Original Article
, Volume: 16( 1)Kinetics and Mechanism of Ruthenium (III) Chloride Catalyzed Oxidation of Sulfanilic Acid by Thallium (III) in Acid Perchlorate Medium
- *Correspondence:
- Sailani R, Department of Chemistry, University of Rajasthan, Jaipur-302004, India, Tel: 0141 270 3655; E-mail: l.p_riya@yahoo.co.in
Received: November 18, 2017; Accepted: December 26, 2017; Published: January 05, 2018
Citation: Sailani R, Pareek D, Meena A, et al. Kinetics and Mechanism of Ruthenium (III) Chloride Catalyzed Oxidation of Sulfanilic Acid by Thallium (III) in Acid Perchlorate Medium. Int J Chem Sci. 2018;16(1):230
Abstract
The kinetics and mechanism of oxidation of sulfanilic acid by thallium (III) in acid perchlorate medium have been studied. The reaction exhibits first order with respect to oxidant whereas order with respect to sulfanilic acid is complex. The rate is retarded by hydrogen ion concentration. Accounting for all such observations, a plausible reaction mechanism has been suggested. The activation parameters such as energy and entropy of activation have also been calculated to be (37.61 ± 0.3) kJ mol-1 and (-157.9 ± 1.6) JK-1 mol-1 respectively employing Eyring equation.
Keywords
Kinetics; Mechanism; Ruthenium (III) chloride; Oxidation; Sulfanilic acid; Thallium (III)
Introduction
Sulfanilic acid (p-amino benzene sulfonic acid) is considered to be an important reagent in synthesis of organic dyes [1]. The substituted sulfanilic acid such as sulfonamides is finding extensive applications in synthesis of sulfa drugs [2,3]. A large number of oxidants such as cerium (IV) [4], persulfate [5,6], hydrogen peroxide [7], periodate [8-11], peroxomonophosfate [12,13] and peroxydisulfate [6] have been employed to understand the mechanism of oxidation of the acid. However, there are certain ambiguities in kinetic analysis of the ruthenium (III) and osmium (VIII) catalyzed oxidations respectively of this acid in certain oxidation reactions particularly catalytic oxidation of sulfanilic acid by hexacyanoferrate (III) both in acid and alkaline media.
Thallium (III) considered being an important two-equivalent oxidant has been employed in a large number of kinetic studies [14-17]. Since thallium (II) to be an intermediate in its oxidations in one-equivalent change is also reported, evidence has been adduced and now it is well matured in its oxidation reactions as reactions employing thallium (II) has also been reported. Since we are engaged with kinetic studies of oxidations by thallium (III) in our laboratory [18-30], it is thought proper to study the oxidation reaction of sulfanilic acid by employing this oxidant in view of the purpose whether or not the oxidation reaction takes place via complex formation between oxidant and substrate. Secondly, is there any possibility of finding an intervention of thallium (II) as an intermediate! Also, thallium (III) is known to form strong chlorothallium (III) complexes; the pattern of reactivity of such complexes towards sulfanilic acid is supposed to provide useful information of the reaction mechanism.
Materials and Methods
Thallic oxide (Acros) was employed for the preparation of thallic perchlorate solution. Thallic oxide (Tl2O3) in a black powder form was added in small lots into heated 70% perchloric acid (E. Merck, 70%) and heating was continued until solution becomes colourless. Other reagents and their preparations have already been mentioned elsewhere. Aqueous solution of sulfanilic acid (E Merck) was prepared by dissolving the requisite amount in doubly distilled water and the solution was kept in bottles painted black from the outside at refrigerated temperature ( ~ 5°C) to eliminate decomposition due to diffused photo light. However, it was observed that the solution of sulfanilic acid did not degrade even at ambient temperature ascribing stability of such a solution to be quite appreciable. Further, it has also been observed that the solution of sulfanilic acid is stabilized by addition of small amount of very dilute alkali solution. This is probably due to the presence of –NH3+ and –SO3- groups in the structure of sulfanilic acid as a zwitter ion [3]. In alkaline solution, the strongly basic hydroxide ion pulls hydrogen ion away from the weakly basic –NH2 group to yield the water-soluble p-aminobenzenesulfonate ion (II). Therefore, the solution of sulfanilic acid was prepared by dissolving appropriate amount of the sample in warm water adding very dilute alkaline solution.
Doubly distilled water was employed throughout the kinetics of the reaction; the second distillation was from an alkaline solution of KMnO4 in an apparatus of all glass. Other reagents were employed as supplied without any further treatment. Ru (III) was used by dissolving RuCl3 in dilute HCl (0.3 mol dm-3). Such a solution of Ru (III) was quite stable and stored in a bottle painted black from the outside to eliminate any further decomposition owing to diffused light.
