environment and are factors of the state of the organic system. Inherited - sporinitis, kutinite, suberinitis, resinitis, alginitis - are specific plant tissues (sporin, kutin, suberin, natural resins) performing protective and multiplying functions and algae. They are characterized by their own chemical composition; they have predominantly aliphatic, partly aromatic structure [5-7]. They are characterized by high hydrogen content, high yield of tar and gas. In the transition to coals with an average yield of volatiles, the macerals of the exinite group undergo a rapid change, known as the "carbonation jump" [5]. In the specific oxidation-reduction regime of the organic system and the conditions for the accumulation of phytomass, coals of a specific petrographic composition can be formed: kennel coals, boghedy, kennel-boghedy, rarer varieties: barzassites, kasyanite, rhabdospisite, pyroposisite, suberitoid Potonie, "paper" coal of Indiana. Within the framework of our topic, the geochemical characteristics of these coals are not considered.
Newly formed include two groups of macerals: vitrinite and inertinite. They were formed in the process of humification of organic matter of the biochemical series (phytomass) into the organic matter of the coal series (humic acids, humus, which is diverse in terms of the initial plant mass). This is a complex group of macerals, the formation of which occurs in a relatively short period, beginning with the peat stage and before turning the plant mass into soft coal. The process of transformation is physico-chemical, in which the factor of the state of the organic system is mainly the activity of oxygen.
The process of depolymerization of biochemical polymers was performed with the active participation of microorganisms that release enzymes that specify the humification of plant matter. No oxidative regime, by itself, is able to make such transformations. In sterile (absence of microorganisms) the oxidative regime destroys the organic matter, bypassing the formation of monomers [8]. The scale of the work of microorganisms on the transformation of organic matter of the biochemical series into the organic matter of the coal series is impressive. This invisible world of microorganisms in a short period of time recycles giant masses of plant matter, especially in the era of intense coal formation. Biopolymers of plant tissues are destroyed to monomers, and these transformations constitute the most important stage in the transformation of plant tissues and structures of the biochemical series into organic matter and the structures of the coal series. The highest manifestation of these transformations is the so-called gelification of "oozudnevanie" plant cells - a process leading to a partial or complete loss of textile structures [5,7,9]. At this stage, the role of microorganisms is the determining factor of transformation.
Humification triggers another mechanism of transformation - the condensation of monomers into heteropolycondensates [10], represented by humic acids and humus with a colloidal structure. Humification is the most important stage in the geochemical history of the organic matter of fossil coals, since this is the stage of the formation of highly reactive organic substances - humic acids and humus. Specifically, these products determine the active interaction of the organic system with the external environment.
When gelification from a colloidal mass, permanent structures are formed from the amorphous state to the appearance of short-range and long-range domains. The inorganic constituent of coal is represented by two genetic groups of minerals:
1. Clastogenic minerals and lithoclastes, which is incorporated in the organic system as part of the solid runoff. In coals, this admixture is dominant and its mineral composition is determined by the petrochemical fund of rocks in the conjugated areas of nutrition, but only to the extent that they are preserved as products of the residual weathering crust. Therefore, the clastic impurity in the coals of various basins and deposits is largely monotonous and is represented by grains of quartz and its modifications, varying degrees of pseudomorphized potassium feldspars and plagioclases, sometimes with muscovite plates, scales of amorphized biotite, less often hornblende, pyroxenes, accessory accessory minerals are very rare. It is clear that there are no clastic sulphide, carbonate, oxide minerals that are unstable in the weathering crust.
Clastogenic are some clay minerals represented by layered and skeletal two- and three-layer structural types, with an unbalanced valence [11], which gives them the ability to swell along the normal to stratification axis, to ion exchange, including trace elements. A number of clay minerals (illite-montmorillonite, paragonite-montmorillonite, biotite-vermiculite, trioctahedral chlorite-montmorillonite) are mixed-layered. All this leads to a complication of the mutual relations of the clastic mineral impurity with organic matter, their close germination [5,6], as a result of which the mineral admixture is not flotated in its pure form. Therefore, the flotation of coal fractions is always "contaminated" by the clay fraction and the mineral fractions by organic matter, which creates uncertainty in the identification of the trace element in the coals.
2. The second group is formed by diagenetic minerals formed in the organic system, in situ. This is mainly the allocation of pyrite, marcasite, forming in the coal seams a small impregnation and interspersed zone. Here they are in close sprouting with the coal mass; often form fused strips, with gradual transition into the dispersed zone. There are pyritic coals (Irkutsk basin). Sometimes in coal seams there are so-called "quartz" - interlayers of microcrystalline quartz (Cheremkhovskoye, Abanskoye deposits). Diagenetic in nature are also sub-monomineral kaolinite interlayers - the tonets, which are products of eolian volcanic ash transformation in the organic system.
The diagenetic release of minerals in coals and host rocks reflects the geochemical regime of the organic system. For example, pyrite segregation is associated with the hydrogen sulfide regime, characterized by specific chalcophile paragenesis of microelements [4]. Microelements of diagenetic minerals in coals together with microelements of organic matter a single paragenesis, which is a consequence of the geochemical regime of the organic system.
