Original Article
, Volume: 12( 3)Biosurfactant Producing Microbe Isolation and Effect of Different pH on its Growth
- *Correspondence:
- Arpita Roy, Department of Biotechnology, Delhi Technological University, New Delhi, India, Tel: (+98)-71-32424127; E-mail: arbt2014@gmail.com
Received: October 12, 2017; Accepted: October 31, 2017; Published: November 04, 2017
Citation: Arpita Roy. Biosurfactant Producing Microbe Isolation and Effect of Different pH on its Growth. Res Rev Biosci. 2017;12(3):135
Abstract
Biosurfactants are surface active molecules which contain both hydrophobic and hydrophilic groups. Due to its amphiphilic nature it has the properties like foaming, emulsification, dispersing and detergency. Due to the various advantages of biosurfactants over chemical surfactants such as environment friendly, lower toxicity, biocompatibility, higher biodegradability, higher stability, extreme etc. they are widely utilized. They possess various applications in industries like health care, cosmetics, bioremediation, oil industries, etc. These biosurfactants are produced by wide range of microbes as an extracellular compound. The present study was done to isolate the potential biosurfactant producing bacteria and effect of pH on production of biosurfactant. All the isolates were screened for biosurfactant production and the presence of surfactant activity was determined by the oil displacement technique, emulsification index and CTAB assay. After identifying the potential isolate optimization of pH was done.
Keywords
Biosurfactant; Microbes; Oil displacement; Emulsion; CTAB; PH
Introduction
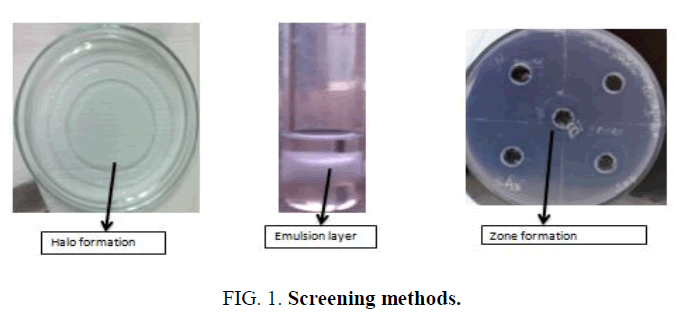
Biosurfactants are surface active compounds which are produced by the microbes either they secreted it extracellular or produced them on their surface. It contains both hydrophobic and hydrophilic portion which are able to reduce the interfacial and surface tension. A biosurfactant can have several structures i.e. glycolipids, polysaccharide-lipid composite, mycolic acid, phospholipid, lipoprotein/lipopeptide, or the microbial cell surface itself [1]. There is an increase attention towards the production of surfactants from biological source due to their potential role in oil industry, food-processing and pharmacology [2]. Production of biosurfactant depends mainly on the particular type of microbe but other than this there are several factors which also influence the biosurfactant produce i.e. pH, temperature, aeration, etc. [3]. Releasing of hydrocarbons into the surrounding whether due to human activities or accidentally leads to the water and soil pollution. Contamination of soil with the hydrocarbons causes damage to the animals and plant tissue as pollutants accumulation in the tissues which leads to the death [4]. Hydrocarbon degrading microbes produced variety of biosurfactant which are of different chemical nature and molecular size. Wide variety of microbes has reported to show biosurfactant activity. Microbes are able to utilize the wide variety of organic compounds as carbon and energy source for their growth. The present study was done to isolate the biosurfactant producing microbes from petroleum contaminated soil and screening the microbes for its biosurfactant activity. Further effect of various pH on the microbial growth was studied (Figures 1 and 2).
Material and Method
Sampling
About 10g of soil sample was collected from petro pump area (Bharat Petroleum, Vishal Enclave, New Delhi). Soil was sampled from 15 cm depth. Sample was placed in sterile falcon tubes and stored at 4°C immediately after they bought to the laboratory.
Isolation
Microbes were isolated in minimal salt medium (MSM). MSM contain (for 1L) 0.9 g of Na2HPO4, 0.7 g of K2HPO4, 2.0 g of NaNO3, 0.4 g MgSO4.7H2O, 0.1 g of CaCl2, 20 g of glucose and 2 g/l of Trace salt (FeSO4 and MnSO4). After adding all the components distilled water was added and pH was adjusted to 7.2 by using 1N Sodium hydroxide. Then medium was autoclave at 121°C and 15 psi pressure for 20 minutes. Bacteria were isolated by using single colony isolation technique. Isolated microbial cultures were stored at 4°C for further use.