Stoichiometry and product analysis
The stoichiometry of the reaction was determined by allowing the reaction mixtures containing excess of thallium (III) concentrations over that of sulfanilic acid in a thermo stated water-bath at (35 ± 0.1)°C for ca for 8 h. The excess concentration of thallium (III) was estimated iodometrically. Such results indicate that two mole of thallium (III) requires a mole of sulfanilic acid exhibiting stoichiometry corresponding to the reaction as represented by eqn. 1.
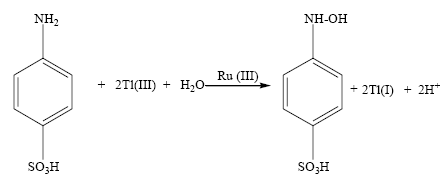
Tl (I) being one of the reaction products was tested as yellow-orange precipitate of thallus iodide. However, the oxidation product of sulfanilic acid was spectrally identified and established to be N-hydroxylaminobenzene-4-sulphonic acid. The reaction product, N-hydroxylaminosulfonic acid was eluted by thin layer chromatography. Thin layer chromatograms were conducted on Merck silica gel G plates in methyl alcohol: ethyl acetate (8:2) and in the column chromatographic fractionations silica gel (60-120 mesh). Spots on TLC plates were visualized by spraying with 2% cerric ammonium sulfate in 2 N H2SO4 or with iodine vapors.
IR spectrum of the product showed absorption band at 3415 cm-1 and 3480 cm-1 indicating two stretching bands of amine. Absorption at 3552 cm-1 indicates the presence of -OH strong stretching band. The bands at 1618 cm-1 and 1643 cm-1 respectively indicate the C=C stretching frequency of aromatic group. The absorption band at 1094 cm-1 shows the presence of stretching frequency of=C-NH.
1HNMR spectrum was also obtained in DMSO-d6 employing 300 MHz spectrometer using TMS as reference NMR signal at δ4.039 (s) ppm is due to -NH group of amine. Two singlets are obtained at δ2.5 ppm and δ2.06 ppm showing the presence of -OH group. The mass spectrum of this product displayed a peak at m/z 187 after the loss of two hydrogens from its molecular ion at 189. The elemental analysis was in agreement with a molecular formula C6H7O4NS. The product N-hydroxyl amino sulfonic acid in acid medium does not undergo any further oxidation under the present kinetics conditions.
Kinetic procedure
Kinetics of the reaction was monitored by assaying thallium (III) iodometrically [31,32]. Reaction mixtures containing all other reaction ingredients except thallium (III) were prepared in glass stoppered Erlenmeyer flasks which were suspended in water-bath thermostated at 35 ± 0.1°C unless stated otherwise. The reaction was initiated by adding temperature pre-equilibrated solution of thallium (III) into the reaction mixture. The time of initiation of the reaction was recorded when half of the contents from the pipette were released. Initial rates (ki, moldm-3 s-1) were computed by employing glass mirror method [33]. The kinetic results in triplicate were reproducible to within ± 5%.
Results
Thallium (III) ion concentration dependence
The concentration of thallium (III) was varied from 1.0 × 10-3 to 5.0 × 10-3 mol dm-3 at three but fixed concentrations of sulfanilic acid (hereto forth written as SA) to be 5.0 × 10-3, 1.0 × 10-2 and 2.0 × 10-2 mol dm-3 respectively keeping constant concentrations of other reaction ingredients viz. [Ru (III)]=2.0 × 10-5 mol dm-3; [H+]=0.5 mol dm-3 and [NaClO4]=0.5 mol dm3 at 35°, 40° and 45°C respectively. Initial rates were computed and then a plot of initial rate (ki, mol dm-3 s-1) vs. concentration of thallium (III) was made that yielded a straight line passing through the origin conforming order with respect to thallium (III) to be one.
Sulfanilic acid concentration dependence
The concentration of sulfanilic acid was varied from 4.0 × 10-3 to 3.0 × 10-2 mol dm-3 at three but fixed concentrations of [H+]=0.5, 1.0 and 1.5 mol dm-3 respectively keeping constant concentrations of other reaction ingredients viz. [Tl (III)]=2.0 × 10-3 mol dm-3; [Ru (III)]=2.0 × 10-5 mol dm-3 and [NaClO4]=0.5 mol dm-3 at 35°C.
The plot of initial rate against the concentration of sulfanilic acid was made which initially shows a straight line passing through the origin and then tended towards a limiting rate at higher concentrations of the organic acid exhibiting variable order with respect to sulfanilic acid.