Catagenesis is the stage of conversion of coal under the influence of temperature and pressure. With regard to trace elements it is destructive, since chemical bonds with organic matter are destroyed and an alkaline regime is established in the organic system.
Organic system and geochemical regimes
The organic system is an open system. It can exchange energy and matter with the external environment. These two forms of exchange are manifested with varying intensity and qualitatively in different forms at different stages in the evolution of organic matter. In the stage of humification, the main is material exchange, which is of great importance in the transformation of the organic matter of the biochemical series into the organic matter of the coal series and the enrichment of the organic system with various chemical elements. There are two forms of material exchange:
By means of a surface solid flow: This way, a mass of clastogenic material is supplied to the organic system, the source of which is a variety of rocks that make up the petrochemical background of the demolition areas. Clastic material, as shown by petrographic studies. it is rather monotonous in various basins: quartz, feldspars, lithoclasts, in the subordinate meaning of mica, less pyroxenes, amphiboles, chlorites. In the rank of accessory, zircon, sphene, monazite, etc. are characteristic. Clastic ore minerals in coals are not noted even in those cases where ore deposits were eroded in the immediate demolition areas. There are many such examples, and they explicitly testify that in humid conditions ore minerals are easily destroyed under conditions of immature weathering crust. This largely impoverishes the clastogenic mineral admixture of microelements.
Exchange of soluble chemical elements, migrating in natural waters, thermal springs, acrotherms: This is a very complex exchange of the external environment with the organic system, due to the peculiarities of the composition of the solutions, the concentrations of microelements in them, the physico-chemical character of the medium, the forms of transport of chemical elements [12], the ways of their migration and their entry into the organic system. These solutions create the geochemical regime of the organic system and determine the rare metal specialization of fossil coals. The concentrations of microelements in the coals created by such solutions were identified as infiltration-organic [13].
The transformation of organic matter is an oxidation-reduction process [14], which determines the quantitative ratios of the newly formed vitrinite (Vt) and fusinite (F) groups, which is a function of oxygen as a factor in the state of the organic system [2,8]:
F/Vt=f (O2) (1)
The organic system consistently passes through aerobic and anaerobic environments, which differ in the state factors and in the corresponding products of the transformation of organic matter [8].
Aerobic environment
It corresponds to the volume of the peat horizon, in which the oxidation-reduction regime is given by the partial pressure of atmospheric oxygen. The reserve of free oxygen here is rapidly consumed for oxidative reactions and the zero value of the partial pressure determines the sole of the peat horizon. This situation is characterized by high activity of microorganisms involved in the transformation of plant material.
Aerobic conditions in the organic system are maintained by incoming solid and liquid runoff. The composition of clastic minerals and lithoclast is determined by petrochemical fund of rocks in the areas of demolition and the degree of weathering; liquid runoff is poor in component composition.
The redox regimen of aerobic conditions can be characterized by the ratio of newly formed macerals-fusinite and vitrinite, which are the main products of this regimen. Their ratio is a function of the partial pressure of atmospheric oxygen (pO2)
F/Vt=ω0 pO2 (2)
where ω0 is the coefficient of proportionality.
It should be emphasized that due to the irregular intake and consumption of free oxygen, the organic substance overcomes the aerobic situation in a state of extremely uneven degree of decomposition and transformation of the organic matter of the biochemical series into the organic matter of the coal series. In aerobic conditions, up to 60% of the total mass of plant material is converted to organic matter in the coal series.
Anaerobic furnishings
These are characterized by a complex set of transformations that causes the depolymerization of large biopolymers to monomers. Of the biopolymers, cellulose is the least resistant, which largely decays, while sugars formed by microbial hydrolysis produce humic acids with amino acids [6]. However, the presence of textitonite structures in peat indicates that xylem cellulose does not completely disappear, while lignin cells remain in the humus. In anaerobic conditions, up to 40% of the volume of biochemical (plant) mass comes in.
Anaerobic environment is the medium of condensation of monomers into heteropolymers, which are humic acids and humus, which open the carbonic series of organic substances. The macerals of the vitrinite and inernitinite groups, which are petrographic constituents of humic coals, are dominant. The oxidation-reduction regime of the organic system is determined by the activity of oxygen [O2], which enters the organic system when the solutions are infiltrated. The activity of oxygen [O2] becomes a factor in the state of the organic system. The oxidation-reduction function takes the form
F/Vt=?0 [O2]
where ?0 is the coefficient of proportionality.
An anaerobic environment receives a very heterogeneous organic material of the biochemical and coal series, that is, incompletely decomposed plant tissues are associated with humic acids and humus. The most stable is the lignin of tree trunks, branches, roots, preserving textitonite structures in brown coals, which are easily recognized by the characteristic birefringence of plant tissues. Cellulose disappears. developing fermentation, which turns organic matter into bituminous products. Resin, wax, fats, kutin, sporopoenin, still remain inert.
