Original Article
, Volume: 18( 1)A RP-HPLC Method for Estimation of Tramadol HCl and Chlorzoxazone in Tablet Formulation-Validation
- *Correspondence:
- Dutta M, , Department of Pharmaceutical Chemistry, Delhi Institute of Pharmaceutical Sciences and Research, Pushp Vihar, Sector-3, M.B. Road, New Delhi-110017, India, Tel: 01129553771; E-mail: manashi.dutta13@gmail.com
Received: January 06, 2018; Accepted: January 10, 2018; Published: January 16, 2018
Citation: Dutta M, Wakode S. A RP-HPLC Method for Estimation of Tramadol HCl and Chlorzoxazone in Tablet Formulation-Validation. Anal Chem Ind J. 2018;18(1):127
Abstract
A simple, rapid and selective method involving reverse phase liquid chromatography is developed for the concurrent determination of Tramadol HCl and Chlorzoxazone in tablet formulation. The chromatographic parameters involved using a Promosil C18 column with dimensions 4.6*250 mm, 5 μ and Acetonitrile and 0.1% Trifluroacetic acid in water in the ratio 60:40 (v/v) as the mobile phase. The flow rate set was of 1.0 mL/min and detected at 275 nm using UV detector. The linearity of Tramadol HCl and Chlorzoxazone were in the range of 25.00 μg/mL-45.00 μg/mL and 125.00 μg/mL-225.00 μg/mL respectively. Standard addition method was used to calculate percentage recovery. The average amount of recovery was found to be 98.8% and 99.7% for Tramadol HCl and Chlorzoxazone respectively. The method was found to be accurate and precise for determining both the drugs simultaneously.
Keywords
HPLC; Tramadol HCl; Chlorzoxazone; Tablet
Introduction
Tramadol hydrochloride (Molecular formula: C16H25O2N.HCl, Molecular weight: 299.8), is an analgesic drug acting centrally and has been shown to be a synthetic analogue of codeine [1]. M1 (O-desmethyl Tramadol) is formed which possesses analgesic properties after Tramadol HCl being metabolized by Cytochrome P450, an enzyme found in liver [2]. It has been used since 1977 for the relief of strong physical pain and has been the most widely sold opioid analgesic drug in the world [3]. Chlorzoxazone (Molecular formula: C7H4ClNO2; Molecular weight: 169.57) [4,5] acts as an muscle relaxant. Chlorzoxazone is a centrally acting muscle relaxant. It is used to treat muscle spasm and the resulting pain or discomfort. It acts on the spinal cord by depressing reflexes. The structural formulas of Tramadol HCl and Chlorzoxazone are shown in Fig. 1 and 2.
Here, in this research paper we are reporting a RP HPLC method for determination of one such combination simultaneously which contains 50 mg Tramadol HCl and 250 mg Chlorzoxazone. This method was developed and validated according to ICH tripartite guidelines [6]. Tramadol HCl and Chlorzoxazone are well known pharmaceutical molecules, when in combination is used for relieving lower back pain and cervical osteoarthritis. After considerable literature search, it was found that though there are method for separation, identification, of both molecules separately [7-11] and simultaneously [12-15] with other analgesics but in case of this combination no published method is still available for appropriate separation and quantification of both the drugs in pharmaceutical preparations by HPLC. Thus on the background of all the earlier experimental studies conducted by several researchers, the experimental design of this present study was drawn to carry out a comprehensive scientific investigation for successful development of the HPLC method for accurate, reliable, most effective, highly sensitive and rapid separation, identification as well as quantitative determination of both the said compounds in the pharmaceutical dosage and formulations so that a comprehensive scientific study would be achieved for faster and effective separation as well as quantification of both molecules in pharmaceutical preparations.
Materials and Methods
The formulation, MUZOX (Stedman Pharmaceuticals Pvt. Ltd) Tablets (containing 50 mg of Tramadol HCl and 250 mg of Chlorzoxazone) were procured from pharmacies. All working standards were obtained from Ranbaxy Laboratories Ltd., Gurgaon and Bhawna Laboratories, Raigad with certificate of analysis. Water, Acetonitrile, Methanol and Trifluroacetic acid used were all HPLC grade. The dilutions were made using calibrated volumetric flasks.