Hydrogen ion dependence
The effect of hydrogen ion concentration was studied by employing perchloric acid. The concentration of hydrogen ion was varied from 0.5 mol dm-3 to 2.0 mol dm-3 at three but fixed concentrations of [SA]=5.0 × 10-3, 1.0× 10-2 and 2.0 × 10-2 mol dm-3 respectively keeping constant concentrations of other reaction ingredients viz. [Tl (III)]=2.0 × 10-3 mol dm-3; [Ru (III)]=2.0 × 10-5 mol dm-3 and I=2.0 mol dm-3 at 35°C. The rate decreases slightly. (‘I’ is ionic strength which was maintained constant by employing sodium perchlorate).
Effect of ionic strength (I)
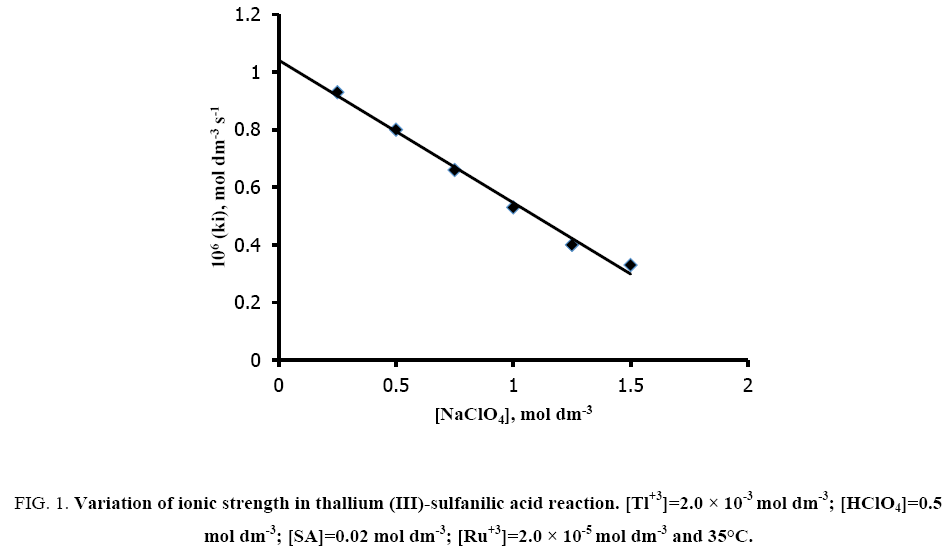
The effect of ionic strength (I) was studied by employing sodium perchlorate at fixed concentrations of reaction ingredients viz. [SA]=2.0 × 10-2 mol dm-3; [Tl (III)]=2.0 × 10-3 mol dm-3; [H+]=0.5 mol dm-3 and [Ru (III)]=2.0 × 10-5 mol dm-3 at 35°C. The rate of the reaction decreases with increasing ionic strength (Figure 1).
Figure 1: Variation of ionic strength in thallium (III)-sulfanilic acid reaction. [Tl+3]=2.0 × 10-3 mol dm-3; [HClO4]=0.5 mol dm-3; [SA]=0.02 mol dm-3; [Ru+3]=2.0 × 10-5 mol dm-3 and 35°C.
Effect of sodium chloride
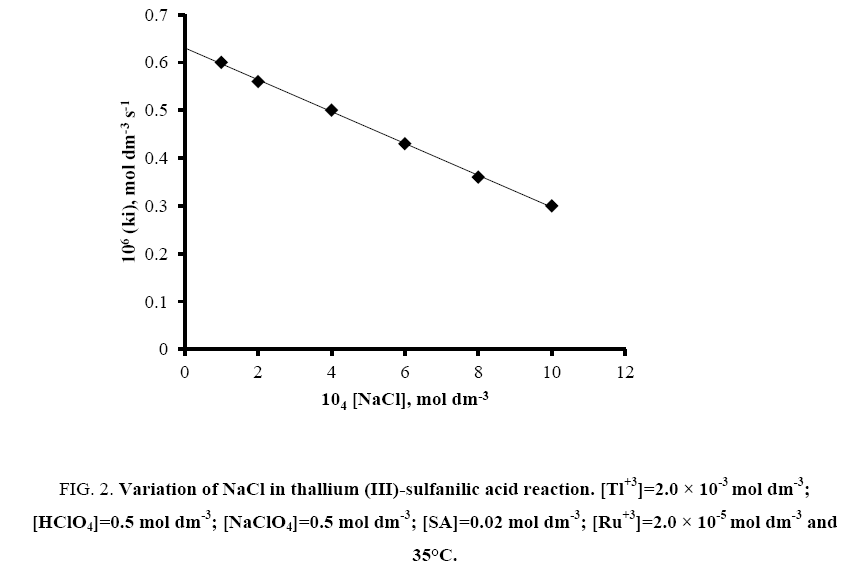
The effect of sodium chloride was also studied by varying its concentration from 1.0 × 10-4 to 1.0 × 10-3 mol dm-3 keeping fixed concentrations of other reaction ingredients viz. [SA]=2.0 × 10-2 mol dm-3; [Tl (III)]=2.0 × 10-3 mol dm-3; [H+]=0.5 mol dm-3; [NaClO4]=0.5 mol dm-3 and [Ru (III)]=2.0 × 10-5 mol dm-3 at 35°C. The rate decreases with increasing concentration of chloride ion (Figure 2).