Anaerobic conditions are the stage of authigenic mineral formation in the organic system. Mineral precipitates are characterized by sulfides (melnikovit, marcasite, pyrite), carbonates (ankerite, calcite, siderite, dolomite). The precipitation of pyrite creates impregnation in a close intergrowth with organic matter, the strengthening of which leads to the formation of zones of a homogeneous fused pyrite. At the same time, the degree of crystallinity increases and in the merged part pyrite becomes coarse-grained. The boundaries with coal are gradual, irregular, but the zones during the ritification as a whole extend along the banding in the coal seams. With intensive development of diagenetic discharges of pyrite, the coals become pyrite. Pyrites in coals reflect the hydrogen sulfide regime of the organic system under anaerobic conditions. In the Irkutsk basin, the source of hydrogen sulfide was saline, red and gypsiferous.
Carbonates form concretions of the "cone-in-cone" type, veins are possible. Dissemination is less characteristic. Often, carbonates develop in the sandstone of the roof, creating carbonization zones that reflect the carbon dioxide regime of the organic system. The carbon dioxide was formed in situ.
A wide spectrum of microelements is associated with coals. The prevalence of the chemical elements of Mendeleyev's periodic table in nature has always intrigued many researchers and the physicochemical laws governing this phenomenon have been formulated. Vernadsky [15,16] defined this phenomenon, known as the principle of universality, reflecting their desire for dispersion. At the same time, the concentrations of microelements can vary in different objects in wide ranges of values, clearly indicating the fact that a natural concentration factor, manifested with varying intensity, operated. Studies show that the specificity of micronutrient concentrations in coals was in close connection with the regimes of the organic system. The isolation of the geochemical regimes of the organic system became possible after the oxidation-reduction function (1) had been established.
The most important mode of action was the activity of [O2], [H2CO3], [HCO-3], [CO2-3], [H2S], [HS-], [S2-], [HCl], [Cl-], [HF], [F-], which were factors of the state of the organic system. Accordingly, the oxygen, carbon dioxide, hydrogen sulphide, chloride and fluoride regimes of the organic system are released [4]. They determined the features of the geochemical behavior of microelements: stability in solution, the formation of a chemical bond with organic matter, the crystallization of minerals [4,17].
Oxygen regime
It is set by two factors:
a) The entry into the organic system of free oxygen, determined by the partial pressure of atmospheric oxygen pO2. Oxygen is consumed in the oxidation process to convert the organic matter of the biochemical series into the organic matter of the coal series F/Vt=ω0pO2. The chemical elements are in the organic system in a low degree of oxidation, for example, Fe3+, Mn6+, U6+. The value pO2=0 determines the sole of the oxidative regime of the organic system - the peat horizon.
b) The activity of oxygen [O2] in the infiltration of solutions. The transformation of the organic matter of the biochemical series that has overcome the aerobic environment into organic matter of the coal series is being carried out, F/Vt=?0 [O2]. Humus is oxidized with the formation of semivitrinite, semifusinite, degradofusinite. The stage of humification is the stage of the greatest chemical activity and aggressiveness of humic acids. Penetrating the rocks of the coal seams, they decompose silicates, converting them to a kaolinite residue, and the interbeds of eolian volcanic ash present in the coal seams - into sub-monomineral kaolinite toteshteyny [18]. The newly formed organic matter - humic acids, humus - become the main substances of interaction with microelements. Fulvic acids are also micronutrient concentrators, but, as light migrants, can carry them out of the organic system.
Carbonic regime
Organic system during the transformation of organic matter of the biochemical series into the organic matter of the coal series produces acidic products (humic acids, humus). Therefore, the carbon dioxide regime is formed by the system itself under anaerobic conditions. At [H+]=3 × 102-, the molecular concentrations of H2CO3 and HCO-3 are equal, the medium is neutral. If the system maintains the ratio of H2CO3>HCO-3, the regime is stable acidic, which is the case with humification. If H2CO3<HCO-3, the medium is a silk. With humification, this ratio will be satisfied if, in the organic system, hydrocarbonate solutions enter that neutralize organic acids. This activates the release of carbonates; Nodules are formed in coal seams and enclosing rocks, especially in the roof of the seams. Such conditions are typical for sedimentary reservoirs in especially in the roof of the seams. Such conditions are typical for sedimentary reservoirs in the areas of development of carbonate strata and the main intrusive complexes.
Carbonic regime becomes dominant in the stage of gelification of organic matter and sediment lithification; the alkaline reserve increases, which is an indicator that the process of converting the organic matter of the biochemical series into the organic matter of the coal series has been stopped, although humification, as geochemical studies show, may not be completed. Fissured forms of carbonate, cryptocrystalline quartz evolution characteristic for catagenesis develop. The state of the organic system in the carbon dioxide regime is determined by the redox function
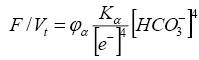 (4)
(4)
where Kα=KH2O/KH2CO3 - constant: KH2O and KH2CO3 are the dissociation constants of water and carbon dioxide, respectively; [e-] - activity of the electron; φα is the proportionality coefficient.