Physical characterization of bacterial isolates
Morphology identification: Identification of morphology of bacterial cells was done by its size, shape and colour of the colony in the nutrient agar plates. The observations were recorded (Table 1).
| Sample name | Gram character | Shape | Morphology |
|---|---|---|---|
| SA1 | Gram positive | Coccobacillus | Translucent, brownish pigment, irregular shape colony |
| SA2 | Gram positive | Long rods | Translucent, brownish pigment, irregular shape colony |
| SA3 | Gram negative | Coccobacillus | Translucent, green pigment, irregular shape colony |
| SA4 | Gram negative | Coccobacillus | Translucent, brownish pigment, irregular shape colony |
| SB1 | Gram negative | Coccobacillus | Translucent, brownish pigment, irregular shape colony |
| SB2 | Gram negative | Rods in chain | White colony, irregular shape colony |
| SC1 | Gram negative | Coccobacillus | Translucent, brownish pigment creamish colony, irregular shape colony |
| SC2 | Gram negative | Small rods | White, irregular shape colony |
| SC3 | Gram negative | Small rods | translucent , pinkish pigment ,light creamish, irregular shape colony |
| SC4 | Gram negative | Big rods | Creamish, irregular shape colony |
Table 1: Physical characterization of bacterial isolates.
Gram staining protocol: A thin smear was prepared and heat fixed and then covered with a thin film of crystal violet for one minute. Then Gram’s iodine was added for one minute and washed with tap water. Then smear was washed with 70% ethanol and again wash with tap water to stop decolourization. After this counterstained of 0.25% safferanine was added for one minute the wash with tap water and blot dry and then examined under light microscope using 100x oil immersion. The observations were recorded (Table 1).
Biosurfactant production screening
All the isolated colonies were tested for their biosurfactant production activity by using three methods.
Oil spreading assay
50 ml of distilled water was added to the petri plate and the 20 μl of diesel was added in to this petri plate to form a thin layer of oil. After this 10 μl of culture supernatant was placed gently on the centre of the oil layer. Displacement of oil layer by formatting a clear zone indicates the biosurfactant presence. Formation of oil displacement was visualized under visible light and measured after 30 seconds, this correlates to the biosurfactant activity of isolates. The observations were recorded (Table 2).
| Sample name | Oil displacement (in cm) | ||
|---|---|---|---|
| 24 h | 48 h | 72 h | |
| SA1 | 2.5 | 2.5 | 3.2 |
| SA2 | 1.5 | 2.0 | 2.6 |
| SA3 | 1.2 | 3.5 | 3.6 |
| SA4 | 2.3 | 2.3 | 3.1 |
| SB1 | 0.0 | 0.0 | 1.5 |
| SB2 | 0.0 | 0.4 | 0.6 |
| SC1 | 1.8 | 1.9 | 3.3 |
| SC2 | 0.6 | 0.6 | 0.8 |
| SC3 | 2.0 | 2.5 | 2.6 |
| SC4 | 0.6 | 1.0 | 1.6 |
Table 2: Oil displacement activity of bacterial isolates.
Emulsification assay
2 ml of diesel was taken in a test tube in which 1 ml of culture supernatant was added and vortexed for 2 minutes for homogenous mixing. Emulsification activity was observed after 24 h and calculated by using the formula:

The observations were recorded (Table 3).
| Sample name | Emulsified layer (in cm) | Total height of solution (in cm) | % of E24 |
|---|---|---|---|
| SA1 | 0.3 | 2.5 | 12 |
| SA2 | 0.2 | 2.5 | 8 |
| SA3 | 0.4 | 2.5 | 20 |
| SA4 | 0.2 | 2.5 | 8 |
| SB1 | 0.1 | 2.5 | 4 |
| SB2 | 0.0 | 2.5 | 0 |
| SC1 | 0.3 | 2.5 | 12 |
| SC2 | 0.0 | 2.5 | 0 |
| SC3 | 0.2 | 2.5 | 8 |
| SC4 | 0.2 | 2.5 | 8 |
Table 3: Emulsification activity of bacterial isolates (after 24 h).
CTAB agar plate
MSM media plate containing CTAB (cetyltrimethylammonium bromide (a cationic surfactant)) and methylene blue dye was used to test this assay. Well were created by using the opposite side of the pipette tip. In these wells 10 μl of culture supernatant was poured and plates were incubated for 48 h. Presence of biosurfactant activity showed the formation of dark blue halos. The observations were recorded (Table 4).
| Sample name | Zone formation (in cm) | Diameter of well (in cm) | Actual zone formation (in cm) |
|---|---|---|---|
| SA1 | 2.1 | 1 | 1.1 |
| SA2 | 2.0 | 1 | 1.0 |
| SA3 | 2.3 | 1 | 1.3 |
| SA4 | 2.1 | 1 | 1.1 |
| SB1 | 2.2 | 1 | 1.2 |
| SB2 | 0.0 | 1 | 0.0 |
| SC1 | 2.1 | 1 | 1.1 |
| SC2 | 0.0 | 1 | 0.0 |
| SC3 | 2.0 | 1 | 1.0 |
| SC4 | 2.2 | 1 | 1.2 |
Table 4: CTAB assay of bacterial isolates (after 24 h).