Figure 2: Variation of NaCl in thallium (III)-sulfanilic acid reaction. [Tl+3]=2.0 × 10-3 mol dm-3; [HClO4]=0.5 mol dm-3; [NaClO4]=0.5 mol dm-3; [SA]=0.02 mol dm-3; [Ru+3]=2.0 × 10-5 mol dm-3 and 35°C.
Effect of thallium (I)
The concentration of thallium (I) was varied from 1.0 × 10-3 to 5.0 × 10-3 mol dm-3 at fixed concentrations of other reaction ingredients. However, rate remains unchanged eliminating any fast equilibrium preceded by the rate determining step.
Test of free radicals
The test of free radicals in reaction mixture was also conducted by adding acrylic acid/acrylonitrile. However, no white sediment was observed during the progress of the reaction which eliminated the probability of polymerization of acrylic acid. Nonetheless, such an exclusive observation does not justify the absence of free radicals. There is every possibility of formation of free radicals in view of the energetically more facile one equivalent process. It appears that the free radicals as soon as are formed, they immediately react with the substrate before finding time to diffuse out of the solvent cage for polymerization.
Effect of temperature
The effect of temperature was studied at 35°C, 40°C and 45°C respectively at constant concentrations of other reaction ingredients viz. [SA]=2.0 × 10-2 mol dm-3; [Tl (III)]=2.0 × 10-3 mol dm-3; [H+]=0.5 mol dm-3; [I]=1.0 mol dm-3 and [Ru (III)]=2.0 × 10-5 mol dm-3. The activation parameters such as energy and entropy of activation were evaluated by employing Eyring equation [34] to be (37.61 ± 0.3) kJ mol-1 and (-157.9 ± 1.6) JK-1 mol-1 respectively.
Discussion
Thallium (III) solutions in acid medium are sufficiently stable but in dilute acid hydrolysis becomes important as represented by eqn. (2).
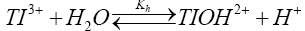 (1)
(1)
The hydrolysis constant (Kh) has been reported [35,36] to be 0.086 at 25°C. Since the rate decreases with increasing hydrogen ion concentration, the hydrolyzed species TlOH2+ appears to be more reactive species than thallic ion (Tl3+ (aq)). Such an observation is not unique more particularly in view of the large number of thallium (III) reactions where hydrolyzed species has been reported to be the reactive species to account for observed hydrogen ion dependence [37]. So far sulfanilic acid is concerned; its protonated form is the only predominant species in the light of small dissociation constant at 25°C. Thus, sulfanilic acid species does not contribute to hydrogen ion dependence in the reaction.
If TlOH2+ to be the species of thallium (III) and sulfanilic acid (heretofore written as SA) are taken into account, the following mechanism consisting of steps (2) to (5) accounting for experimental observations can be envisaged.
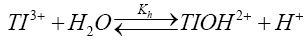 (2)
(2)
 (3)
(3)
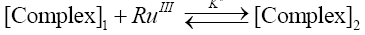 (4)
(4)
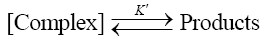 (5)
(5)
Such a mechanism leads to the rate law (6)
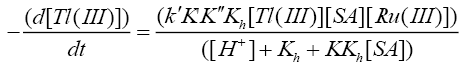 (6)
(6)
[Tl (III)] and [SA] are the gross analytical and free equilibrium concentrations of thallium (III) and sulfanilic acid respectively. Since uncatalyzed path also simultaneously occurs, the rate law accounting for such a path can be given by eqn. (7).
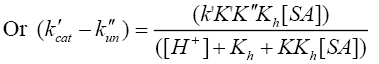 (7)
(7)
Where, k'cat and k"un are first order rate constants for catalyzed and uncatalyzed paths. Since equilibrium constant K' for ternary complex is insignificant i.e kK=k’K’K”, the rate law (7) is further reduced to eqn. (8).
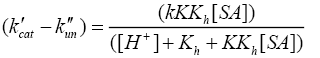 (8)
(8)
The double reciprocal of eqn. (8) on re-arrangement yields eqn. (9).