It follows from equation (3) that if the activity of an electron is maintained at a constant level, then an increase in the activity of [HCO-3] enhances the oxidation state in the organic system; Fusenization of organic matter is increasing. In the presence of CO2, the Ca2+ and Mg2+ cations are stable. As organophilic elements, they bind to humic acids and humus and form humates. Ash content of such coals is characterized by high contents of CaO and MgO, thus affecting the technological quality of coals. We note that calcium and magnesium ions peptize humic acids, increasing their mobility. Therefore, in such coals, dopplerite can be found - a lithiated natural extract of colloidal humic solution, displaced and lithified, and performing cavities in the coal seam [19] or in the fissures of the enclosing rock.
Hydrogen sulfide regime
Defined by the receipt in the organic system of hydrogen sulfide solutions associated with acrotherms and thermal springs. Hydrogen sulfide H2S is the weakest of mineral acids; it is weaker than humic acid. With weak alkalization of solutions, the crystallization of sulphides of many metals is enhanced. The hydrogen sulphide regime of the organic system creates a so-called hydrogen sulphide barrier [20], which affects the behavior of trace elements.
In the Irkutsk basin, the coals of many deposits are pyritized, and for some the content of pyrite sulfur may exceed 10%. Coals become pyrites and, despite their large reserves, are unsuitable even as an energy fuel. Here, the source of hydrogen sulphide solutions was the underlying salt-bearing, gypsiferous, carbonate and red-colored deposits of the platform cover.
The oxidation-reduction function of the hydrogen sulfide regime has the form:
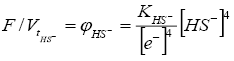 (5)
(5)
where KHS-=KH2O/Ks4 is a constant.
Since hydrogen sulphuric acid is weak, in the presence of humic acid, the concentrations of HS- are low. This greatly reduces the oxidative effect of H2S; fusenization is poorly developed even in coal pyrite. At the same time, due to the dissociation of H2S=2H+ + S2-, the hydrogen sulfide medium serves as a medium for the liberation of sulphide minerals and, above all, marcasite and pyrite.
Chloride regime
Chlorine ions are widely distributed in natural waters, but in surface waters their concentrations are negligible. In organic matter, chlorine is not accumulated, but can give salts that are easily soluble in water. Chloride solutions play a significant role in the geochemical migration of metals [21]. The influence of chloride solutions is associated with the formation of hydrochloric coals [22].
The oxidation-reduction state of the organic system under the chloride regime is given by the condition
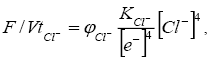 (6)
(6)
Where KCl-=KH2O/K4Cl- is a constant; φCl- is the coefficient of proportionality.
It follows from equation (6) that an increase in the activity of Cl- contributes to fusenization of the organic matter.
Fluoride regime
Fluoride solutions and emanations are characteristic for volcanic belts and activation regions. Fluidite deposits and zones of near-ore changes are associated with their manifestation. There are known finds of fluorite in the coal-bearing sediments of the Transbaikal, but it was not found in the coals. We cannot ignore the possibility of developing a fluoride regime in coals in the areas of synchronous volcanism and thermal springs. In this case, the oxidation-reduction state of the organic system is determined as
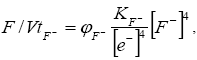 (7)
(7)
Where KF-=KH2O/K4F- is a constant; ?F- is the coefficient of proportionality.
Associations and parageneses of microelements in coals
It was shown above that the organic system evolves physico-chemically in its development. Organic matter of the biochemical series is transformed into organic matter of the coal series, the organic system interacts with the external environment as an open system, exchanging matter and energy. It receives solid and liquid runoff, enriching it with clastogenic material and dissolved components. The formed reactive organic matter of the coal series, - humic acids and humus, - concentrate the dissolved components of infiltration solutions to form a chemical bond. In coal, thus, microelements, coming in various carriers: mineral impurity, organic matter of the coal series, accumulates. Some part in the balance of microelements in coals is made by chemical elements of the initial plant mass. These genetic groups of microelements have different economic significance; have different effects on the technology of processing and use of coals.
Information on the concentrations and distribution of microelements in coal is delivered by analytical methods: spectral and chemical analyzes. A feature of such data is gross contents, which are the total values of concentrations in various carriers in coals. This is a disadvantage of the determination methods, since the various chemical forms of the trace elements in the coals have a different effect on processing technologies, environmental assessments and the potential value of coals. There are no quantitative analytical methods for determining the concentrations of microelements in the main carriers. The method used to divide coal into fractions of different densities gives only a general tendency for the distribution of microelements, due to incomplete fractionation of the organic and mineral parts of coal.
The accumulation of trace elements in coal, regardless of the form of the bond, constitutes an association [23]. From the geochemical point of view, the associations of microelements in coals of different deposits and coal seams are always poly-element, due to the principle of Vernadsky's universality.
The association of microelements in coals is a collective concept, since it includes chemical elements that differ in the forms of location: in the organic matter of the coal series, in the clastogenic mineral impurity, in authigenic minerals, biogeochemical concentrations in the coal-forming vegetation. Each of these types of carriers can be significant and give elevated levels in the coals. In associations, the concentrations of trace elements due to different carriers reflecting different forms of migration are not delineated. From analytical methods, semi-quantitative spectral analysis gives the most extensive, albeit incomplete, set of trace elements that make up the association. Therefore, the association of trace elements in the coals of different deposits and basins is always the same. Nevertheless, studies on the geochemistry of trace elements are limited to the analysis of associations.