The analysis was performed on Waters HPLC having Waters 515 HPLC pump, equipped with Waters 717 plus auto sampler and a Waters 2998 PDA detector. The chromatographic data were collected through Empower2 Chromatographic software.
Preparation of working standard
Standard solution S1 (1 mg/ml) and standard solution S2 (5 mg/ml) for Tramadol HCl and Chlorzoxazone respectively were prepared using HPLC grade methanol. Then final working standard S3 (0.1 mg or 100 μg Tramadol/ml+0.5 mg or 500 μg Chlorzoxazone/ml) was prepared by taking 10 ml each of Standard S1 and Standard S2. The solution is mixed, then kept for sonication for 10 min and using 0.22 μm Millipore filter unit the solution is filtered. The chromatographic separation was performed using isocratic elution mode of mobile phase containing acetonitrile and 0.1% trifluroacetic acid in water (60:40, v/v), pH 1.5 on a column, Promosil C18 with dimensions 250 mm × 4.6 mm × 5 μm and a flow rate of 1.00 mL/min at 275 nm using PDA detector.
Calibration curves for Tramadol HCl and Chlorzoxazone
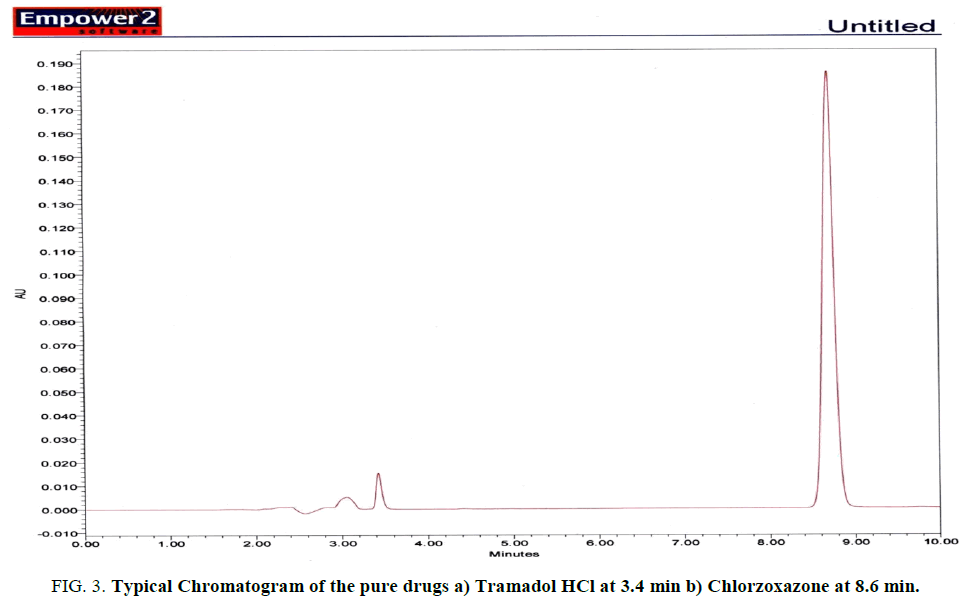
Five different concentrations containing mixture of Tramadol HCl and Chlorzoxazone were prepared in methanol from stock solution using 10 mL volumetric flasks. The drugs concentration range was found to be 25.00 μg/mL to 45.00 μg/mL for Tramadol HCl and 125.00 μg/mL to 225.00 μg/mL for Chlorzoxazone respectively. An injection of 10 μL of each solution was made in triplicates. The chromatograms and peak areas were recorded and calculated. When a curve between peak areas (average peak areas of three replicates) versus concentrations was drawn a linear relationship is observed for Tramadol HCl and Chlorzoxazone in the above linearity range. This range that is found is set as linear range for further method development of the two components Fig. 3.
Figure 3: Typical Chromatogram of the pure drugs a) Tramadol HCl at 3.4 min b) Chlorzoxazone at 8.6 min.