Effect of different pH on growth of the potential biosurfactant producing microbe
Bacterial isolates (A3) was inoculated in 25 ml of Erlenmeyer flasks containing MSM media at different pH i.e. 6.6, 7.0, 7.6 and 8.0 and then cultures were incubated at the temperature of 37°C in an orbital shaker at 140 rpm for 72 h. The observations were recorded (Table 5).
| Sample A3 | |||||||||
|---|---|---|---|---|---|---|---|---|---|
| 24 h | 48 h | 72 h | |||||||
| pH | OD (cm) | EL (cm) | CZ (cm) | OD(cm) | EL (cm) | CZ (cm) | OD (cm) | EL (cm) | CZ (cm) |
| 6.6 | - | - | - | 2.4 | 1.6/3.2=50% | 0.8 | 4.8 | 2.2/4=55% | 0.9 |
| 7.0 | - | - | - | 2.1 | 1.1/3.2=34% | 0.9 | 4.6 | 2.1/4=52.5% | 1.0 |
| 7.6 | - | - | - | - | - | 0.7 | 4.5 | 1.9/4=47.5% | 0.8 |
| 8.0 | - | - | - | - | - | 0.6 | 3.9 | 2.2/4=55% | 0.7 |
OD: Oil Displacement EL: Emulsion Layer CZ: CTAB Zone Formation
Table 5: Effect of different pH on growth.
Results and Discussion
Soil from petroleum contaminated area was collected. Biosurfactant producing bacterial isolates were screened for their potential activity. Out of ten isolates eight of them show good oil displacement activity, emulsifying activity and CTAB activity which indicates their potential role in oil degradation activity. Out of those eight one isolates which was given highest oil displacement activity, emulsifying activity and CTAB activity was chosen for the pH optimization experiment. pH optimization showed that the culture SA3 produce highest amount of biosurfactant activity at pH 6.6 and the biomass obtained was 0.30724 g and at pH 7.6 it showed lower biosurfactant activity than pH 6.6 but the biomass was higher i.e. 0.93172 g. It was also observed that higher pH i.e. pH 8 decrease the growth of the bacterial biomass i.e. 0.24301 g as well as the biosurfactant activity [5]. Isolated Bacillus lichneformis in mineral salt medium and showed that the microbes have the capacity of biodegrading crude oil which was the only carbon and energy source for their growth. [6] isolated five microbes and screened them for their biosurfactant activity by using oil displacement and blood haemolysis method [7] isolated 42 microbes and screened them for their biosurfactant activity and out of these isolates only six of them showed highest CFU and production of biosurfactant.
Conclusion
The present study concluded the potential ability of bacterial isolates as a biosurfactant producing agents. They were grown on MSM medium and showed potential oil degradation and biosurfactant production activity. Out of ten isolated eight of them showed the biosurfactant activity and one isolate i.e. SA3 which showed the maximum activity were further utilized for the pH variation study. It was also observed that increase in pH inhibits the growth of microbes as well as the production of bio surfactant.
Conflict of Interest
The authors declare that there are no conflicts of interest.
References
- Ma Y, Oliveira RS, Freitas H, et al. Biochemical and molecular mechanisms of plant-microbe-metal interactions: Relevance for phytoremediation. Frontiers in Plant Science. 2016;7:918.
- Muthusamy K, Gopalakrishnan S, Ravi TK, et al. Biosurfactants: Properties, commercial production and application. Curr Sci. 2008;94:736-47.
- Santos DKF, Rufino RD, Luna JM, et al. In: Biosurfactants: Multifunctional biomolecules of the 21st Century. Taubert A, editor. International Journal of Molecular Sciences. 2016;17:401.
- Alvarez PJJ, Vogal TM. Substrate interactions of benzene, toluene and oara-xylene during microbial deyredation by pure cultures and mixed culture quifer slurries. Applied and Environmental Microbiology. 1991;57:2931-85.
- Sheshtawy HS, ElTabei AS, Kobisy AS, et al. Application of biosurfactant produced by Bacillus lichneformis and chemical surfactant in biodegradation of crude oil: Part 1. Bioscien Biotech Research Asia. 2013;10:515-26.
- Anandarj B, Thivakaran. Isolation and production of biosurfactant producing organism from oil spilled soil. J Biosci Tech. 2010;1:120-26.
- Vijaya B, NR Jayalakshmi, K Manjunath. Enumeration of biosurfactant producing microorganisms from oil contaminated soil in and around Bangalore (India). Int J Curr Sci. 2013; 5:86-94.