Recently, the concept of paragenesis of trace elements in coals has been introduced [23]. Isolation and investigation of the paragenesis of chemical elements in coals is a more complex problem than the establishment of mineral paragenesis. The mineral paragenesis are diagnosed visually and under microscopic examination, the paragenesis of microelements is solely based on analytical definitions of the contents in the coals. However, these contents are gross, polygenetic, and for the establishment of paragenesis it is necessary to differentiate them into aggregates associated with their carriers in the coals. In this case, the change in the geochemical regimes of the organic system leads to different paragenesis, which differ in the sets of trace elements and levels of their contents. There is a need for geochemical differentiation of coal deposits and to assess their potential rare metality.
Systematic determinations of the contents of trace elements in coal persuade that their paragenesis are determined by the chemical form of bonding with organic matter, as their main carrier. The authigenic minerals present in the coal crystallized from the same solutions with which the organic system and microelements were supplied. Therefore, microelements of authigenic minerals make up a single paragenesis with microelements of organic matter. Their separate estimation as carriers of microelements is solved by petrographic study and analytical analysis.
Isolation of paragenesis of microelements from associations in coals
As noted above, the trace element associations are determined analytically and are gross. There are no analytical paths for determining the paragenesis of analytic paths. The peculiarity of the paragenesis of microelements is the levels of their contents; it was thus possible to isolate them from associations by the statistical method [24]. The content of the microelement, given by the geochemical Clark, is determined in the coal by the quantity (Cl × Ad/100), where Cl is a clarke of a microelement, according to [25]; Ad - ash content of coal.
For large samples [26], the statistical moments of the trace element contents can be determined by assuming that they are distributed in a mineral impurity in a normal manner. Further, according to selected estimates, using a microelement as the mean Clarke, it is possible to determine a confidence interval with a confidence factor of 1 - α. Then it is possible to assert with 95% probability that the content of each microelement, associated with a mineral impurity, falls within a given confidence interval. These background contents are caused by a clastogenic mineral impurity. Their variations do not go beyond the boundaries of trust.
It can also be argued that content beyond the upper limit of the confidence interval does not belong to the confidence interval with the same probability, that is, they do not belong to the background. Concentrations exceeding the upper limit confidence interval, are elevated, including, anomalous. They, in the composition of gross contents are due to infiltration of solutions. and named confidence interval, are elevated, including, anomalous. They, in the composition of the gross contents, are due to infiltration of solutions. and are called infiltration-organic concentrations (Cio) [13,27]. Hence, we obtain:
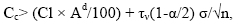 (8)
(8)
Where σ is the standard deviation of the microelement content in the sample; n is the sample size; τν is the Student's index, with ν=m - 1 degrees of freedom; α is the standard deviation. The values of τν (1-α/2) are plotted according to the nomogram [28], depending on the volume of the sample.
Confidence interval allows with a 95% chance to estimate the background and limiting the contents of the trace element. Microelements with contents of the upper limit, with a probability of 95%, do not belong to the confidence interval, and are due to the infiltration mechanism of entry into the organic system. Microelements with such contents in the coal make paragenesis.
If the relation (8) is fulfilled, and regardless of the ash content, then such contents are associated with an organic substance, otherwise with a mineral impurity. The criterion for the microelement to belong to the paragenesis is, therefore, the systematic fulfillment of the relation (8). Elevated contents in single point, cannot be, it is important to emphasize.
Containing trace element in mineral impurity
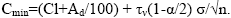 (9)
(9)
These contents in coal vary depending on the amount of mineral impurity, i. E. from ash content. The upper limit value is the content at Ad=100% (pure siltstone rock), the lower limit is the content at Ad ≤ 3 (ultrapure coals).
The content of microelements in organic matter is defined as:
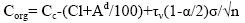 (10)
(10)
It follows from relation (10) that, the smaller the Cc, the lower the concentration of traces elements in the organic part. It is determined by the solutions supplied into the organic system. The value of Corg is an indicator of the degree of the connection between the organic system and the external environment. Obviously, it can be determined either by the limited infiltration of the solution, or by the low concentration of trace elements in the solution. This is assessed in each case on the basis of geochemical studies. Values of Corg are infiltration-organic concentrations and serve as the basis for assigning a trace element to paragenesis. Table 1, the statistical method of isolating specific paragenesis of trace elements from associations by the example of some coal deposits in Transbaikalia. Digital data show that paragenesis is represented in germanium-bearing coals by a wide range of microelements. The larger the potential, the scarcity of the carbon deposit.