Analysis of formulation
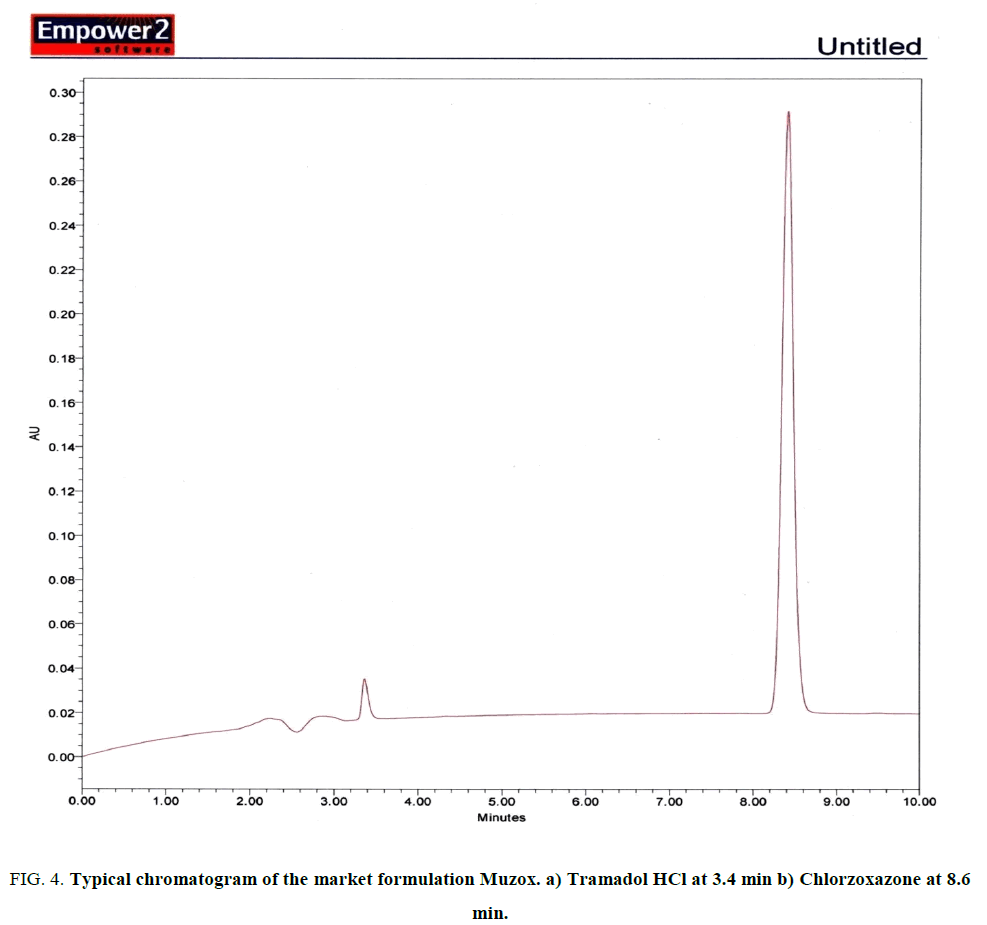
For preparation of sample solution, 20 tablets were crushed and weighed in an electronic balance and the average weight was taken. These tablets were then powdered. The weight that is equivalent to one tablet was taken in a 50 ml volumetric flask, and dissolved using HPLC grade methanol and diluted up to the mark with methanol. Sample solution was then sonicated for 15 min for complete dissolution of active ingredients. The solution prepared was then filtered through Millex (0.22 μm) syringe driven filter unit. Then one ml of filtrate was taken and diluted to 50 ml with HPLC grade methanol to get 20 μg/mL of Tramadol HCl and 100 μg/mL of Chlorzoxazone respectively Fig. 4.
Figure 4: Typical chromatogram of the market formulation Muzox. a) Tramadol HCl at 3.4 min b) Chlorzoxazone at 8.6 min.
Method validation
Using optimized chromatographic parameters from the above sample solution, 10 μL was injected in triplicate along with the same concentration of standard solution to Waters HPLC system. The values of peak area of Tramadol HCl, and Chlorzoxazone were calculated. The amount of Tramadol HCl and Chlorzoxazone present was then estimated using regression equation (calibration curve method). Results of assay are displayed in Table 1.
| Name of Drug | Amount of labeled claim (mg) | Amount found (mg) | % Recovery | ± CI |
|---|---|---|---|---|
| Tramadol HCl | 50 | 49.4 | 98.8 | 0.079 |
| Chlorzoxazone | 250 | 249.5 | 99.7 | 0.159 |
Table 1: Result of assay experiment.