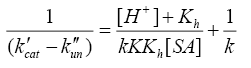 (9)
(9)
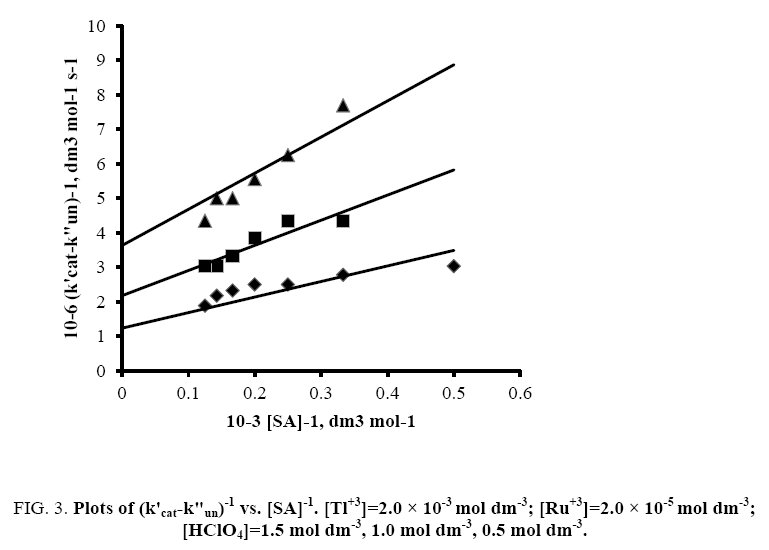
A plot of (1/k'cat-k"un) vs. 1/[SA] was made from eqn. (9) that yielded a straight line with non-zero intercept (Figure 3) k was calculated from the intercept to be 3.05 × 10-4 dm3 mol-1 s-1 at 35°C. The gradient (G) of the line can be represented by eqn. (10) or (11).
Figure 3: Plots of (k'cat-k"un)-1 vs. [SA]-1. [Tl+3]=2.0 × 10-3 mol dm-3; [Ru+3]=2.0 × 10-5 mol dm-3; [HClO4]=1.5 mol dm-3, 1.0 mol dm-3, 0.5 mol dm-3.
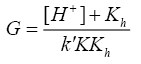 (10)
(10)
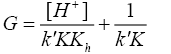 (11)
(11)
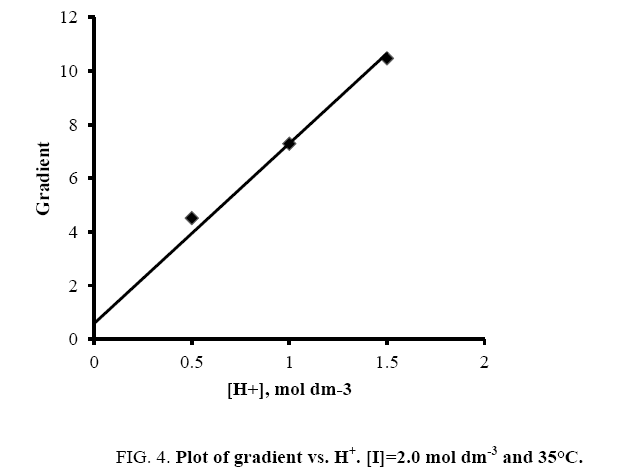
A plot of ‘G’ vs. [H+] was further made from eqn. (11) that also yielded a straight line with non-zero intercept (Figure 4).
Kh was calculated from the ratio of intercept and slope to be (0.086 ± 0.002) in good agreement with the earlier reported hydrolysis constant of thallium (III). Since k was determined from eqn. (9) and Kh from eqn. (11), K was calculated from the slope of eqn. (11) by employing these values of k and Kh to be 1.24 ± 0.01 × 103 dm3 mol-1 at I=2.0 mol dm-3 and 35°C.
The activation parameters such as energy and entropy of activation calculated by employing Eyring equations are (37.61 ± 0.3) kJ mol-1 and (-157.9 ± 1.6) JK-1 mol-1 respectively. The lower energy of activation is in favor of formation of an intermediate complex. The (-) ve entropy of activation favors more compact transition state. So far, the proposed intermediate complex in step (3) of the reaction mechanism is concerned; kinetic evidence supports such a proposal. Further thallium (III) is transparent in visible spectral region, no such complex was observed between oxidant and substrate spectrally. However, a slight shift in UV region at 210 nm of the oxidant on addition of the substrate might be an indication of complexation. Nevertheless, rate of the reaction is strongly retarded by chloride ion concentration. Such an observation might be taken as strong indirect evidence to favor the formation of an intermediate complex between thallium (III) and sulfanilic acid.
Chloride ions are known [38] to form quantitatively strong chloro-thallium (III) complexes in a successive manner as follows where K1>K2>K3>K4.
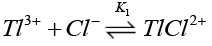 (12)
(12)
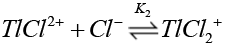 (13)
(13)
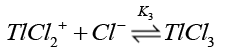 (14)
(14)
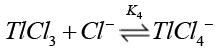 (15)
(15)
Since chloride ion blocks the co-ordination sites of the metal-ion, the chances of incorporation of sulfanilic acid in the coordination shell of thallium (III) are significantly reduced. This is obviously not the case as chloride ion ligand forms chlorothallium (III) complex with formation constant of the order of ~ 105 and thus scores over sulfanilic acid for occupation of coordination sites of thallium (III). Had it not been the situation, sulfanilic acid would have been in the co-ordination shell of the metal ion. This is exactly what we observed in the title reaction that an intermediate complex between oxidant and the substrate is supported by the kinetic evidence.