| Clark.(g/?) by [25] |
Deposits | ||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| ?harbagataisk | Mordoisk | Althansk | Kharanorsk | ||||||||||||||||||
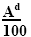 |
C?s | σ | C?in | ?org | 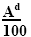 |
C?s | σ | C?in | ??rg | 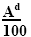 |
C?s | σ | C?in | ??rg | 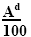 |
C?s | σ | C?in | ??rg | ||
| Be | 7 | 0.127 | 24.5 | 1.217 | 1.4 | 23.1 | 0.210 | 2.2 | 1.568 | 2.2 | - | 0.262 | 3.6 | 1.603 | 2.2 | 1.4 | 0.136 | 2.0 | 1.658 | 1.6 | 0.4 |
| V | 130 | 35.3 | 1.513 | 17.1 | 18.2 | 7.5 | 1.584 | 27.9 | - | 25.5 | 1.095 | 34.4 | - | 2.9 | 1.841 | 18.4 | - | ||||
| Cr | 160 | 18.2 | 1.187 | 20.7 | - | 1.4 | 1.414 | 35.8 | - | 5.3 | 2.512 | 42.7 | - | 1.1 | 1.149 | 22.1 | - | ||||
| Co | 23 | 19.1 | 2.264 | 3.8 | 15.3 | 2.2 | 1.887 | 5.8 | - | 4.7 | 1.495 | 6.5 | - | 7.4 | 2.884 | 3.5 | 3.9 | ||||
| Ni | 95 | 18.2 | 1.584 | 12.7 | 5.5 | 3.2 | 1.778 | 20.5 | - | 6.2 | 1.497 | 23.1 | - | 8.3 | 1.587 | 13.5 | - | ||||
| Cu | 57 | 37.0 | 1.187 | 7.7 | 29.3 | 8.7 | 1.568 | 12.6 | - | 27.7 | 1.171 | 14.0 | 13.7 | 2.8 | 2.427 | 8.6 | - | ||||
| Zn | 80 | 41.1 | 2.456 | 11.0 | 30.1 | 2.3 | 2.066 | 17.5 | - | 11.8 | 1.842 | 19.8 | - | - | - | - | - | ||||
| Pb | 20 | 47.5 | 1.584 | 3.1 | 44.4 | 1.3 | 1.334 | 4.7 | - | 1.2 | 1.428 | 5.8 | - | 1.0 | 1.174 | 3.2 | - | ||||
| Ga | 40 | 20.3 | 1.584 | 5.6 | 14.7 | 1.7 | 1.568 | 9.0 | - | 9.3 | 1.679 | 16.6 | - | 1.8 | 1.136 | 5.9 | - | ||||
| Ge | 2 | 35.7 | 2.851 | 1.3 | 34.4 | 1.3 | 1.134 | 0.8 | 0.5 | 7.7 | 1.549 | 0.6 | 7.1 | 1.1 | 1.136 | 0.7 | 0.4 | ||||
| Mo | 2 | 20.8 | 1.229 | 0.7 | 20.1 | 2.3 | 1.584 | 1.0 | 1.3 | 2.4 | 1.568 | 1.1 | 1.3 | 5.5 | 1.949 | 1.0 | 4.5 | ||||
| W | 2 | 51.4 | 2.398 | 1.1 | 50.3 | 3.4 | 1.995 | 1.1 | 2.3 | 20.6 | 2.917 | 1.6 | 19.0 | - | - | - | - | ||||
| Paragenesisis | Be, V, Co, Ni, Cu, Zn, Pb, Ga, Ge, Mo, W | Ge, Mo, W | Be, Cu, Ge, Mo, W | Be, Co, Ge, Mo | |||||||||||||||||
Table 1: Statistical parameters of the distribution microelements in the coals and their paragenesisis (n=30).
Decomposition of gross contents of microelements in coals by main carriers
The gross contents of trace elements in coal are determined by the total contents in the main carriers:
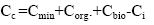 (11)
(11)
where Cmin, Corg., Cbio - the trace element content in the main in the plant mineral (in the plant material, paleophytomass); Ci - of the trace element content in soluble organic compounds (fulvic acids) removed from the organic system.
Equation (11) is the equation of the material balance of the trace element content in the organic system introduced by independent substances; it is closed. However, the value of Ci is unknown and is mainly due to soluble organic compounds, for example, fulvic acids [6,7]. This dilution factor can be neglected. Then, we obtain:
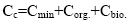 (12)
(12)
Analytic methods, these contents in carriers are indeterminate. A statistical method was used to estimate the contents of trace elements in the main carriers, which is as follows [13]. The trace element content in the mineral and organic components is determined by equations (9) and (10) and are reflected in the diagram (Figure 1). Obviously, the content of Corg is characterized by its own distribution function with an average value of C * org. Corg oscillations are confined to a confidence interval limited by the lower and upper limits. The lower limit is determined by the quantity C*org-[(Cl × Ad/100) + τν(1-α/2) σ/√n],
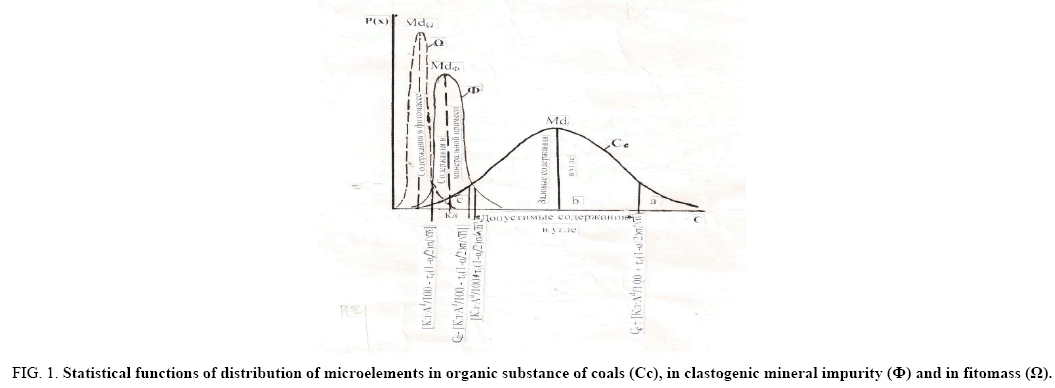
Figure 1: Statistical functions of distribution of microelements in organic substance of coals (Cc), in clastogenic mineral impurity (?) and in fitomass (Ω).