To study the accuracy and precision of the optimized method, recovery experiments were carried out which checks for the presence of interferences from excipients present in the formulation. The standard addition method was used for performing recovery experiment. The recovery of the added standard was studied at three different levels namely 60%, 100% and 120% of the drug and results were calculated for each set of recovery. The results of recovery experiment are displayed in Tables 2 and 3.
| Conc. of Formulation (μg/mL) | Conc. of pure drugs spiked(μg/mL) | Total Conc. of the drug (μg/mL) | Mean Conc. (μg/mL) | SD | % Recovery | ± CI |
|---|---|---|---|---|---|---|
| 40 | 80 | 60 | 59.7 | 0.054 | 99.5 | 0.053 |
| 40 | 100 | 70 | 69.5 | 0.115 | 99.2 | 0.112 |
| 40 | 120 | 80 | 80.1 | 0.054 | 100.1 | 0.052 |
Table 2: Recovery of the analytical method by standard addition method for tramadol HCl.
| Conc. of Formulation (μg/mL) |
Conc. of pure drugs spiked (μg/mL) |
Total conc. of the drug (μg/mL) |
Mean Conc. (μg/mL) |
SD % Recovery ± CI | ||
|---|---|---|---|---|---|---|
| 40 | 80 | 60 | 59.6 | 0.112 | 99.3 | 0.112 |
| 40 | 100 | 70 | 70.2 | 0.05 | 100.4 | 0.048 |
| 40 | 120 | 80 | 79.7 | 0 | 99.6 | 0 |
Table 3: Recovery of the analytical method by standard addition method for Chlorzoxazone.
The precision study of Tramadol HCl and Chlorzoxazone was carried out by calculating the corresponding responses 3 times on the same day (Intraday) and on 3 different days (Interday) for the concentration of 25 μg/mL, 35 μg/mL and 45 μg/mL for Tramadol HCl and 125 μg/mL, 175 μg/mL and 225 μg/mL for Chlorzoxazone. Results are displayed in Table 4.
| Parameters calculated (Units) | Tramadol HCl | Chlorzoxazone |
|---|---|---|
| Linearity Range (μg/mL) | 25-45 | 125-225 |
| r2 | 0.998 | 0.9990 |
| Slope | 9611 | 32820 |
| Intercept | 29265 | 16914 |
Table 4: Calibration curves linear regression data.
System suitability tests to check the system reproducibility were performed by injecting the drug solutions repetitive times and the results for these tests are displayed in Table 5.
| Parameters studied (Units) | Tramadol HCl | Chlorzoxazone |
|---|---|---|
| Linearity Range (μg/mL) | 25-45 | 125-225 |
| Correlation coefficient | 0.998 | 0.999 |
| Recovery (%) | 98.8 | 99.7 |
| Precision (%RSD) | - | - |
| Intraday (n=4) | 0.08 | 0.14 |
| Interday (n=4) | 0.06 | 0.12 |
| Robustness | Robust | Robust |
| Retention time | 3.4 | 8.6 |
| Theoretical plates | 3495 | 14070 |
| Tailing factor | 1.1 | 1.0 |
Table 5: Results of validation and system suitability parameters.
Results and Discussion
Single pure drugs chromatogram was run in different composition mobile phases containing methanol, acetonitrile, water and different buffers in different ratios. The resultant retention time and tailing factor for each drug was calculated. Finally a mobile phase comprising of Acetonitrile and 0.1% Trifluroacetic acid in water in the ratio 60:40 (v/v) and Promosil C18 column was selected which showed a sharp and symmetrical peak with minimum amount of tailing. Calibration curve was found to be linear in the range of 25.00 μg/mL to 45.00 μg/mL, and 125.00 μg/mL to 225.00 μg/mL for Tramadol HCl and Chlorzoxazone respectively.
Five different concentration solutions containing mixture of the two drugs in above given range were prepared and from each solution 10 μl was injected into HPLC. By regression method analysis of the calibration data obtained for the two drugs showed that the dependent variable (peak area) and the independent variable (concentration) were found to be y=9611x-29265 and y=32820x+16914 for Tramadol HCl and Chlorzoxazone respectively.