Since all such chloride ion decelerated [39,40] reactions of thallium (III) are reported to occur via an intermediate complex, the title reaction should also occur via an intermediate complex. It is now well matured that thallium (II) is an intermediate in oxidation of thallium (I) or reduction of thallium (III). However, test of free radical is negative in the reference reaction; there is less probability of formation of thallium (II) as an intermediate mainly on account of the following facts:
The rate of disproportionate [41,42] of thallium (II) is much larger than that of electron transfer reactions (16, 17).
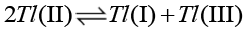 (16)
(16)
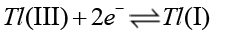 (17)
(17)
Had it not been the situation, Tl (II) would have been trapped by a monomer. The standard oxidation potential [43] (vs. NHE) for TIII/TII redox couple (3.14 V) is much larger than that of TlIII/TlI redox couple (1.45 V), showing Tl (II) to be a much stronger oxidant than Tl (III). As soon as thallium (II) is formed as an intermediate, in all probability it remains in the solvent cage and immediately attacks the substrate before it comes out of the cage for polymerization of the monomer.
Thallium (III) is substitution labile in view of high exchange rate [44,45] of water for aqua thallium (III) (~ 3 × 108 s-1). Thus, the substitution rate of aqua ligand by sulfanilic acid must be much higher than the observed exchange rate for thallium (III)- sulfanilic acid complex formation to be the rate controlling step. Since this is not an observed situation in the title reaction, the reaction in all probability is redox controlled and not substitution controlled [46,47]. So far the mode of electron transfer from the substrate to the oxidant is concerned; the reaction events can be understood by the following SCHEME 1.
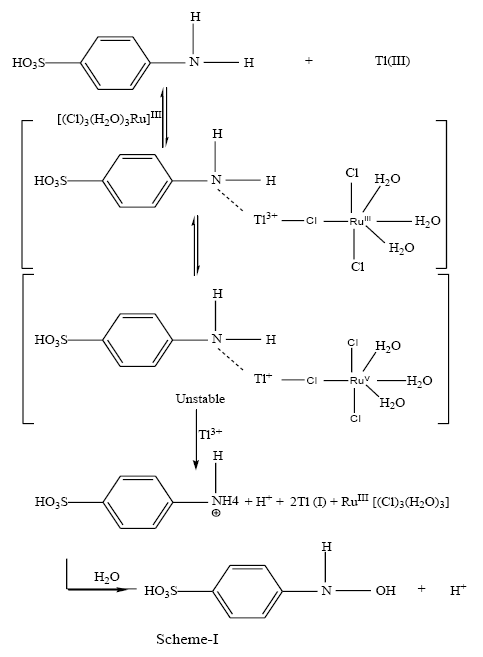
Conclusion
Therefore, the proposals as suggested on the premise of experimental observations are more logical in the light of the following observations:
 If oxidation potentials of Tl3+/Tl+ and Tl2+/T+ redox couples are taken into account, Tl (II) is certainly a stronger oxidant than thallium (III). It is, therefore, probable that Tl (II) intermediate, in the solvent cage to immediately reacts with substrate free radical. This appears to be the reason of not finding free radical formation in the reaction kinetics. Since the reaction in all probability is redox controlled and not substitution controlled.
If oxidation potentials of Tl3+/Tl+ and Tl2+/T+ redox couples are taken into account, Tl (II) is certainly a stronger oxidant than thallium (III). It is, therefore, probable that Tl (II) intermediate, in the solvent cage to immediately reacts with substrate free radical. This appears to be the reason of not finding free radical formation in the reaction kinetics. Since the reaction in all probability is redox controlled and not substitution controlled.
 Rate of the reaction is strongly retarded by chloride ion concentration. Such an observation might be taken as strong indirect evidence to favor the formation of an intermediate complex between thallium (III) and sulfanilic acid.
Rate of the reaction is strongly retarded by chloride ion concentration. Such an observation might be taken as strong indirect evidence to favor the formation of an intermediate complex between thallium (III) and sulfanilic acid.
Acknowledgement
This work was supported in part by the University Grant Commission, New Delhi, India through senior research fellowship for financial support. Acknowledgment is also made to the co-authors and Head of Department of Chemistry, UOR, Jaipur, Rajasthan, India.
References
- Brewster RQ, Mcewen WE. Organic Chemistry. 6th edn. Prantice-Hall of India, New Delhi. 1971.