Upper C*org+[(Cl × Ad/100) + τν(1-α/2) σ/√n].
Md - The arithmetic mean (modal) content of the microelement, corresponding to distributions in the main carriers; gross contents of microelements, with respect to the boundaries of the confidence interval: a - exceeding the upper limit; b - for gross contents within the limits of the confidence interval; c - less than the lower limit. The permissible limits of gross contents in coal and the content in the mineral impurity are shown by functions.
The contents of Corg in these boundaries characterize the intrinsic concentrations of microelements in the organic part of the coal, are the total infiltration-organic concentrations and concentrations in plant matter (paleophytomass). It is possible with a probability of 95% to assert that the contents that go beyond the limits of the confidence interval do not belong to the contents of the confidence interval. Those of them that go beyond the upper boundary are due to the increased concentration of microelements in the coals, are elevated.
The content is less than the lower limit of the confidence interval, with the same probability; do not belong to the contents of the confidence interval. They are significantly lower than the latter, therefore, due to a different source of microelements and could not arise in the situation of infiltration of solutions. The only source of such accumulations of trace elements in coal was the paleophytomass.
These contents are biogeochemical concentrations associated with the accumulation "during the life of plants" [29]. Their possible fluctuations are within the limits of:
0<Cbio<{C*c-[(Cl × Ad/100) + τν(1-α/2) σ/√n]} (13)
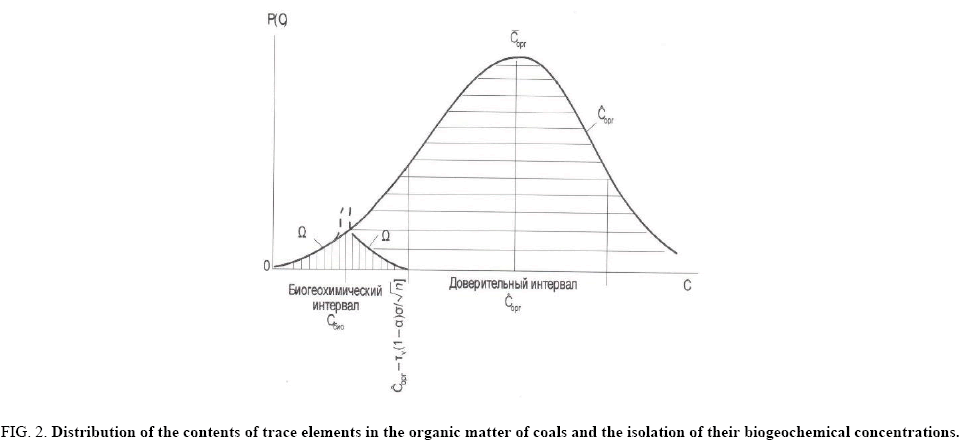
Biogeochemical concentrations are low, characterized by a small dispersion. It can be assumed that the left branch of the distribution curve in the considered interval (Figure 2) is part of the proper normal distribution of biogeochemical concentrations of microelements. The right side of this curve is complicated by the influence of infiltration-organic concentrations, somewhat obscuring biogeochemical concentrations, and these contents can be estimated [30].
Figure 2: Distribution of the contents of trace elements in the organic matter of coals and the isolation of their biogeochemical concentrations.
Ωο - The isolated function of distribution of biogeochemical concentrations of microelements; ? - Arithmetic mean (modal) content; Corg - Content in the organic part of coal; ?bio - Biogeochemical concentrations. Vertical hatching is the distribution density of biogeochemical concentrations of microelements, excised from gross contents in organic matter of coal.
Discussion
Since the biogeochemical concentrations are distributed according to the normal law, and the left-hand part of the branch of the curve is identified as the proper biogeochemical normal distribution, the right half of this branch of the distribution curve can be found by the mirror symmetry method.
The mirror plane is perpendicular to the content axis. The modal position of the mirror plane is found by moving it along the content axis. It is fixed when the right branch is a mirror image of the left one, at the same time, ending on the abscissa of the boundary of the confidence interval C*c-[(Cl × Ad/100) + τν(1-α/2) σ/√n] (Figure 2). In this case, the right branch of the distribution curve becomes a mirror image of the left branch of the proper distribution, and then this half of the curve will appear to belong to the normal distribution curve, by definition. The fixed position of the mirror plane immediately gives the mean (modal) value of biogeochemical concentrations equal to {C*c-[(Cl × Ad/100) + τν(1-α/2) σ/√n]}/2, and the curve as a whole is a proper normal distribution of biogeochemical concentrations of the microelement.