The correlation of coefficient (r2) was found to be 0.9988 and 0.9990 for Tramadol HCl and Chlorzoxazone respectively. It was seen that the concentration range showed a good relationship and therefore proves the sensitivity of the optimised method for the drugs. The % assay (average amount) of Tramadol HCl and Chlorzoxazone was found to be 98.8%, and 99.7% respectively in the formulation (tablet). The % recovery for Tramadol HCl, and Chlorzoxazone was found to be 100.1%, and 100.4% respectively which shows that method is free from any interference from excipients which are present in the formulation. Similarly, the low values of standard deviation and coefficient of variation at each level of the recovery experiment determined indicate the method is of high precision.
Conclusion
The HPLC method for the determination of Tramadol HCl and Chlorzoxazone from their fixed dosage form was found to be precise and accurate. The proposed RP-HPLC method to determine simultaneously Tramadol HCl and Chlorzoxazone by PDA detector is found to be a simple, precise, rapid and selective and which may be employed for its assay in both bulk drugs and formulation.
Acknowledgement
We are thankful to Ranbaxy Laboratories Ltd. Gurgaon and Bhawna Laboratories, Raigad for providing the gift samples of Chlorzoxazone and Tramadol HCl. We are also thankful to Mr. Aneesh Jain, Nimisha Pharmaceuticals, Mumbai for his help in this project.
References
- Lewis KS, Han NH. Tramadol: A new centrally acting analgesic. Am J Health Syst Pharm. 1997;54(6):643-52.
- Budd K, Langford R. Tramadol revisited. Br J Anaesth. 1999;82(4):493-495.
- Lintz W, Barth H, Osterloh G, et al. Pharmacokinetics of Tramadol and bioavailability of parenteral Tramadol formulations. Drug Res. 1998;48(11);889-99.
- Budavari S, Maryadele Neil JO, Smith A, et al. The Merck Index, Merck Research Laboratories. 13th ed. 2001.
- Lunn G, Schmuff NR, Wiley J, et al. HPLC methods for pharmaceutical analysis. 1999;25:367.
- Chuan GY, Faurett JP. Improved HPLC method for simultaneous determination of Tramadol and O-desmethyl Tramadol in human plasma. J Chromatogr B. 2005;82:240-43.
- Chang YG, Thau SM, Lin YC, et al. High performance liquid chromatographic method of determination of Tramadol in human plasma. J Chromatogr B. 1999;723:247-53.
- Thomas AB, Dumbre NG, Nanda RK, et al. Simultaneous determination of tramadol and ibuprofen in pharmaceutical preparations by first order derivative spectrophotometric and LC methods. Chromatographia. 2008;68:843-47.
- Aysel K, Yucel K. Determination of Tramadol HCl in ampoule dosage form by using UV spectrophotometry and HPLC-DAD methods in methanol and water media. II Farmaco. 60;(2):163-169.
- Sastry CSP, Chintalapati. Spectrophotometric methods for determination of Chlorzoxazone in tablets. Indian J Pharm Sci. 2001;63(3)260-63.
- Reddy BP. Simultaneous estimation of Nimesulide and Chlorzoxazone in pharmaceutical formulations by RP-HPLC. J Chemech Research. 2009;1(2):283-85.
- Pawar UD, Abhijit V, Sulebhavikar AV, et al. Simultaneous determination of Acelofenac, Paracetamol and Chlorzoxazone by HPLC. E J Chem. 2009; 6(1):289-94.
- Goyal A, Jain S. Simultaneous estimation of Paracetamol, Chlorzoxazone and Diclofenac Sodium in pharmaceutical formulation by novel HPLC method. Acta Pharmaceutica Scientia. 2007;49:147-51.
- Ravishankar S, Vasudevan M, Gandhimathi M, et al. Reversed phase HPLC method for estimation of Acetaaminophen, Ibuprofen and Chlorzoxazone in pharmaceutical formulations. Talanta. 1998;46:1577-81.
- Puranik M, Hirudkar A, Wadher SJ, et al. Development and validation of spectrophotometric methods for simultaneous estimation of Tramadol HCl and Chlorzoxazone in tablet dosage form. Indian J Pharm Sci. 2006;68(6):737-39.