- Diaz M, Luiz M, Bertolotti SG, et al. Scavenging of photogenerated singlet molecular oxygen and superoxide radical anion by sulpha drugs-kinetics and mechanism. Can J Chem.2004;82:1752-9.
- Morrison RT, Boyd RN. Organic Chemistry. 6th edn. Prantice-Hall of India, New Delhi.2001;pp:862-4.
- Dziegiec J. Kinetics and mechanism of oxidation of sulfanilic acid with cerium(IV) in perchloric acid. Pol J Chem.1979;53:1821-8.
- Alexiev AA, Bontschev PR, Burdarov VI. Catalytic effect of copper(II) on the oxidation of sulfanilic acid with potassium persulfate. KhimFaku.1975;66:237-50.
- Alexiev AA, Bontschev PR. Mechanism of silver(I) catalyzed sulfanilic acid oxidation by persulfate. J InorgNucl Chem.1970;32:2237-46.
- Angelova M, Stoyanova A, Alexiev A. Catalytic spectrophotometric determination of cobalt (II) by sulfanilic acid-hydrogen peroxide system. Trak J Scien.2008;6:12-7.
- Srivastava SP, Bhattacharjee G, Gupta VK, et al. Kinetics and mechanism of oxidation of sulfanilic acid by periodate ion in aqueous medium. ReacKinet Cat Lett.1980;13:231-7.
- Gupta VK, Pal S, Kumar A, et al. Thermodynamic and related studies of the oxidation of p-aminobenzoic acid, sulfanilic acid and anthranilicacid by periodate. ThermochimActa.1989;140:197-202.
- Alexiev AA, Mutaftchiev KL. A selective catalytic spectrophotometric determination of manganese in subnanogram amounts throughoxidation of sulfanilic acid by potassium periodate with 1,10-phenanthroline as activator.MikrochimActa.1985;2:115-25.
- Caro A, Cortes G, Cerda V. Kinetic-thermometric determination of manganese(II) based on its catalytic action on the oxidation ofsulfanilic acid by periodate. Analyst (London).1990;115:753-5.
- Panigrahi GP, Nayak RN. Kinetic and mechanistic studies on the oxidation of sulfanilic acid by peroxomonophosphoric acid. ReacKinet Cat Lett.1982;21:283-7.
- Panda J, Panigrahi GP. Kinetic investigation of the oxidation of sulphanilic acid by peroxomonophosphoric acid in anionic surfactant sodium lauryl sulphate. Ind J Chem.2003;42:1636-8.
- Varale A, Hilage N. Oxidation of nicotinic acid hydrazide by thallium (III) in acidic medium: A kinetic and mechanistic study. Int J Chem Sci.2009;7:2173-82.
- Chimatadar SA, Basavaraj T, Nandibewoor ST. Ruthenium(III) mediated cerium(IV) oxidation of thallium(I) in aqueous sulphuric acid-a kinetic andmechanistic study. InorgReac Mech.2002;4:209-19.
- Bhat KR, Jyothi K, Gowda BT. Mechanism of Ru(III) catalysedTl(III) oxidation of di- and tri-substituted phenols in aqueous acetic acid. Akinetic study. OxidnCommun.2002;25:117-41.
- Asthana SK, Mishra SK, Nand KC. Kinetics of oxidation of glutamic acid by thallium(III) perchlorate in the presence of metal ion catalysts. OxidnCommun.1997;20:132-8.
- Gupta P, Sharma PD, Gupta YK. Kinetics and mechanism of oxidation of lactic and mandelic acids by thallium(III) in acid perchlorate solution. Ind J Chem.1984;23:392-6.
- Gupta P, Sharma PD, Gupta YK. Kinetics and mechanism of the oxidation of glycolic and glyoxylic acids by thallium(III) in acidic perchlorate solutions. J ChemSoc Dalton Trans.1984;9:1867-71.
- Sharma PD, Mishra SK, Gupta YK. Kinetics and mechanisms of thallium(III) oxidation reactions. RasayanSamiksha.1986;1:20-44.
- Rao I, Mishra SK, Sharma PD. Kinetics and mechanism of electron-transfer reactions of aquothallium(III) and coordinated thallium(III),oxidation of malonic acid with thallium(III) in perchlorate medium. Ind J Chem.1991;30: 773-6.
- Sharma N, Mishra SK, Sharma PD (1992) Kinetics and mechanism of oxidation of uric acid with thallium (III) in acetate buffers. Trans Met Chem. 17: 224-227.
- Gupta C, Mishra SK, Sharma PD. Trace metal ion catalysis. Part 2. Kinetics and mechanism of ruthenium (III) chloride-catalyzed oxidation of butane-1,4-diol by thallium(III) in acid perchlorate medium. J Chem Res.1993;7:254-6.