Obviously, all the gross contents of trace elements in the organic matter of coal include biogeochemical concentrations accumulated in the organic matter of the biochemical series, and infiltration-organic concentrations accumulated in the organic matter of the coal series and, thus, are complex. The mechanism of infiltration of solutions in the organic system is also due to the release of diagenetic minerals. The contents of microelements of diagenetic minerals constitute, together with infiltration-organic concentrations, the paragenesis of microelements in the coals. The contents of trace elements in the cluster mineral impurity were formed independently, and accumulated due to a solid runoff, i.e., into a pre-angle stage of the evolution of the organic system.
Conclusion
The contents of microelements, the figurative points of which are above the normal distribution curve, are due to the influence of infiltration solutions that obscure biogeochemical concentrations. They are easily calculated graphically. The isolation of parageneses and the study of the distribution of microelements along the main carrier in coals give more complete geochemical information about the processes of their accumulation in coals than the traditional analysis of gross contents. Such studies are effective in assessing the potential rare metality of coals.
References
- Henderson P. Inorganic geochemistry. ?oskow: Mir. 1985;339.
- Admakin LA. The relationship between the content of trace elements and ash. KokeKhim. 2004;9:8-11.
- ?dmakin L?. Factors of the condition of the organic system and petrogenesis of the fossil coals. Coke Chem. 2010;53(8):281-9.
- ?dmakin L?. Basic corporative property of the organic system and mikroelements in the fossil coals. Coke Chem. 2014;57(8):321-31.
- Stach E, Macquowsky M, Teichmuller M, et al. Pethrology of coal. ?: ?ir. 1978;556.
- ??nskaya SM, Drozdova TV. Geochemistry of organic matter. Mósków: Nauka. 1964;316.
- Given PH. An essay on the organic geochemistry of coal. Coal Sci. 1984;3:63-252.
- Admakin LA. Relations between the organic system and petrology of coal. Coke Chem. 2009;52(10):424-8.
- Zhemchuzhnikov YuA, Ginsburg AS. Principles of coal petrography. Moskow: Akad. Nauk SSSR. 1960;400.
- Degens EN. Geochemistry of sedimentary deposits. ?oskow: ?ir. 1967;300.
- Frank-Kateetsky VA. (ed) X-ray of basic types of rock-building minerals (layered and framework silicates). Leningrad: Nedra. 1983;359.
- Krynov SR, Ryshenko BN, Shvets VM. Geochemistry of interground waters. Theoretical, applied and ecological aspects. M Nauka. 2004;677.
- ?dmakin LA. Analysis of the microelement content in coal. Coke and Chem. 2017;60(8):311-5.
- Admakin LA. Oxidation-reduction regimen of the medium and transformation of the organic matter of fossil coals. 2008; 51(4):119-124.
- Vernadsky VI. Essays on geochemistry. Part 1: Introduction to Geochemistry. Moskow: Nauka. 1994;495.
- Vernadsky VI. Selected researchin studies. ?oskow: AcadNauk. SSSR. 395-410.
- Admakin LA. Geochemical regimens of the organic system and paragenesis of microelements in coal. Coke Chem. 2015;58(11):411-8.
- Admakin LA. Accumulation and post-sedimentation transformations of Tonshtein. Lithology and Mineral Deposits. 2002;37(1):68-76.
- Admakin LA. Features of the material composition of fossil dopplerite. Lithology and Mineral Deposits. 1998;33(1):92-5.
- Perelman AI. Geochemistry of elements in the hypergenicZona. Mósków: Nauka. 1972;288.
- Helgeson G. Complexation in hydrothermal solutions. Moscow: Mir. 1967;184.
- Ivanova FI, Krivega IN. Salted coals of the Western Donbass. Kiev: NaukovaDumka. 1985;288.
- ?dmakin LA. Associations and paragenesesvicroelements in coals. Coke and Chem. 2016;59(12):444-50.
- ?dmakin LA. Basic fundamental dependencies in the petrology of fossil coals. KoksKhim. 2016;9:2-13.
- Vinogradov AP. Geochemistry of rare and dispersed chemical elements in soils. ?oskow: AkadNauk SSSR. 1957;238.
- Hudson DJ. Statistics for Phiziks. ?oskow: ?ir. 1970;103.
- ?dmakin L?. Individual contributions to the overall microelement concentrations in coals. Coke and Chemi. 2017;60(6):226-30.
- Jenkins G, Watts D. Spectral analysis and its applications. ?oskow: ?ir. 1961;1:316.
- Gjldschmidt VV. Collection of researchpapers on geochemistry of rare elements.Jb'edNauchno-Tech Izd. 1938:244.
- Admakin LA. The verify valuation of the biogeochemical concentrations of microelements in coal. Coke Chem. 2017;60(10):13-6.