- Nahar S, Sharma PD. Kinetics and mechanism of electron-transfer reaction of aquothallium(III) and coordinated thallium(III).Oxidation of metol with thallium(III) in acid perchlorate medium. J Phy Org Chem.1994;7:117-21.
- Bhasin M, Sharma I, Sharma PD. Kinetics and mechanism of ruthenium(III) chloride catalyzed oxidation of propane-1,3-diol by thallium(III) in acid perchlorate medium. J Chem Res.1999;1:201-18.
- Binyahia AR, Dubey S, Sharma PD. Kinetics and mechanism of electron transfer reactions in acid perchlorate medium. Hydrogen ion dependence on the rate of oxidation of formic acid by thallium perchlorate catalyzed by ruthenium (III) chloride in acid aqueous medium. OxidnCommun.2000;23:246-54.
- Bhasin M, Bansal S, Sharma PD. Kinetics and mechanism of ruthenium(III) chloride catalyzed oxidation of hypophosphorous acid bythallium(III) in acid perchlorate medium. OxidnCommun.2000;23:515-23.
- Bhasin M, Dubey S, Sharma I, et al. Kinetics and mechanism of ruthenium(III) chloride catalyzed oxidation of phosphorous acid by thallium(III) in acid perchlorate medium:Role of tautomeric forms of the phosphorous acid. Ind J Chem.2000;39:1036-40.
- Vijay N, Bhasin M, Sharma I, et al. Kinetics and mechanism of electron transfer reactions in acetate buffers: Ruthenium (III) chloride catalysed oxidation of nitrite by thallium (III) acetate. Ind J Chem.2001;40:708-13.
- Hemkar S, Sailani R, Khandelwal CL, et al. Kinetics and mechanism of electron-transfer reactions: Oxidation of butanone and cyclohexanone by thallium (III) in acid perchlorate medium:An appraisal of keto form reactivity. Am J Phy Chem.2013;2:73-9.
- Kolthoff IM, Belcher R, Stengar R, et al. Volumetric Analysis.Interscience.1957;3:212-4.
- Senger HGS, Gupta YK. Kinetics of silver catalysed oxidation of Tl (I) by persulphate. J IndChem Soc.1966;43:223-4.
- Latshaw M. A simple tangentimeter. J Am Chem Soc.1925;47:793-4.
- Gabor L, Istvan F, Poe AJ. A common misconception about the Eyringequation. New J Chem. 2005;29:759-60.
- Nord G. Oxidation of some tris (2,2’-bipyridyl) and tris (1,10-phenenthroline) osmium (II) salts by thallium (III) in aqueous acid. Inorg Chem.1976;15:1921-5.
- Thakuria BM, Gupta YK. Kinetics and mechanism of electron-transfer reactions of aqueous and co-ordinated thallium(III). Part XII. Reduction of hexa-aquathallium(III) by hydrazine. J ChemSoc Dalton Trans.1975;pp:2541-5.
- Thakuria BM, Gupta YK.Kinetics and mechanism of electron-transfer reactions of aqueousand co-ordinated thallium(III). Part IX. Stoichiometry and kinetics of reduction of hexa-aquathallium(III) by hydroxylamine. J ChemSoc Dalton Trans.pp:77-81.
- Harbottle G, Dodson RW. The exchange reaction between the two oxidation states of thallium in solution1. J Am Chem Soc.1951;73:2442-7.
- Gilks S, Rogers TE, Waind GM. Salt effects on the rate of exchange of thallous and thallic ions in water and heavy water. Part 2.The effect of chloride on the solvent reaction. Trans. Faraday Soc.1961;57:1371-6.
- Duke FR, Bornong B. The Fe(II)-Tl(III) reaction at high chloride concentration. J Phys Chem.1956;60:1015-6.
- Sharma PD, Gupta YK. Reduction of chlorothallium (III) complexes by arsenic (III). Austr J Chem.1973;26:2115-22.
- Sharma PD, Gupta YK. Kinetics and mechanism of the reduction of thallium (III) by arsenic (III) in perchlorate acid solution. J ChemSoc Dalton Trans.1972;pp:52-5.
- Catherino HA, Jordan J. Bivalent thallium and the mechanism of the electrode reaction Tl3++ 2e ?Tl+.Talanta1964;11:159-73.
- Catherino HA, Jordan J. Divalent thallium. J Phys Chem.1963;67: 2241-2.
- Lefort M, Tarrago X. Reduction of thallic ions in acid solutions by alpha and gamma rays. Effects of thallous ion concentration. J Chim Phys.1960;57:38-46.
- Banyai I, Glaser J. Equilibrium dynamics in the thallium(III)-chloride system in acidic aqueous solution. J Am Chem Soc.1989;111:3186-94.
- Banyai I, Glaser J. Equilibrium dynamics in the thallium(III)-bromide system in acidic aqueous solution. A thallium-205 NMR study. J Am Chem Soc.1990;112:4703-10.